Unit 5 Renewable Energy – Biodiesel Lab - Wolf...
Transcript of Unit 5 Renewable Energy – Biodiesel Lab - Wolf...
Unit 5 Renewable Energy – Biodiesel Lab
LESSON OUTLINE: I. “Everything depends on energy
– How you use it changes the world.”/Notes a. Energy transformation and
fuels b. What are fossil fuels c. Fuels and electricity d. Renewable energy sources
i. Solar ii. Geothermal iii. Nuclear
e. Carbon Cycle f. Energy Conservation
II. NOVA Power Surge III. Biodiesel Lab IV. “Car Fieldtrip” KEY CONCEPTS: Everything depends on energy. How you use it changes the world. How can you reduce your energy footprint? As a society, energy conservation can reduce part of our dependence on fossil fuels, however, to solve the climate change issues brought up in Unit 4 – Acid Rain, renewable energy sources are needed to combat climate change. The sun, being the indirect source of all fossil fuel energy, could be directly accessed through photosynthesis and stored in plant seeds. If we convert these seeds to oil, it can be converted to a usable fuel for our current automobiles. OUTCOMES:
Upon completion of this lesson, students will be able to:
1. describe how electricity is made from fossil fuels.
2. describe how crude oil can be made into different types of products for different uses.
3. explain the difference between renewable energy sources and fossil fuels.
4. make biodiesel from seed oil. 5. measure and compare the
energy value of biodiesels made from the different types of oil.
6. identify different types of conservation efforts already done by automobile makers.
GRADUATION STANDARDS:
The following MN graduation standards apply: 8.3.4.1.1 Describe how mineral and fossil fuel resources have formed over millions of years, and explain why these resources are finite and non-renewable over human time frames. 9.1.3.3.1 Describe how values and constraints affect science and engineering. For example : Economic, environmental, social, political, ethical, health, safety and sustainability issues. 9.1.3.3.3 Describe how scientific investigations and engineering processes require multi-disciplinary contributions and efforts. 9.2.4.1.1 Compare local and global environmental and economic advantages and disadvantages of generating electricity using various sources or energy.
Unit 5 Renewable Energy – Biodiesel Lab
Learning Activities:
I. Everything depends on energy. How you use it changes the
world. Notes: (2 hours) a. When fuels are burned, the chemical energy that is released can
be used to generate another form of energy, such as heat, light, motion, or electricity.
b. Coal is a solid fossil fuel formed from plant remains. c. Crude oil is first pumped out of the ground and then refined. In
the refining process, crude oil is heated and separated to make different products.
d. Passive and active solar systems convert solar energy into heat and electricity in a solar house.
e. A geothermal power plant uses heat from Earth’s interior as an energy source to produce electricity.
f. In a nuclear power plant, the heat released from fission reactions is used to change water into steam. The steam then turns the blades of a turbine to generate electricity.
g. In nuclear fusion, two hydrogen nuclei combine to create a helium nucleus, which has slightly less mass than the two hydrogen nuclei. The lost mass is converted to energy.
II. Carbon Cycle: (1 hour) Have students take notes on the key terms
during the playing of this animation. a. carbon exchange b. photosynthesis c. cellular respiration d. decomposers e. terrestrial ecosystems f. human activities g. carbon sinks h. deforestation i. fossil fuel use j. CO2 graph and global warming k. greenhouse gasses l. greenhouse effect
III. Nova Power Surge: (2 hours) “Can emerging technology defeat
global warming? The United States has invested tens of billions of dollars
Unit 5 Renewable Energy – Biodiesel Lab
in clean energy projects as our leaders try to save our crumbling economy and our poisoned planet in one bold, green stroke. Are we finally on the brink of a green-energy "power surge," or is it all a case of too little, too late?”
a. Have the students watch the NOVA episode “Power Surge” to understand the factors involved in global warming and our energy needs as a country.
