UvA-DARE (Digital Academic Repository) Heparins, cancer ... · longer period of time. They were...
Transcript of UvA-DARE (Digital Academic Repository) Heparins, cancer ... · longer period of time. They were...

UvA-DARE is a service provided by the library of the University of Amsterdam (http://dare.uva.nl)
UvA-DARE (Digital Academic Repository)
Heparins, cancer and thrombosis: clinical and experimental studies
Smorenburg, S.M.
Link to publication
Citation for published version (APA):Smorenburg, S. M. (2000). Heparins, cancer and thrombosis: clinical and experimental studies.
General rightsIt is not permitted to download or to forward/distribute the text or part of it without the consent of the author(s) and/or copyright holder(s),other than for strictly personal, individual use, unless the work is under an open content license (like Creative Commons).
Disclaimer/Complaints regulationsIf you believe that digital publication of certain material infringes any of your rights or (privacy) interests, please let the Library know, statingyour reasons. In case of a legitimate complaint, the Library will make the material inaccessible and/or remove it from the website. Please Askthe Library: https://uba.uva.nl/en/contact, or a letter to: Library of the University of Amsterdam, Secretariat, Singel 425, 1012 WP Amsterdam,The Netherlands. You will be contacted as soon as possible.
Download date: 31 Aug 2020

IN VIVO TREATMENT OF RATS WITH UNFRACTIONATED HEPARIN OR LOW MOLECULAR WEIGHT HEPARIN DOES NOT
AFFECT EXPERIMENTALLY INDUCED COLON CARCINOMA METASTASIS
Susanne M. Smorenburg, Roel Vink, Merijn te Lintelo, Wikky Tigchelaar, Adrie Maas, Harry R.
Büller, Cornells J. F. van Noorden
CLINICAL & EXPERIMENTAL METASTASIS, 1999; 17:451-456

ABSTRACT
Recent randomized trials have suggested that treatment with low molecular weight heparin
(LMWH) improves survival of cancer patients with venous thromboembolism, as compared to
treatment with unfractionated heparin (UFH). Experimental studies have shown that UFH has
activities besides its anticoagulant function which may affect progression of malignancy, including
stimulation of new blood vessel formation. In contrast, LMWH has been suggested to inhibit
angiogenesis. In the present study, we compared quantitatively the effects of treatment with UFH,
LMWH or placebo on the development of experimentally induced colon carcinoma metastases in
rat liver and on tumor-associated angiogenesis. It is shown that UFH and LMWH in therapeutic
dosages neither affect development of metastases nor tumor blood vessel formation in this animal
model. These results indicate that heparins do not affect colon cancer metastasis in liver. Further
studies in other animal models are required to establish the mechanisms by which heparins
potentially affect cancer.
INTRODUCTION
Patients with malignancy frequently suffer from thromboembolic complications. As a consequence,
numerous cancer patients are treated with anticoagulants such as unfractionated heparin (UFH) or
low molecular weight heparin (LMWH). It is a matter of debate whether these agents interfere with
cancer progression and alter prognosis of patients with malignancies.
Experimental studies have shown that UFH is endowed with multiple activities besides its
anticoagulant function, including inhibition of the immune system during inflammation, cell
proliferation, cellular adhesion and invasion, and enhancement of effects of various growth factors
and proteases (1-3). In addition, UFH may stimulate angiogenesis, an essential process in tumor
growth, by the release of angiogenic growth factors from the extracellular matrix and by induction
of their activity through binding to the high affinity receptors (4). As a consequence, UFH treatment
may alter the prognosis of patients with cancer, either in a positive or negative way.
Randomized trials, which compared the efficacy and safety of LMWH with UFH in the treatment
of patients with venous thromboembolism, have created renewed interest in the potential of UFH
and its fractions to affect cancer progression (5-8). It was shown that cancer patients with venous
thromboembolism who were treated with LMWH had a significantly improved 3-6 month survival
as compared to UFH recipients. Recurrence of thromboembolic and bleeding complications was
identical in both patient groups and the difference in mortality was not found in patients with venous
thromboembolism without cancer. The difference was observed in a wide variety of malignancies,
including gastro-intestinal, genito-urinary, breast, haematological and central nervous system
malignancies (8). Although these results were obtained with a post hoc analysis, they suggest that
90

either LMWH affects survival of patients positively and/or UFH negatively. However, in a recent
systematic review of clinical studies it was shown that UFH had no effect on prognosis of patients
with cancer (9).
