Unit 2 - Heatpalmarin.weebly.com/uploads/2/3/0/7/23075168/ch4_print_version.pdf · Unit 2 - Heat...
Transcript of Unit 2 - Heatpalmarin.weebly.com/uploads/2/3/0/7/23075168/ch4_print_version.pdf · Unit 2 - Heat...

Unit 2 - HeatChapter 4 - Thermochemistry & Thermodynamics
Mr. Palmarin Chapter 4 - Thermodynamics 1 / 56

The concept of heat bridges the gap between chemistry and physics.
→ Chemistry deals with microscopic phenomena (on the nanoscale).→ Physics deals with macroscopic phenomena in general.
Heat is a macroscopic property that is caused by microscopic phenomena.
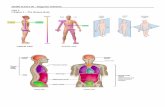
Heat flowing out versus heat flowing into a system
Mr. Palmarin Chapter 4 - Thermodynamics 2 / 56

Section 4.1 - Thermochemistry
Energy - the capacity to do work or to transfer heat.
Heat - is energy used to cause the temperature of an object to increase.
Enthalpy - is essentially heat energy. It is specifically a measure of howmuch “internal energy” is present in a system.
Change in enthalpy is denoted as ∆H and it is usually measured inJoules (J) or Kilojoules (kJ).
Mr. Palmarin Chapter 4 - Thermodynamics 3 / 56

Thermochemical equation: a balanced chemical equation that includesthe physical states of reactants and products and the value of ∆H.
Example:
Hot pack: 4Fe(s) + 3O(g) −−→ 2Fe2O3(s) ∆H = -1625 kJ
Cold pack: NH4NO3(s) −−→ NH +4 (aq) + NO –
3 (aq) ∆H = +27 kJ
The hot pack releases energy =⇒ the system loses energy (negative∆H). This is an exothermic reaction.
The cold pack absorbs energy =⇒ the system gains energy (positive∆H). This is an endothermic reaction.
Mr. Palmarin Chapter 4 - Thermodynamics 4 / 56

Potential Energy Diagrams
These diagrams show the stored energy in the reactants and productsrather than the heat energy absorbed or released.
Mr. Palmarin Chapter 4 - Thermodynamics 5 / 56

Examples: Identify the following reactions as exothermic or endothermicand draw a potential energy diagram to communicate the enthalpy change.
1) H2(g) + Br2(g) −−→ 2 HBr(g) ∆H = -36 kJ
2) 6 FeCl3(s) −−→ 6 FeCl2(s) + 3 Cl2(g) ∆H = +173 kJ
1) Exothermic 2) Endothermic
Mr. Palmarin Chapter 4 - Thermodynamics 6 / 56

Examples: Identify the following reactions as exothermic or endothermicand re-write them as thermochemical equations.
1) Fe2O3(s) + 3 CO(g) −−→ 3 CO2(g) + 2 Fe(s) + 25kJ
Basically, we just want to write the equation so that it includes ∆H.We do this by removing the “kJ term”. If that term is in the reactants,the equation is endothermic (absorbing energy). If it is in theproducts, the equation is exothermic (releasing energy).
Here, this is exothermic (so ∆H is negative).
Answer: Fe2O3(s) + 3 CO(g) −−→ 3 CO2(g) + 2 Fe(s) ∆H = −25 kJ
2) 2 HCl(g) + 96kJ −−→ H2(g) + Cl2(g)
96 kJ is in the reactants =⇒ endothermic
Answer: 2 HCl(g) + 96kJ −−→ H2(g) + Cl2(g) ∆H = 96 kJ
3) 4 NH3(g) + 5 O2(g) −−→ 4 NO(g) + 6 H2O(l) + 1170kJ
Answer: 4 NH3(g) + 5 O2(g) −−→ 4 NO(g) + 6 H2O(l) ∆H = −1170 kJ
Mr. Palmarin Chapter 4 - Thermodynamics 7 / 56

Example: Use the enthalpy diagram below to answer the followingquestions:
1) Is this an endothermic or exothermic reaction?
∆H is negative =⇒ exothermic
2) Is energy lost or gained?
Look at the overall shape of the graph. Energy is clearly decreasingafter the reaction arrow. Therefore, energy is lost.
3) Write out the thermochemical equation for the above reaction.
2 H2 + O2 −−→ 2 H2O ∆H = −285.8 kJ
4) How much energy would result if 6 mol H2 reacted instead of two.
6 mol H2
(−285.8 kJ
2 mol H2
)= −857.4 kJ
Mr. Palmarin Chapter 4 - Thermodynamics 8 / 56