IV. Biodiesel Lab a. Experimental Question: (1 hour) “How does oil type
(corn oil, sunflower seed, vegetable oil,…) affect biodiesel energy output?”
i. Have students write these parts of the lab report in their notebook. Start with the experimental question above, and the following purpose statement. “The purpose of this experiment is to measure the heating value of a fuel (calories) (MJ/kg).” The biodiesel generated in the class will be compared to fossil fuel diesel.
Unit 5 Renewable Energy – Biodiesel Lab
b. Make biodiesel: (8 Hours) Biodiesel is a mixture of methyl esters of fatty acids (commonly found in plants & animals). It can be made easily from vegetable cooking oil. Enough fuel can be produced from this lab to burn although it is not pure enough to actually be used as a fuel in a car or truck. The synthesis is a simple chemical reaction that produces biodiesel and glycerol. Have the students choose one of the oils available (Flax, Corn, Vegetable, Sunflower, Safflower, Canola, Sesame,…) and make a biodiesel sample to test in the calorimeter.
i. Materials 1. Lab balance 2. 1 250ml Erlenmeyer flask 3. 1 100ml beakers 4. 1 25ml graduated cylinder 5. 2 100ml graduated cylinder 6. Distilled water 7. 100ml vegetable oil 8. 20ml methanol 9. 0.5g potassium hydroxide (KOH) pellets
i i. Safety 1. You MUST wear goggles and gloves 2. Methanol is flammable and poisonous 3. KOH is corrosive; will cause severe burns to skin
and eyes ii i. Procedure
1. Measure 20ml of methanol using the small graduated cylinder.
2. Pour the methanol into the 250ml Erlenmeyer flask using a funnel.
3. Carefully add 0.5g of KOH to the methanol and cap quickly with the stopper.
4. Swirl the mixture for 10 minutes to form methoxide (CAUTION – this reaction is exothermic i.e. GETS HOT)
5. Measure 100ml of warmed (55oC) vegetable oil using the graduated cylinder.
6. Pour this into the methoxide, replace the stopper and swirl occasionally over the next 20 minutes keeping the mixture on the warming plate.
7. Allow the mixture to sit and separate for 20 minutes.
Unit 5 Renewable Energy – Biodiesel Lab
8. Carefully decant the top layer using a into a 100ml beaker.
9. Wash the product with 10ml of distilled water. Mix. 10. Allow the mixture to sit and separate. 11. Carefully decant the top layer into a
clean100ml graduated cylinder. 12. Measure the amount of biodiesel you have
collected using a graduated cylinder and compare it to the amount of oil you started with.
c. Calorimeter Testing of Biodiesel: Use the following
apparatus in a well-ventilated area to test the energy content of your biodiesel.
i. Materials i i. Safety
1. Put your safety goggles on and tie back any long hair.
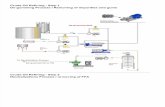
i i i. Procedure 1. Record your fuel type in the Data Table. 2. Assemble the calorimeter (see the figure below).
3. Use approximately 20mL of your fuel into the bottom half of the soda can calorimeter. It should be about .5 to 1 inch deep.
4. Roll the cotton gauze wick and insert through drinking hole in can top. Drip a few drops of fuel onto the top of the wick to make sure there is fuel to burn at the top.
5. Assemble the calorimeter carefully (edges are sharp).
Unit 5 Renewable Energy – Biodiesel Lab
d. Measuring the energy content of the biodiesel: The energy that is used to raise the temperature of the water can be calculated based on the specific heat of water. Use the following formula to calculate the calories of energy in each biodiesel sample.
i. Q = m*c*ΔT
1. Where: Q is the heat flow (calories) 2. m is the mass of the water (grams) 3. c is the specific heat of water (c = 1 calorie/gram) 4. ΔT is the temperature change (°C)
ii. Example data table:
b. Lab Report Criteria List or Conclusion Letter: Have the students write a lab report or a conclusion letter depending on the time available and teacher preference.
i. Conclusion Letter: Each student should write a three-paragraph letter by finishing these sentences with enough detail to explain to the reader what they did, how they did it, and what they found out.