Studies on the effects of LMWH on tumor progression are limited. LMWH, in contrast to UFH, has
been shown to suppress angiogenesis in vivo (10-12). Angiogenesis is stimulated by heparin-
binding growth factors, such as basic fibroblast growth factor (bFGF) and vascular endothelial
growth factor (VEGF) (13). Heparin oligosaccharides containing less than 10 saccharide residues
have been found to inhibit biological activity of bFGF, whereas heparin fragments with less than 18
saccharide residues have been reported to inhibit the binding of VEGF to its receptors on
endothelial cells (12, 14). These findings implicate that the size of the heparin molecule may be a
critical determinant for the binding of bFGF and VEGF to their receptors, and that the effects of
LMWH on blood vessel formation may differ from those of UFH.
In the present study, we compared quantitatively the effects of treatment with UFH, LMWH or
placebo on development of experimentally induced colon carcinoma metastasis in rat liver as well
as on tumor-associated angiogenesis.
MATERIALS AND METHODS
CANCER CELLS AND INDUCTION OF LIVER METASTASES
A colon adenocarcinoma cell line, CC531s, was obtained from a moderately differentiated and
weakly immunogenic colon adenocarcinoma after experimental induction in WAG-Rij rats by
treatment with 1,2-dimethylhydrazine (15). Cells were cultured in vitro at 37°C as monolayers in
Dulbecco's modified Eagle's medium (ICN Biomedicals, Irvine, Ayrshire, UK) supplemented with
10% (v/v) fetal bovine serum, 2 mmol/1 glutamine, 100 IU/ml penicillin, and 100 mg/ml
streptomycin. Cells were washed with phosphate buffered saline (PBS) and after detachment with
0.05% trypsin in PBS and centrifugation (250 g, room temp, 10 min), single cell suspensions were
obtained with a viability of at least 95%. After anaesthesia with 0.07 ml/10 g body weight Fentanyl-
Fluanizone-Midazolam mixture (FFM; 1ml Hypnorm, Janssen, Beerse, Belgium; 1ml Midazolam,
Roche, Mijdrecht, The Netherlands and 2 ml water), colon carcinoma metastasis was induced in rat
liver by injection of a single cell suspension containing 1 x 105 cancer cells into the portal vein of
54 mature female WAG-Rij rats, weighing 130-150 g (Broekman, Someren, The Netherlands) as
described previously (16). To prevent peritoneal seeding, the puncture site was covered with
Spongostan (Medical Workshop, Groningen, The Netherlands) until complete haemostasis was
obtained.
CHAPTER 7 EFFECTS OF HEPARINS ON COLON CARCINOMA METASTASIS 91

TREATMENT PROTOCOL
In the first experiment, 3 groups of 10 rats each were treated intraperitoneally (i.p.) with either 0.9
% NaCl once daily, 2.0 mg/kg LMWH ( Reviparin, 7000 IU aXa/ml; Knoll, Ludwigshafen,
Germany) dissolved in 0.9% NaCl once daily or 2.0 mg/kg UFH (5000 IU/ml; Leo, Weesp, The
Netherlands) dissolved in 0.9% NaCl twice daily, respectively. These dosages correspond with the
human, therapeutic dose of UFH or LMWH. Treatment was started one day before operation of the
animals and was continued up to 7 days after administration of cancer cells. On the day of operation,
the compounds were given one h before inoculation of cancer cells in all treatment groups. To
measure anti-activated factor X (aXa) plasma levels, 0.5 ml blood was sampled from the tail vein
of all rats on the 5th day after inoculation, at 3, 6, 9, and 12 h after injection. Seven days after
administration of cancer cells, animals were weighed and sacrificed with an overdose of FFM
mixture.