Example:
1) The standard heat of formation, H◦f , for sulfur dioxide (SO2) is
-296.8 kJ/mol. How many kJ of energy are given off when 25.0 g ofSO2(g) is produced from its elements?
25.0 g
(1 mol
64.07 g
)(−296.8 kJ
1 mol
)= −116 kJ
2) The heat of reaction for the combustion of 1.0 mol of ethanol,C2H5OH(l), is −9.50× 102 kJ. How much heat is produced when 11.5g of alcohol is burned?
11.5 g
(1 mol
46.07 g
)(−950 kJ
1 mol
)= −240 kJ
Mr. Palmarin Chapter 4 - Thermodynamics 9 / 56

Practice: The ∆H for the complete combustion of 1 mol of propane(C3H8) is −2.22× 103 kJ. Calculate the heat of reaction for thecombustion of 33.0 g of propane. [See Section 4.1 Video]
C3H8(g) + 5 O2(g) −−→ 3 CO2(g) + 4 H2O(l)
Mr. Palmarin Chapter 4 - Thermodynamics 10 / 56

Enthalpies of Formation
∆Hformation = H◦f (measured in kJ/mole) is the heat lost or gained when
one mole of a substance is formed from its elements.
Note: The H◦f of any single element = 0 kJ/mol.
Steps for writing H◦f reactions:
Reference a Thermochemistry Data Table to find the heat offormation for the particular compound. This can be downloaded fromMoodle.
Remember that the unit is in kJ/mole.
Mr. Palmarin Chapter 4 - Thermodynamics 11 / 56

Example: What is the heat of formation for the following compounds?
1) H2O(l)
Reference the data table. Be sure to find H2O(l). Then,∆Hf = −285.8 kJ/mol
2) CuCl(s)
∆Hf = −137.2 kJ/mol
3) N2H4(l)
∆Hf = 50.6 kJ/mol
4) NH4Cl(s)
∆Hf = −314.4 kJ/mol
Mr. Palmarin Chapter 4 - Thermodynamics 12 / 56

Enthalpies of Reactions ∆H
Potential energy diagrams led us to basic enthalpies of reactions where∆H was given. We now will calculate ∆H:
∆H =∑
H◦f (products)−
∑H◦
f (reactants)
Note: This read as “the sum of the heat of formation of the productsminus the sume of the heat of formation of the reaction”.
Example: Calculate ∆H for the following reaction:
Cl2(g) + 2 HBr(g) −−→ 2 HCl(g) + Br2(g)
∆H = (2(−92.3) + 0)− (0 + 2(−36.4)) = −184.6− (−72.8) = −111.8 kJ
Recall: All of these values are found on your thermochemical data sheet.You must remember to add up the products first and then subtract thesum of the reactants.
Mr. Palmarin Chapter 4 - Thermodynamics 13 / 56

Practice: Calculate ∆H for the synthesis reaction between ammonia gasand gaseous hydrochloric acid to create solid ammonium chloride.
[See Section 4.1 Video]
NH3(g) + HCl(g) −−→ NH4Cl(s)
Mr. Palmarin Chapter 4 - Thermodynamics 14 / 56

Section 4.2 - Kinetic Theory
Kinetic Molecular theory explains all of the effects produced by heat. Itcan be summarized by:
1 Particles are held together by electric forces.
2 Particles are in constant motion and therefore possess kinetic energy(energy of motion).
3 Particles do not lose energy in collisions but transfer the energybetween one another.
4 When heat is added to matter, molecules absorb the energy and movefaster (kinetic energy increases). When heat is removed, themolecules move slower (kinetic energy decreases).
Watch: “Kinetic Molecular Theory”
Mr. Palmarin Chapter 4 - Thermodynamics 15 / 56

Thermal Energy
Thermal energy is the sum of the kinetic and electric potential energypossessed by the molecules of an object. A hot object has more thermalenergy than a cold object because the molecules are moving faster in thehot object.
E(thermal) = E(kinetic) + E(electric potential)
Mr. Palmarin Chapter 4 - Thermodynamics 16 / 56

