Tyndall Effect
-
Upload
paul-muljadi -
Category
Documents
-
view
37 -
download
11
Transcript of Tyndall Effect

Tyndall effect 1
Tyndall effect
Flour suspended in water appears to be bluebecause only scattered light reaches the viewerand blue light is scattered by the flour particles
more strongly than red.
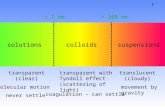
The Tyndall effect, also known as Tyndall scattering, is lightscattering by particles in a colloid or particles in a fine suspension. It isnamed after the 19th century physicist John Tyndall. It is similar toRayleigh scattering, in that the intensity of the scattered light dependson the fourth power of the frequency, so blue light is scattered muchmore strongly than red light. An example in everyday life is the bluecolour sometimes seen in the smoke emitted by motorcycles,particularly two stroke machines where the burnt engine oil providesthe particles.
Under the Tyndall effect, the longer-wavelength light is moretransmitted while the shorter-wavelength light is more reflected viascattering. An analogy to this wavelength dependency is that longwaveelectromagnetic waves such as radio waves are able to pass through thewalls of buildings, while shortwave electromagnetic waves such aslight waves are stopped and reflected by the walls. The Tyndall effectis seen when light-scattering particulate-matter is dispersed in anotherwise light-transmitting medium, when the cross-section of anindividual particulate is the range of roughly between 40 and 900nanometers, i.e., somewhat below or near the wavelength of visiblelight (400–750 nanometers).
The Tyndall effect is commercially exploited to determine the size anddensity of particles in aerosols and other colloidal matter; seeultramicroscope and turbidimeter.
Difference from Rayleigh scattering
The Tyndall effect in opalescent glass: It appearsblue from the side, but orange light shines
through.[1]
Rayleigh scattering is defined by a mathematical formula that requiresthe light-scattering particles to be far smaller than the wavelength ofthe light. For a dispersion of particles to qualify for the Rayleighformula, the particle sizes need to be below roughly 40 nanometers;and the particles may be individual molecules. Colloidal particles arebigger, and are in the rough vicinity of the size of a wavelength oflight. It follows from scattering theory that Tyndall scattering (bycolloidal particles) is much more intense than Rayleigh scattering. Theimportance of the size factor for intensity can be seen in the largeexponent it has in the mathematical statement of the intensity ofRayleigh scattering. There is no equivalent mathematical statement forTyndall scattering. But, if the colloid particles are spheroid, Tyndallscattering is mathematically analysable in terms of Mie theory, which admits particle sizes in the rough vicinity ofthe wavelength of light.

Tyndall effect 2
Blue irises
A blue iris
A blue iris in an eye is due to Tyndall scattering in a turbid layer in the iris.Brown and black irises have the same layer except with more melanin in it.The melanin absorbs light. In the absence of melanin, the layer is translucent(i.e., the light passing through is randomly and diffusely scattered) and anoticeable portion of the light that enters this turbid layer re-emerges via ascattered path. That is, there is backscatter, the redirection of the lightwavesback out to the open air. Scattering takes place to a greater extent at theshorter wavelengths. The longer wavelengths tend to pass straight through theturbid layer with unaltered paths, and then encounter the next layer furtherback in the iris, which is a light absorber. Thus, the longer wavelengths are
not reflected (by scattering) back to the open air as much as the shorter wavelengths are. Since the shorterwavelengths are the blue wavelengths, this gives rise to a blue hue in the light that comes out of the eye.[2] The blueiris is an example of a structural color, in contradistinction to a pigment color.
Weather
Ribbons of light due to Tyndall Scattering
On a day when the sky is overcast, the sunlight passes through theturbid layer of the clouds, resulting in scattered, diffuse light on theground. This does not exhibit Tyndall scattering because the clouddroplets are larger than the wavelength of light and scatter all colorsapproximately equally. On a day when the sky is cloud-free, the sky'scolor is blue in consequence of light scattering, but this is not termedTyndall scattering because the scattering particles are the molecules ofthe air, which are much smaller than the wavelength of the light. Onoccasion, the term Tyndall effect is incorrectly applied to lightscattering by macroscopic dust particles in the air. However, this ismore like reflection, not scattering, as the macroscopic particlesbecome clearly visible in the process.
References[1] http:/ / www. webexhibits. org/ causesofcolor/ 14B. html[2] For a short overview of how the Tyndall Effect creates the blue and green colors in animals see uni-hannover.de (http:/ / www. itp.
uni-hannover. de/ ~zawischa/ ITP/ scattering. html#tyndalleffekt) and for information in greater detail see Colourandlife.com (http:/ / www.colourandlife. com/ ).

Article Sources and Contributors 3
Article Sources and ContributorsTyndall effect Source: http://en.wikipedia.org/w/index.php?oldid=505366934 Contributors: Abdull, Adambisset, Adithyak1, Afluegel, Alvis, Annielogue, Archelon, Armeria, Avathar,Belg4mit, Berland, Burn, CERminator, CPMcE, Chenzw, Conscious, D'Agosta, DanMS, Dante Alighieri, Derek1G, Drphilharmonic, Eddie Nixon, Fito, Gene s, GreatWhiteNortherner,Grogdamighty, Gruts, HappyApple, Henriquevicente, Honza Záruba, Ian Dunster, Icairns, Inourbedroomafterthewar, Inter, Jag123, Jmnbatista, Julian herrera, Karol Langner, Lamarque,Limideen, Lkjhgfdsxcvb, Lotje, M1ss1ontomars2k4, Materialscientist, Mathwhiz 29, Michael Hardy, Minna Sora no Shita, Obradovic Goran, Ozo, Palfrey, Peter Karlsen, Petergans, Pjacobi,Pradeep717, Puffin, Renata3, Renski, Rifleman 82, Rigadoun, Ruy Pugliesi, Sabri76, Seanwal111111, Shanes, Shinkolobwe, Sintaku, Slicky, Srleffler, Sundar, Taxiarchos228, Tinton5, Vonzepp,Wiki.Goodash, Wjbeaty, YanA, Yancyfry jr, 124 anonymous edits
Image Sources, Licenses and ContributorsImage:WaterAndFlourSuspensionLiquid.jpg Source: http://en.wikipedia.org/w/index.php?title=File:WaterAndFlourSuspensionLiquid.jpg License: GNU Free Documentation License Contributors: Chris 73, DrJunge, Ies, Lobo, Saperaud, Superm401, WstFile:Why is the sky blue.jpg Source: http://en.wikipedia.org/w/index.php?title=File:Why_is_the_sky_blue.jpg License: Creative Commons Attribution-Sharealike 2.0 Contributors: optickImage:Bluishgrayeye.JPG Source: http://en.wikipedia.org/w/index.php?title=File:Bluishgrayeye.JPG License: Copyrighted free use Contributors: en:User:Nick4gwenImage:TyndallEffect.JPG Source: http://en.wikipedia.org/w/index.php?title=File:TyndallEffect.JPG License: Creative Commons Attribution-Sharealike 3.0 Contributors: Pradeep717
LicenseCreative Commons Attribution-Share Alike 3.0 Unported//creativecommons.org/licenses/by-sa/3.0/