In the second experiment, 3 groups of 8 rats received higher doses of LMWH and UFH and for a
longer period of time. They were treated with either 0.9% NaCl once daily, 2.5 mg/kg LMWH once
daily, or 2.5 mg/kg UFH once daily, respectively, using a similar set up as in the first experiment,
but treatment was continued up to 24 days after administration of cancer cells. Blood was sampled
on the 20th day, at 1, 3, and 6 h after the injection. Animals were weighed and sacrificed at 24 days
after cancer cell administration. The animal experiments were performed according to guidelines of
the University of Amsterdam.
MEASUREMENT OF P L A S M A A X A LEVELS
Blood samples were collected in EDTA, centrifuged (15 min, 10.000 g, 4°C) and plasma was stored
at -80°C until use. Plasma UFH and LMWH levels were assayed by measuring inhibition of factor
Xa, using the chromogenic substrate S-2222 (Chromogenix, Mölndal, Sweden) as described
previously (17). For calibration, UFH and LMWH in normal rat plasma was used.
PREPARATION OF LIVER TISSUE AND EVALUATION OF TUMOR GROWTH
Livers were removed immediately after sacrifice and the individual liver lobes were divided and cut
into small pieces (up to 5 mm thick) according to a rigid dissection protocol (18). Tissue fragments
were frozen in liquid nitrogen and the material was kept at -80°C until further use. To quantify the
number of metastases after 7 days of growth, 21 sites were randomly selected from all lobes of each
liver before experiments were carried out. From these sites, sections (8 mm thick) were cut on a
motor-driven cryostat with a rotary retracting microtome (Bright, Huntingdon, UK) at a constant
speed and a cabinet temperature of-25°C.
The number of metastases after 24 days were quantified by counting metastatic nodules on the liver
surface. It appeared in a previous study that the number of macroscopically detectable nodules on
the surface of the livers was a valid and reproducible measure of tumor load in the livers (19). The
92

pieces of liver that contained macroscopically visible metastases were frozen in liquid nitrogen and
stored at -80°C until further use. To evaluate size and vessel density of the tumors, 20 sections (8
mm thick) were cut at the site where the tumors had the largest diameter. Sections were mounted
on glass slides and stored at -20°C until use.
IMMUNOHISTOCHEMICAL PROCEDURES
To facilitate recognition of cancer cells, cryostat sections were stained immunohistochemically
using the lectin Ulex Europaeus agglutinin I (UEA-1), a selective marker of CC531s cells in rat as
described previously (16). Briefly, sections were air dried at room temp for 1 h and fixed with
aceton for 10 min at 4°C. Endogenous peroxidase activity was blocked with 0.3% (v/v) hydrogen
peroxide and 0.1% NaN3 in PBS for 15 min at room temp. After preincubation with normal goat
serum (Dako, Glostrup, Denmark: 10% v/v in PBS for 10 min at room temp), sections were
incubated for 60 min using UEA-1 (Dako) diluted 1:100 in PBS, then 30 min with rabbit anti-UEA-
1 conjugated with horseradish peroxidase (Dako) diluted 1:50 in PBS in the presence of 10% AB
serum (CLB, Amsterdam, The Netherlands). Sections were stained for peroxidase activity using
3,3-diaminobenzidine-tetrachloride and hydrogen peroxide as described previously (16).
Endothelial cells were detected with a rat endothelial cell-specific monoclonal antibody (RECA-1),
kindly provided by Dr. A. M. Duijvestijn, University of Maastricht, The Netherlands (20). Sections
were air-dried at room temp for 1 h, then fixed 10 min in 4% formaldehyde in 0.1 M phosphate
buffer (pH 7.4). Endogenous peroxidase activity was blocked with 0.3% (v/v) hydrogen peroxide
and 0.1% NaN3 in PBS for 15 min. Sections were incubated for 60 min with RECA-1 diluted 1:10
in PBS containing 0.2% (w/v) bovine serum albumin, then for 60 min with rabbit anti-mouse IgG
peroxidase (Dako) diluted 1:200 in PBS in the presence of 0.2% bovine serum albumin and 5%
normal rat serum. Sections were stained for peroxidase activity using 3,3-diaminobenzidine-
tetrachloride and hydrogen peroxide (16). Control sections were incubated in the absence of the first
antibody.