Temperature
Temperature is a measure of the average kinetic energy of the moleculesof a substance.
Suppose we have two objects: one is cold and the other is hot. Whenplaced in contact, the average kinetic energy of the cooler object increasesas the kinetic energy of the warmer object decreases.
Eventually, the two objects have the same kinetic energy and are said tobe in thermal equilibrium.
Mr. Palmarin Chapter 4 - Thermodynamics 17 / 56

Thermometers
Thermometers are used to measure temperature. Most thermometerscontain either alcohol or mercury. Both have their advantages anddisadvantages.
Alcohol thermometers cannot measure high temperatures (low boilingpoint of 78 ◦C) and mercury thermometers cannot measure very coldtemperatures (high freezing point of −39 ◦C).
Mr. Palmarin Chapter 4 - Thermodynamics 18 / 56

Temperature Scales
Temperatures can vary considerably. While no upper boundary oftemperature exists, a lower boundary does exist. The lowest temperaturethat can exist is called absolute zero. At absolute zero nomolecular motion occurs. If no molecular motion occurs, then no thermalenergy exists.
In practice, it is in fact impossible to reach true absolute zero. This issimply because a complete zero state of energy does not exist!
Note on significant figures: For the sake of simplicity, we will nowalways consider temperatures to be at least 2 significant figures; e.g.40 ◦C is considered 2 s.f. (40.0 ◦C is still 3 s.f. and so on).
Watch: “Absolute Zero”Mr. Palmarin Chapter 4 - Thermodynamics 19 / 56

Three temperature scales exists.
1 Fahrenheit: -459 ◦F is absolute zero, 32 ◦F is freezing point of waterand 212 ◦F is the boiling point of water.
2 Celsius: −273.15 ◦C is absolute zero, 0 ◦C is the freezing point ofwater and 100 ◦C is the boiling point of water.
3 Kelvin: 0 K is absolute zero, 273.15 K is the freezing point of waterand 373 K is the boiling point of water.
◦C = (◦F − 32)× 5
9◦F =
(9
5
)◦C + 32 K = ◦C + 273.15
Mr. Palmarin Chapter 4 - Thermodynamics 20 / 56

Examples: Convert the following:
1) 32 ◦C into ◦F
We are given a temperature in degrees Celsius and wish to move toFahrenheit. Therefore, since we are looking for Fahrenheit, we use thesecond formula from the above slide.
◦F =(9
5
)◦C + 32 =⇒ ◦F =
(9
5
)(32) + 32
◦F = 89.6 = 90 ◦F Recall: We’re assuming that this is 2 s.f.
2) -54.0 ◦F into K
I have not provided you with a direct formula to convert fromFahrenheit to Kelvin. If you wish, you can construct one from theformulas in the above slide. If not, then this is a 2 step procedure.
◦C = (◦F − 32)× 5
9=⇒ ◦C = (−54.0− 32)× 5
9= −47.77 ◦C (this is an intermediate step; i.e. take one extra s.f.)
K = ◦C + 273.15 =⇒ K = −47.77 + 273.15 = 225 K
Mr. Palmarin Chapter 4 - Thermodynamics 21 / 56

Practice: Convert the following: [See Section 4.2 Video]
1) 112 K to ◦C
2) 212 K into ◦F
Mr. Palmarin Chapter 4 - Thermodynamics 22 / 56

Zeroth Law of Thermodynamics
“If two thermodynamic systems are each in thermal equilibriumwith a third, then they are in thermal equilibrium with each other.”
The Zeroth law is so named as it came after the other three laws.However, it turns out to be so fundamental that it was placed before theother three.
What does the law actually mean?
Basically, if A = B and C = B, then A = C. This may seem obvious, butthe definition and our understanding of temperature requires us to acceptthis law.
Mr. Palmarin Chapter 4 - Thermodynamics 23 / 56

First Law of Thermodynamics
“The total amount of energy in an isolated system is conserved.”
When energy is converted into thermal energy, total energy isconserved. If the thermal energy is converted to another form of energy,the amount of energy developed will be exactly the same as the originalamount of energy.
Mr. Palmarin Chapter 4 - Thermodynamics 24 / 56

Second Law of Thermodynamics
“The entropy of an isolated system not in equilibrium will tend toincrease over time, approaching a maximum value at equilibrium.”
Entropy is a very important thing in thermodynamics. Essentially, entropyis the measure of disorder and randomness in a system.
When considering heat, this law essentially states that heat flows naturallyfrom a hot object to a cold object and does not flow naturally from a coldobject to a hot object.
If you tossed bricks off a truck, which kind of pile of bricks would you more likely
produce?
Mr. Palmarin Chapter 4 - Thermodynamics 25 / 56