Unit 5 Renewable Energy – Biodiesel Lab
ii. In our experiment we found that …(explain how each of the different biodiesels performed)
iii. We thought that ______ oil would produce the most heat. We determined this by … (summarize the procedure in sentence form)
iv. If we were to do this experiment again, my partner and I would…(explain sources of error) because…
b. Biodiesel Lab Grading Sheet (See Appendix 1)
IX. Car Fieldtrip: (1 hour) Students will go to the parking lot or front of school where they will look at, hear abit about and ask questions of their owners about the three different types of cars present. The cars are all current models from major manufacturers who have engineered some way to reduce auto emission reduction.
a. Toyota Prius (hybrid) b. Chevrolet Volt (hybrid) c. Nissan Leaf (electric) d. Subaru Outback (partial zero emissions)
Unit 5 Renewable Energy – Biodiesel Lab
RESOURCES:
Prentice Hall Science Explorer ©2009 Earth Science http://www.sumanasinc.com/webcontent/animations/content/globalcarboncycle.html NOVA Power Surge http://www.pbs.org/wgbh/nova/tech/power-surge.html Biodiesel Lab Procedure – Calorimeter set up http://www.clarkson.edu/highschool/k12/project/documents/energysystems/LP_4%20-biofuels%20complete.pdf EQUIPMENT:
Lab balance 250ml Erlenmeyer flask 100ml beakers graduated cylinder graduated cylinder Distilled water vegetable oil canola oil sunflower seed oil safflower seed oil sesame seed oil corn oil flax seed oil methanol potassium hydroxide (KOH) pellets
GLOSSARY:
Carbon Cycle: the series of processes by which carbon compounds are interconverted in the environment, chiefly involving the incorporation of carbon dioxide into living tissue by photosynthesis and its return to the atmosphere through respiration, the decay of dead organisms, and the burning of fossil fuels. Fossil Fuels: a natural fuel such as coal or gas, formed in the geological past from the remains of living organisms. Carbon Dioxide (CO2): a colorless, odorless gas produced by burning carbon and organic compounds and by respiration. It is naturally present in air (about 0.03 percent) and is absorbed by plants in photosynthesis. Greenhouse Effect: the trapping of the sun's warmth in a planet's lower atmosphere due to the greater transparency of the atmosphere to visible radiation from the sun than to infrared radiation emitted from the planet's surface. Biodiesel: a biofuel intended as a substitute for diesel.
Unit 5 Renewable Energy – Biodiesel Lab
Appendix 1: Biodiesel Lab Report Grading Sheet
Points Self Teacher Possible Grading Grading Introduction (Biofuels are… Biodiesel is made from… Biodiesel properties… ) 5 _______ _______ Experimental Question& Purpose Statement (clear and concise sentence describing 5 _______ _______ dependent and independent variables) List of Materials 5 _______ _______ - include brand names & scientific names - include detailed descriptions
Unit 5 Renewable Energy – Biodiesel Lab
Procedure 10 _______ _______
- descriptive step by step list of instructions - repeatable based on information given - design of experiment clear - sample size stated - brand names & scientific names included - explain how and what you measured as your results
Results/Data Table 5 _______ _______
- include units - Label columns & rows
- title & label Discussion 10 _______ _______ - How do your results compare to the heating values of diesel, and biodiesel? - How does the experiment we performed differ from an ideal controlled experiment? - Where do you think the biggest errors came from? - Why is the heating value of biodiesel reported as a range? - Which fuel (diesel, biodiesel, or vegetable oil) is most “efficient” in a vehicle? Explain your answer? Conclusion 5 _______ _______ - at least one page of analysis of data and explanation of the outcome of your experiment - statement comparing your data to your hypothesis - sources of experimental error - how could you improve on the project if you did it again (try harder is not an answer) TOTAL 40 _______ _______