MORPHOMETRIC ANALYSIS
The number of tumors after 7 days as detected with UEA-1 was analyzed quantitatively by
morphometry in cryostat sections obtained from the 21 selected sites in the livers (18, 21). The
percentage area of cancer cells was expressed as the ratio of the area of cancer cells and the total
area of the liver tissue sections including cancer cells (xlOO) ± standard error of the mean (SEM).
After 24 days, the largest area of each tumor was measured using sections that were stained with
UEA-1. The values were averaged to determine the mean area of tumors ± SEM per group. Vessel
density of tumors was determined in 2 sections adjacent to the section with the largest area and the
values were averaged. Vessel density was determined as the ratio of the area of RECA-1-positive
endothelial cells in the tumor and the total area of UEA-1-positive tumor (xlOO) ± SEM.
CHAPTER 7 EFFECTS OF HEPARINS ON COLON CARCINOMA METASTASIS 93

Analysis of the number of tumors and their vessel density was performed without knowledge of the
treatment group. Statistical analysis of the percentage areas of tumors after 7 days was performed
with the Kruskall-Wallis test, whereas ANOVA was used to determine statistical differences in
number, size, and vessel density of metastases after 24 days. Values of p<0.05 were considered to
indicate significant differences.
RESULTS
A X A LEVELS IN PLASMA
The aXa plasma levels measured in UFH- and LMWH- treated rats in both experiments are
presented in Fig. 1. Therapeutic plasma levels (>0.3 aXa IU/ml) were measured for at least 4 h after
administration of UFH or LMWH, both in rats that received 2.0 or 2.5 mg/kg of either UFH or
LMWH. One h after injection of 2.5 mg/kg UFH, a mean plasma level of 0.9 (± 0.2) aXa IU/ml was
found, whereas LMWH-treated rats (2.5 mg/kg) had a mean level of 1.5 (± 0.3) aXa IU/ml at 1 h
after administration, indicating that high concentrations of the anticoagulants were present during
cancer cell administration. No bleeding complications were observed during either administration
of cancer cells or throughout the experiments.
1 3 6 9 12
hours after injection
1 3 6 9 12
hours after injection
Figure 1. aXa plasma levels of UFH- (dashed line) and LMWH-treated rats (solid line) in experiment 1 (A) after 5 days of treatment with 2.0
mg/kg UFH b.i.d. or with 2.0 mg/kg LMWH o.d., and in experiment 2 (B) after 20 days of treatment with 2.5 mg/kg UFH or LMWH o.d.
C O L O N CANCER METASTASIS IN RAT LIVER
Seven days after CC531s cells had been administered, livers contained tumors of variable size (Fig.
2A). The median volume of tumors, expressed as percentage area of tumors, was 0.04% (range,
0.01-0.99%) in placebo-treated rats. Treatment with either UFH or LMWH did not affect the
volume of tumors significantly after 7 days (Table 1). After 24 days, on average 4.1 tumors were
found on the liver surface of placebo-treated rats, with a mean area of 6.0 mm2 (Fig. 2C). The
average numbers of metastases in UFH-treated and LMWH-treated rats were 4.4 and 5.4,
94

respectively, with a mean area of 5.0 mm2 in UFH-treated rats, and 3.1 mm2 in LMWH-treated rats
(Table 2). Number and size of the tumors did not differ significantly among the treatment groups.
Metastases were not found in other organs of the rats in either of the 3 treatment groups.
Treatment
Placebo
UFH
LMWH
Table 1. Volume (expressed as percentage area) of tumors in livers of UFH-, LMWH-, and placebo-treated rats at 7 days after administration
ofCC531s colon cancer cells in the portal vein. a Percentage area is expressed as the ratio of the area of cancer cells and the total area of liver tissue sections including cancer cells xlOO
(%). Median percentage per rat and range between brackets are presented. Each group consisted of 10 rats. Percentage areas of tumor did
not differ significantly between treatment groups (p= 0.7).