Third Law of Thermodynamics
“As temperature approaches absolute zero, the entropy of a systemapproaches a constant minimum.”
This law provides an absolute reference point for measuring entropy. At 0K, the value of entropy is essentially 0.
Recall: In practice, absolute zero is impossible to reach. As one attemptsto reach absolute zero it becomes more difficult to get any closer to it.
This is due to the fact that thermal energy from the surroundings transfersto the lower temperature that was created. The greater the difference intemperature the more difficult it becomes to attain absolute zero.
Mr. Palmarin Chapter 4 - Thermodynamics 26 / 56

Section 4.3 - Thermal Expansion
Most substances expand when heated and contract when cooled. Thisincrease in temperature means the molecules are moving faster causingthem to collide with each other. Therefore, the substance’s volume andsize expands.
This increase in length of a solid in one direction is called linearexpansion.
Mr. Palmarin Chapter 4 - Thermodynamics 27 / 56

The amount of linear expansion of a solid depends on two factors:
1 Initial length of the solid.
2 Temperature change.
We use the following formula to determine the amount of expansion:
∆L = α · L ·∆T
- α is the coefficient of linear expansion (varies for different materials).The unit is ◦C−1 or 1
◦C . You can find a list of α for certainmaterials on the backside of your Thermochemical Data Table.
- ∆L is the change in length
- L is the initial length
- ∆T is the change in temperature
Note: ∆T = Tfinal − Tinitial
Mr. Palmarin Chapter 4 - Thermodynamics 28 / 56

Example: The longest continuous bridge in Saskatchewan is a 380 m longsteel bridge in North Battleford. The temperature in the area varies from−40 ◦C to 30 ◦C. What is the change in length of the bridge if thecoefficient of linear expansion of steel is 12× 10−6 ◦C−1?
We first identify the variables that we know and what we’re looking for:
α = 12× 10−6◦C−1 L = 380 m
∆T = Tfinal − Tinitial = 30 ◦C−−40 ◦C = 70 ◦C
∆L =?
∆L = α · L ·∆T =⇒ ∆L = (12× 10−6◦C−1)(380 m)(70 ◦C)
∆L = 0.32 m
This result means that the bridge’s length can vary by 0.32 m.
Mr. Palmarin Chapter 4 - Thermodynamics 29 / 56

Practice: A piece of aluminum house siding is 3.66 m long on a coldwinter day (−28 ◦C). How much longer is it on a very hot summer day(39 ◦C)? The coefficient of linear expansion of aluminum is25× 10−6 ◦C−1. [See Section 4.3 Video]
Mr. Palmarin Chapter 4 - Thermodynamics 30 / 56

Section 4.4 - Specific Heat Capacity
Heat is the thermal energy transferred from one object to another due todifferences in temperature.
This transfer of energy is called heat or heat energy. This heat energy isdenoted with a Q and is measured in Joules (J). The amount of heattransfer is affected by the size and material type of the objects.
The specific heat capacity refers to the amount of energy that must beadded to raise the temperature of 1 kg of a substance 1 ◦C or 1 K.
Mr. Palmarin Chapter 4 - Thermodynamics 31 / 56

The heat gained or lost by an object as its temperature changes is foundusing the formula:
Q = m · C ·∆T
- Q is the amount of heat gained or lost in Joules (J)
- m is the mass of the object in kg
- C is the specific heat capacity of the object in J/(kg ◦C)
You can find a list of the specific heat capacity of certain materialson the backside of your Thermochemical Data Table.
- ∆T is the change in temperature in Kelvin (K) or Celsius (C)
Mr. Palmarin Chapter 4 - Thermodynamics 32 / 56

Examples:
1) Determine the specific heat capacity of 1.0 kg of water if it contains288000 J of heat and has a temperature change of 66 ◦C.
Like in thermal expansion, we will identify which variables we know andwhat we’re looking for.
m = 1.0 kg Q = 288 000 J ∆T = 66 ◦C C =?
Q = mC∆T
288 000 J = C (1.0 kg)(66 ◦C)
=⇒ C =288 000 J
(1.0 kg)(66 ◦C)= 4363... = 4400 J/(kg ◦C)
Note: In situations were I directly ask you to solve for C , you don’tneed to reference the table. Your answer may not always match thetable; i.e. the values given in this question may not be very preciselymeasured!
Mr. Palmarin Chapter 4 - Thermodynamics 33 / 56

