Top 14 Photochemical Smog (Part 2) (1)
-
Upload
kenneth-leong -
Category
Documents
-
view
35 -
download
0
Transcript of Top 14 Photochemical Smog (Part 2) (1)

CHEM115 Envrionmental Chemistry 1
TOPIC 14(part 2)
SMOG & OZONE DEPLETION

CHEM115 Envrionmental Chemistry 2
SMOG

CHEM115 Envrionmental Chemistry 3
Introduction
It is a general term first used in 1905 for a mixture of smoke and fog.
A kind of urban air pollution.
2 major types:Photochemical smogSulfurous smog (Classical smog)

CHEM115 Envrionmental Chemistry 4
Photochemical Smog
Solar radiation is important for formation of photochemical smog.
The reactions that occur is complex and involve nitrogen oxides (NOx) and hydrocarbons.
It is directly related to automobile use.

CHEM115 Envrionmental Chemistry 5
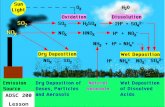
Formation of Photochemical Smog
Sun
Nitrogen oxides (NOx) + Hydrocarbons
Photochemical Smog
Solar radiation
Pollutants are trapped in an inversion layer.
Source: Botkin&Keller.2003. Environmental Science, 4th ed.

CHEM115 Envrionmental Chemistry 6
Example: Photochemical Fog Formation over Los Angeles Area
Source: Botkin&Keller, 2003. Environmental Science. 4 th ed. Chapter 23.

CHEM115 Envrionmental Chemistry 7Source: Botkin&Keller, 2003. Environmental Science. 4 th ed. Chapter 23.
The city of Los Angeles, on a clear day (left) and a smoggy day (right)

CHEM115 Envrionmental Chemistry 8
Sulfurous Smog (Classical Smog)
It is mainly due to burning of coal or oil at large power plants.
Sulfur oxides and particulates combine to produce sulfurous smog.

CHEM115 Envrionmental Chemistry 9
Formation of Sulfurous SmogBurning of coal or oil
Sulfur oxides + particulates
releases
Concentrated sulfurous smog
•stagnant and stable air•sufficient relative humidity•cloud cover •formation of inversion layer

CHEM115 Envrionmental Chemistry 10
What are the consequences?
• Reduce visual range and atmospheric clarity• Damage plant tissues• Increase lung disease and respiratory system
disease such as bronchitis• Aggravate chronic disease such as asthma and
emphysema

CHEM115 Envrionmental Chemistry 11
Control of Smog
• Stricter emission control for automobiles• Requirement for more making fuel cleaner• Improvement in public transportation and
incentives for people to use it• Enforcing mandatory carpooling• Increase control on industrial and household
activities

CHEM115 Envrionmental Chemistry 12
Relevant Regulations and Guidelines
Clean Air ActNational Ambient Air Quality Standards (NAAQS)Air Quality IndexClean Air RegulationsAir Pollution Index

CHEM115 Envrionmental Chemistry 13
OZONE DEPLETION

CHEM115 Envrionmental Chemistry 14
Introduction
Ozone is a triatomic form of oxygen.
In the lower atmosphere, ozone is a pollutant produced by photochemical reactions which involve sunlight, nitrogen oxides, hydrocarbons, and oxygen.
In the upper atmosphere, ozone acts as a sunscreen against UV radiation.

CHEM115 Envrionmental Chemistry 15
Formation of Stratospheric Ozone
Source: Botkin&Keller, 2003. Environmental Science. 4 th ed. Chapter 25.

CHEM115 Envrionmental Chemistry 16
Ozone Depletion
CFCs are considered responsible for most of the ozone depletion.
CFCs, unlike other pollutants cannot be broken down by sunlight, rain-out and oxidation. CFCs are transparent to sunlight, insoluble in water, and non-reactive in oxygen-rich at lower atmosphere.

CHEM115 Envrionmental Chemistry 17
When CFCs wander to the upper atmosphere, the highly energetic UV splits up the CFCs, releasing chlorine. Then, ozone depletion occurs.
Cl + O3 -> ClO + O2
ClO + O -> Cl + O2
The series of reactions is known as catalytic chain reaction.

CHEM115 Envrionmental Chemistry 18
Formation and Destruction of Ozone
Source: Botkin&Keller, 2003. Environmental Science. 4 th ed. Chapter 25.

CHEM115 Envrionmental Chemistry 19
What are the consequences?
• Damage on food chain• Increase in skin cancer, cataracts and
suppression of immune system

CHEM115 Envrionmental Chemistry 20
Control
• Montreal Protocol, 1987• Substitutes for CFC.

CHEM115 Envrionmental Chemistry 21










![Chapter 4 · photochemical smog and, on a global scale, to a radiative forcing of climate [IPCC, 1994; WMO, 1998]. CO is formed by photochemical breakdown of methane and higher hydrocarbons,](https://static.fdocuments.in/doc/165x107/608ce6a910760c5c395a9e8e/chapter-4-photochemical-smog-and-on-a-global-scale-to-a-radiative-forcing-of-climate.jpg)