Treatment
HHHHMHHH Placebo
UFH
LMWH
Table 2. Number and size of tumors at the liver surface of UFH-, LMWH-, and placebo-treated rats at 24 days after administration ofCC531s
colon cancer cells in the portal vein.
< Data are given as mean number ± SEM and mean area of metastases ± SEM (mm-) per rat. Each group consisted of 8 rats. Number and size
of metastases did not differ significantly between treatment groups (number, p=0.7; size, p= 0.4).
VESSEL DENSITY O F METASTASES
At 7 days after cancer cell administration, newly formed blood vessels were found to be hardly
present in liver metastases irrespective of treatment (Fig. 2B). Only a few large metastases
contained small vessels, again independently of the treatment given. After 24 days, metastases were
well-vascularized (Fig. 2D). Vessel density of tumors in placebo-treated rats was 3.1 %, whereas in
UFH-treated and LMWH-treated rats the vessel density was 3.7 % and 3.3 %, respectively. Again,
significant differences were not found among the rats in the 3 treatment groups (Table 3).
Treatment Tumor vessel density
(%)perrat>
Placebo 3.1 ± 0.3
UFH 3.7 ± 0.3
LMWH 3.3 ± 0.2
Table 3. Vessel density in tumors at the liver surface of UFH-, LMWH-, and placebo-treated rats at 24 days after administration of CC531s
colon cancer cells in the portal vein.
" Vessel density is expressed as mean area of endothelial cells per tumor x 100 ± SEM (%). Data are given as mean percentage vessel density
of tumors per rat. Each group consisted of 8 rats. Tumor vessel density did not differ significantly between treatment groups (p = 0.2).
ercentage area (%)•
0.04 (0.01-0.99)
0.06 (0.01-0.48)
0.05 (0.00-0.29)
Number of metastases Size of metastases per rat • per rat •
4.1 ±1.4 6.0 ±2.5
4.4 ± 0.5 5.0 ± 0.7
5.4 ±1.2 3.1 ±0.9
CHAPTER 7 EFFECTS OF HEPARINS ON COLON CARCINOMA METASTASIS 95

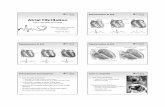
Figure 2. Photomicrographs ofcryostat sections of rat liver containing colon carcinoma metastases after staining with UEA-1 to detect cancer
cells (A, C) (16) and RECA-1 to detect endothelial cells (B, D) (20). Endothelial cells are scarcely present in tumors after 7 days of growth
(B), whereas after 24 days tumors are well vascularized (D). t, tumor; p, liver parenchyma; arrows, endothelial cells in tumor and *,
endothelial cells in liver parenchyma after staining with RECA-1. Bar = 100 fxm (A, B) or 200 fJm (C, D.)
DISCUSSION
Various experimental studies have reported that anticoagulants such as UFH inhibit metastasis (22-
24). The rationale for the use of UFH as an antimetastatic agent was the assumption that it prevents
clot formation around cancer cells, and thereby protects cancer cells in the circulation (25, 26).
However, effects of UFH on progression of cancer or metastasis may not solely be related to its
anticoagulant function. Recent studies have suggested that chemically modified heparins without
anticoagulant activity inhibit metastasis as well (27-29). It was suggested that UFH and heparin-like
molecules effectively reduce metastasis by inhibiting heparanases, which are matrix degrading
enzymes utilized by metastatic cancer cells to break down the basement membrane during invasion
(30). In contrast to these findings, we did not observe any inhibitory or stimulatory effects of UFH
96

or LMWH on the development of colon carcinoma metastases in rat liver. These contradictory
findings cannot be explained by differences in plasma levels of the anticoagulants. Both UFH and
LMWH were given in therapeutic concentrations, and plasma concentrations of UFH and LMWH
were above 0.3 aXa IU/ml for at least 4 h in the treated rats, both at 5 and 20 days after starting the
treatment. In the previous studies, similar or lower concentrations of UFH inhibited metastasis
effectively after being injected either subcutaneously or intraperitoneally (27, 28, 31-33).