2) How many Joules of heat are needed to raise the temperature of 3.0 kgof aluminum from 10 ◦C to 50 ◦C? The specific heat capacity ofaluminum is 903 J/(kg ◦C).
m = 3.0 kg ∆T = 50 ◦C− 10 ◦C = 40 ◦C C = 903 J/(kg ◦C)
Q =?
Q = mC∆T
Q = (3.0 kg)(903 J/(kg ◦C))(40 ◦C)
Q = 108360 = 110 000 J
Notice how the units cancel to give joules. This is the benefit ofwriting the units in the calculation. I’d suggest mirroring this examplein your work.
Mr. Palmarin Chapter 4 - Thermodynamics 34 / 56

Practice: [See Section 4.4 Video]
1) If a mass of aluminum takes 108 kJ of heat to raise the temperaturefrom 10 ◦C to 50 ◦C, what is the mass of the aluminum?
2) When 2.0 kg of iron is cooled from 80 ◦C to 15 ◦C, it releases 58500 Jof heat. What is the specific heat capacity?
Mr. Palmarin Chapter 4 - Thermodynamics 35 / 56

Section 4.5 - Transfer of Heat
1 Conduction: Process of heat transfer in which heat energy is passedon from one end of the solid to the other end through the collisionsbetween the particles.
The transfer of heat depends on the temperature differencebetween the two surfaces.The greater the area the greater the heat transfer.The greater the thickness the slower the heat transfer.
2 Convection: Process of heat transfer by the movement of themolecules from one place to another.
3 Radiation: Energy that is transferred through space in the form ofelectromagnetic waves.
Watch: “Conduction vs Convection”
Mr. Palmarin Chapter 4 - Thermodynamics 36 / 56

Calorimetry is how we will measure heat transfer. If one object loses heatthe other gains heat. This transfer of energy can be summarized using theformula:
Tf =maCaTa,i + mbCbTb,i
maCa + mbCb
- Tf is the final temperature
- ma and mb are the masses of objects a and b
- Ca and Cb are the specific heat capacities of objects a and b
- Ta,i and Tb,i are the initial temperatures of objects a and b
Here are two other useful arrangements of the same formula:
Ta,i =Tf (maCa + mbCb)−mbCbTb,i
maCaCa =
mbCbTb,i − Tf mbCb
Tf ma −maTa,i
Mr. Palmarin Chapter 4 - Thermodynamics 37 / 56

How is heat transfer experimentally measured?
A calorimeter is a device used to measure changes in thermal energy.
A solid sample is placed on the stirring stick, which is submerged inwater.
The initial temperature of the solid sample and the water is knownbefore submergence.
The masses are known and the specific heat capacities are constants.
After the two samples have reached thermal equilibrium, the finaltemperature can be measured directly.
Mr. Palmarin Chapter 4 - Thermodynamics 38 / 56

Example: Determine the final temperature of the system if 0.0677 kgpiece of brass at 132 ◦C is placed into a container that contains 0.1584 kgof water at 20 ◦C.
While the formula is large, we simply identify what we know and what isunknown. In the formula, I’ll let b = brass and w = water .
Tf =? mbrass = 0.0677 kg mwater = 0.1584 kg Tbrass,i = 132 ◦C
Twater ,i = 20 ◦C Cbrass = 376 J/(kg ◦C) Cwater = 4180 J/(kg ◦C)
Note: We must reference the specific heat capacities using the table.
Tf =mbCbTb,i + mwCwTw ,i
mbCb + mwCw
Tf =(0.0677 kg)(376 J/(kg ◦C))(132 ◦C) + (0.1584 kg)(4180 J/(kg ◦C))(20 ◦C)
(0.0677 kg)(376 J/(kg ◦C)) + (0.1584 kg)(4180 J/(kg ◦C))
Tf =16 602.3264 J
687.5672 J/◦C=⇒ Tf = 24 ◦C
Mr. Palmarin Chapter 4 - Thermodynamics 39 / 56

Practice: A 0.500 kg sample of water is at 15.0 ◦C. A 0.0400 kg block ofzinc is placed in the water. If the final temperature of the water is15.73 ◦C, determine the original temperature of the piece of zinc.
[See Section 4.5 Video]
Mr. Palmarin Chapter 4 - Thermodynamics 40 / 56