It is possible that our data differ from that of previous experimental studies as a consequence of the
different tumor types or animal models that were used. Effects of therapeutic doses of UFH on colon
carcinoma metastasis have not been investigated thus far. One study that evaluated low doses of
UFH (3 IU twice daily, s.c.) on growth and metastasis of subcutaneously implanted colon carcinoma
tumors in nude mice reported that there was no inhibitory effect either (34). Studies in which UFH
was found to inhibit metastasis generally evaluated pulmonary metastases of intravenously
administered mammary or melanoma cancer cells (22, 27, 28, 31). It has been postulated that
interactions between cancer cells and haemostasis differ between tumor types. Pathological studies
suggest that the fibrinolytic system is of more importance than coagulation activation in colon
carcinomas (35). In addition, clinical trials evaluating UFH, warfarin or fibrinolytic agents have
indicated that various tumor types respond differently to anticoagulants (36-39). On the other hand,
randomized trials which compared LMWH with UFH for the treatment of venous
thromboembolism showed an improved survival in LMWH-treated patients with a variety of
malignant diseases, including colon cancer (10). These latter studies thus suggest a rather general
effect of LMWH or UFH on cancer progression, irrespective the tumor type.
Various studies have reported that UFH stimulates and that LMWH inhibits blood vessel formation,
but the effects of the heparins on cancer-associated angiogenesis have not been investigated thus far
(11, 12). In the present study, we also quantitatively evaluated effects of UFH and LMWH on blood
vessel formation in the tumors, by comparing the density of RECA-1 positive endothelial cells in
tumors of the 3 groups. Overt effects of the heparins on cancer-associated angiogenesis were not
found.
In conclusion, the results of these experiments indicate that heparins do not affect colon carcinoma
metastasis in liver. Outgrowth of tumors and intra-tumor vessel density were both not affected by
heparin treatment in this animal model. As a consequence of the numerous biological activities of
UFH and LMWH, it is possible that heparin treatment in vivo does not substantially enhance or
reduce cancer progression and tumor-associated angiogenesis. Nevertheless, one cannot exclude
that the negative results of the present study are related to the specific tumor type or model used and
that heparins affect outgrowth of tumors which are highly vascularized. Therefore, further
experiments are warranted to provide detailed insights into the mechanisms by which heparins may
affect malignancy. The results of these experiments may guide the selection of appropriate clinical
settings for definitive testing of effects of LMWH in human cancer.
CHAPTER 7 EFFECTS OF HEPARINS ON COLON CARCINOMA METASTASIS 97

ACKNOWLEDGEMENT
We thank Mrs. W.M. Morriën for measurements of plasma aXa levels, Dr. A.M. Duijvestijn for the
supply of RECA-1, Mrs. K.S. Bosch for her technical assistance, Prof.Dr. C.H.N. Veenhof and Dr.
R .0 . Schlingemann for their scientific advice, and Mr. J. Peeterse and Mr. C. Gravemeijer for
photographic work.
REFERENCES 1 Jackson RL, Busch SJ, Cardin AD. Glycosaminoglycans: molecular properties, protein interactions,
and role in physiological processes. Physiol Rev 1991;71:481-539.
2 Tyrell DJ, Kilfeather S, Page CR Therapeutic uses of heparin beyond its traditional role as an anticoagulant. Trends Pharmacol Sci 1995;16:198-204.
3 Zacharski LR, Ornstein DL. Heparin and cancer. Thromb Haemost 1998;80:10-23.
4 Folkman J, Taylor S, Spillberg C. The role of heparin in angiogenesis. Ciba Found Symp 1983;100:132-149.
5 Siragusa S, Cosmi B, Piovella F, Hirsh J, Ginsberg JS. Low-molecular-weight heparins and unfractionated heparin in the treatment of patients with acute venous thromboembolism: results of a meta-analysis. Am J Med; 100:269-277.
6 Green D, Hull RD, Brant R, Pineo GF. Lower mortality in cancer patients treated with low-molecular-weight versus standard heparin. Lancet 1992;339:1476.
7 Prandoni P, Lensing AW, Biiller HR, Carta M, Cogo A, Vigo M, Casara RA, Ten Cate JW. Comparison of subcutaneous low-molecular-weight heparin with intravenous standard heparin in proximal deep-vein thrombosis. Lancet 1992;339:441-445.
8 Hettiarachchi RJK, Smorenburg SM, Ginsberg J, Levine M, Prins MH, Büller HR. Do heparins do more than just treat thrombosis? Thromb Haemost 1999;82:947-952.
9 Smorenburg SM, Hettiarachchi RJK, Vink R, Biiller HR. The effects of unfractionated heparin on survival in patients with malignancy. A systematic review. Thromb Haemost 1999;82:1600-1604.
10 Norrby K, Ostergaard P. Basic-fibroblast-growth-factor-mediated de novo angiogenesis is more effectively suppressed by low-molecular-weight than by high-molecular-weight heparin. Int J Microcirc Clin Exp 1996;16:8-15.
11 Norrby K. Heparin and angiogenesis: a low-molecular-weight fraction inhibits and a high-molecular-weight fraction stimulates angiogenesis systemically. Haemostasis 1993;23 Suppl 1:141-149.
12 Jayson GC, Gallagher JT. Heparin oligosaccharides: inhibitors of the biological activity of bFGF on Caco-2 cells. Br J Cancer 1997;75:9-16.
13 Iruela-Arispe ML, Dvorak HF Angiogenesis: a dynamic balance of stimulators and inhibitors. Thromb Haemost 1997;78:672-677.
14 Soker S, Goldstaub D, Svahn CM, Vlodavsky I, Levi BZ, Neufeld G. Variations in the size and sulfation of heparin modulate the effect of heparin on the binding of VEGF165 to its receptors. Biochem Biophys Res Commun 1994;203:1339-1347.
15 Marquet RL, Westbroek DL, Jeekel J. Interferon treatment of a transplantable rat colon adenocarcinoma: importance of tumor site. Int J Cancer 1984;33:689-692.
16 Smorenburg SM, Griffini P, Tiggelman AB, Moorman AFM, Boers W, Van Noorden CJF Alpha2-Macroglobulin is mainly produced by cancer cells and not by hepatocytes in rats with colon carcinoma metastases in liver. Hepatology 1996;23:560-570.
98

17 Ten Cate H, Lamping RJ, Henny CP, Prins A, Ten Cate JW. Automated amidolytic method for determining heparin, a heparinoid, and a low-Mr heparin fragment, based on their anti-Xa activity. Clin Chem 1984;30:860-864.
18 Van Noorden CJF, Jonges TG, Van Marie J, Bissell ER, Griffini P, Jans M, Snel J, Smith RE. Heterogeneous suppression of experimentally induced colon cancer metastasis in rat liver lobes by inhibition of extracellular cathepsin B. Clin Exp Metast 1998;16:159-167.
19 Griffini P, Smorenburg SM, Verbeek FJ, Van Noorden CJF. Three-dimensional reconstruction of colon carcinoma metastases in liver. J Microsc 1997;187:12-21.
20 Duijvestijn AM, Van Goor H, Klatter F, Majoor GD, Van Bussel E, Van Breda VP Antibodies defining rat endothelial cells: RECA-1, a pan-endothelial cell-specific monoclonal antibody. Lab Invest 1992;66:459-466.
21 Griffini P, Fehres O, Klieverik L, Vogels IMC, Tigchelaar W, Smorenburg SM, Van Noorde CJF. Dietary omega-3 polyunsaturated fatty acids promote colon carcinoma metastasis in rat liver. Cancer Res 1998;58:3312-3319.
22 Sciumbata T, Caretto P, Pirovano P, Pozzi P, Cremonesi P, Galimberti LF, Marcucci F. Treatment with modified heparins inhibits experimental metastasis formation and leads, in some animals, to long-term survival. Inv Metast 1996;16:132-143.