Section 4.6 - Change of State
The three most common states of matter are solids, liquids, and gases.
As the temperature of a solid is raised, the forces between the particlesare no longer strong enough to hold them in fixed positions. Eventually,the particles become free enough to slide past each other. At this point,the substance has changed from a solid to a liquid. The temperature thatthis occurs at is called the melting point.
The specific latent heat of fusion is the quantity of heat needed to melt1 kg of a solid without change of temperature.
Mr. Palmarin Chapter 4 - Thermodynamics 41 / 56

After the substance has melted an increase in temperature of the liquidallows some of the particles in the liquid to acquire enough energy to breakfree from other particles.
When the liquid reaches its boiling point, additional energy causes theliquid to turn into a vapour or gaseous state.
The specific latent heat of vapourization is the quantity of heat neededto vapourize 1 kg of a liquid without change of temperature.
Mr. Palmarin Chapter 4 - Thermodynamics 42 / 56

Specific Latent Heat of Fusion
The equation used to calculate the amount of heat necessary to change acertain mass from a solid to a liquid is:
Q = m · Hf
- Q is the amount of heat in joules (J)
- m is the mass of the substance in kg
- Hf is the heat of fusion in J/kg
Note: Hf can be determined if Q and m are given; however, usually weare looking for Q. In this case, you must reference Hf from the table onthe backside of the Thermochemical data sheet.
Mr. Palmarin Chapter 4 - Thermodynamics 43 / 56

Examples:
1) How much heat is required to change 400 g of silver from a solid to aliquid?
Solid to a liquid =⇒ melting, therefore the heat required is the heatof fusion. Reference the table for the Hf for silver.
Hf = 1.04× 104 J/kg m = 400 g = 0.400 kg (must be in kg)
Q = mHf =⇒ Q = (0.400 kg)(1.04× 104 J/kg) = 4160 = 4000 J
2) How much heat is required to change 410 g of water at 0 ◦C is changedinto ice at 0 ◦C?
Remember, fusion implies change of state from solid to liquid. Whenmoving the opposite direction (liquid to solid), we must introduce asign in front of Hf to inform the reader of the direction.
Hf = −3.34× 105 J/kg m = 410 g = 0.410 kg
Q = mHf =⇒ Q = (0.410 kg)(−3.34× 105 J/kg) = −136940 =
−140 000 J
Mr. Palmarin Chapter 4 - Thermodynamics 44 / 56

Specific Latent Heat of Vapourization
The equation used to calculate the amount of heat necessary to change acertain mass from a liquid to a gas is:
Q = m · Hv
- Q is the amount of heat in joules (J)
- m is the mass of the substance in kg
- Hv is the heat of vapourization in J/kg
Note: Once again, Hv can be determined if Q and m are given; however,usually we are looking for Q. In this case, you must reference Hv fromthe table on the backside of the Thermochemical data sheet.
Mr. Palmarin Chapter 4 - Thermodynamics 45 / 56

Examples:
1) How much heat is needed to change 60.0 g of water at 100 ◦C tosteam at 100 ◦C?
This is identical to fusion. Here, m = 60.0 g = 0.0600 kg andHv = 2.26× 106 J/kg
Q = mHv =⇒ Q = (0.0600 kg)(2.26× 106 J/kg) = 135 600 J =
140 000 J
2) Determine Q (heat required) when 150 g of steam is changed intowater.
Vapourization is liquid to gas, but here we’re going from gas to liquid.Therefore, this is opposite direction and so we will introduce a sign infront of Hv .
Q = mHv =⇒ Q = (0.150 kg)(−2.26× 106 J/kg) = −339 000 J =
−340 000 J
Mr. Palmarin Chapter 4 - Thermodynamics 46 / 56

Multi-step Questions with Fusion & Vapourization
Often, we wish to find the heat required to melt and possibly vapourize inone question. For example, suppose we wish to calculate the heat requiredto change 10 g of ice at −15 ◦C to steam at 140 ◦C. How would we dothis?
This is a five step question (the longest type possible). When thetemperature is changing, we use Q = mC∆T to find Q. When we arein a phase change; i.e. fusion or vapourization, we use Q = mHf orQ = mHv . We then add each step’s value of Q together.
Temperature change (Q = mC∆T ) →Phase change (Q = mHf or Q = mHv ) →and so on ...
(See next slide)
Mr. Palmarin Chapter 4 - Thermodynamics 47 / 56