23 Engelberg H. Actions of heparin that may affect the malignant process. Cancer 1999;85:257-272.
24 Hejna M, Raderer M, Zielinski CC. Inhibition of metastases by anticoagulants. J Natl Cancer Inst 1999;91:22-36.
25 Koike A. Mechanism of blood-borne metastases. Cancer 1963;17:450-460.
26 Fisher B, Fisher ER. Experimental studies of factors which influence hepatic metastases. VIII. Effect of anticoagulants. Surgery 1961;50:240-247.
27 Coombe DR, Parish CR, Ramshaw IA, Snowden JM. Analysis of the inhibition of tumour metastasis by sulphated polysaccharides. Int J Cancer 1987;39:82-88.
28 Vlodavsky I, Mohsen M, Lider O, Svahn CM, Ekre HP, Vigoda M, Ishai-Michaeli R, Peretz T Inhibition of tumor metastasis by heparanase inhibiting species of heparin. Inv Metast 1994;14:290-302.
29 Irimura T, Nakajima M, Nicolson GL. Chemically modified heparins as inhibitors of heparan sulfate specific endo-beta-glucuronidase (heparanase) of metastatic melanoma cells. Biochemistry 1986;25:5322-5328.
30 Nakajima M, Irimura T, Nicolson GL. Heparanases and tumor metastasis. J Cell Biochem 1988;36:157-167.
31 Lee AE, Rogers LA, Jeffery RE, Longcroft JM. Comparison of metastatic cell lines derived from a murine mammary tumour, and reduction of metastasis by heparin. Clin Exp Metast 1988;6:463-471.
32 Gorelik E, Bere WW, Herberman RB. Role of NK cells in the antimetastatic effect of anticoagulant drugs. Int J Cancer 1984;33:87-94.
33 Nagawa H, Paris P, Chauffert B, Martin F. Treatment of experimental liver metastases in the rat by continuous intraportal infusion of 5-fluorouracil and heparin: a pilot study. Anticancer Drugs 1990;1:149-156.
34 Antachopoulos CT, Gagos S, Iliopoulos DC, Karayannacos PE, Tseleni-Balafouta S, Alevras P, Koundouris C, Skalkeas GS. Low-dose heparin treatment does not inhibit SW480 human colon cancer growth and metastasis in vivo. In Vivo 1996;10:527-531.
35 Zacharski LR, Costantini V, Wojtukiewicz MZ, Memoli VA, Kudryk BJ. Anticoagulants as cancer therapy. Semin Oncol 1990;17:217-227.
CHAPTER 7 EFFECTS OF HEPARINS ON COLON CARCINOMA METASTASIS 99

36 Calvo FA, Hidalgo OF, Gonzalez F, Rebollo J, Martin AS, Ortiz de Urbina D, Brugarolas A. Urokinase combination chemotherapy in small cell lung cancer. A phase II study. Cancer 1992;70:2624-2630.
37 Wereldsma JC, Bruggink ED, Meijer WS, Roukema JA, van Putten WL. Adjuvant portal liver infusion in colorectal cancer with 5-fluorouracil/heparin versus urokinase versus control. Results of a prospective randomized clinical trial. Cancer 1990;65:425-432.
38 Chahinian AP, Propert KJ, Ware JH, Zimmer B, Perry MC, Hirsch V, Skarin A, Kopel S, Holland JF, Comis RL. A randomized trial of anticoagulation with warfarin and of alternating chemotherapy in extensive small-cell lung cancer by the Cancer and Leukemia Group B. J Clin Oncol 1989;7:993-1002.
39 Zacharski LR, Henderson WG, Rickles FR, Forman WB, Cornell jr CJ, Forcier RJ, Edwards RL, Headley E, Kim SH, O'Donnell JF. Effect of warfarin anticoagulation on survival in carcinoma of the lung, colon, head and neck, and prostate. Final report of VA Cooperative Study :75. Cancer 1984;53:2046-2052.
100