Both of these diagrams are very useful. Note: you won’t always have5-steps questions (sometimes 2, 3, or 4). Since we will always (formulti-step calculations) be working with water, you can simply mark whereyou are starting and ending on one of these graphs, and it will help youdecide how many steps there are and which formula to use (and when).
Used when temperature is increasing Used when temperature is decreasing
Each line segment requires a calculation. The formula to use is written above each
segment. The temperatures of 0 ◦C and 100 ◦C implies that we are working with
water.
Mr. Palmarin Chapter 4 - Thermodynamics 48 / 56

Examples:
1) Calculate the quantity of heat required to change 50.0 g of ice at−10 ◦C into water at 0 ◦C. Hint: Use Q = mC∆T and Q = m · Hf
Let’s draw a diagram (when doing this, you can reference theappropriate diagram above). In this case, the temperature is increasing,so we have:
Step 1: Q = mC∆T = (0.0500 kg)(2060 J/(kg ◦C))(10 ◦C) = 1030 J(don’t round yet, but this is only accurate to 2 s.f.)
Step 2: Q = mHf = (0.0500 kg)(3.34× 105 J/kg) = 16700 J(once again don’t round - this is accurate to 3 s.f.)
Add Steps 1 & 2: 1030 J + 16700 J = 17 700 J
Here, we are significant to the hundreds place in both numbers;therefore, we round our answer to the hundreds place.
Mr. Palmarin Chapter 4 - Thermodynamics 49 / 56

2) How much heat is released (transferred) when 100 g of steam at130 ◦C is condensed and cooled to 50 ◦C?
Step 1: Q = mC∆T = (0.1 kg)(2020 J/(kg ◦C))(−30 ◦C) = −6060 J
Step 2: Q = mHf = (0.1 kg)(−2.26× 106 J/kg) = −22600 J
Step 3: Q = mC∆T = (0.1 kg)(4180 J/(kg ◦C))(−50 ◦C) = −20900 J
Add Steps 1, 2, & 3: -6060 J + -22600 J + -20900 = −50 000 J
Here, the least certain measurement is to the 10,000’s place; therefore, weround our answer to the 10,000’s place.
Mr. Palmarin Chapter 4 - Thermodynamics 50 / 56

Practice: Calculate the heat required to change 10 g of ice at −15 ◦C tosteam at 140 ◦C. [See Section 4.6 Video]
Mr. Palmarin Chapter 4 - Thermodynamics 51 / 56

Section 4.7 - Combustion Engines
Combustion Engines are devices that convert large amounts of heat intomechanical energy, which is the sum of the kinetic and potential energieswithin the object.
1 The External Combustion Engine - the fuel is burned outside ofthe location where the thermal energy (heat) created is convertedinto mechanical energy.
2 The Internal Combustion Engine - the fuel is burned inside thecylinder(s) where the thermal energy (heat) created is converted intomechanical energy.
Mr. Palmarin Chapter 4 - Thermodynamics 52 / 56

The drawbacks to this conversion of energy:
The conversion of thermal energy (heat) into mechanical energy is notefficient. The energy transformation produces useable and non-useableenergy.
Automobiles
In a car engine the thermal energy produced by the engine (expanding gas)is used to move the pistons, then the crankshaft, transmission and finallythe tires, but only a small portion of this energy is used to do this. Themajority of the thermal engine is lost as waste heat. This heat is removedby the cooling system or lost through heat transfer through the engine.
Note: Only about 25% of the energy produced by a car engine isuseable energy.
Mr. Palmarin Chapter 4 - Thermodynamics 53 / 56

Mr. Palmarin Chapter 4 - Thermodynamics 54 / 56

Electric Cars - Example: The Tesla Model S
Battery: 90 kWh battery
Battery Range: 435 km
Acceleration: 0 - 100 km/h in 2.8 seconds
Top Speed: 250 km/h
Power: 762 hp; 713 lb-ft (dual motor)
Charge Time: 40-60 min at supercharging station (free); 8 hours athome plug-in
Mr. Palmarin Chapter 4 - Thermodynamics 55 / 56

Points of Interest:
In the Tesla, there are no gears. Power is directly applied to the rotationof the axial(s). Therefore, the electric motor maximizes efficiency.
Also, as the car slows down, the kinetic energy in the moving car isharnessed by the braking system, converted to electricity, and stored inthe batteries.
Watch: “How it works” “Review” “Demo”
Mr. Palmarin Chapter 4 - Thermodynamics 56 / 56