Thomson’s Model of Atom - :: Vishwa Bharti Public … and...1 Atom and Nucleus by Umesh Tyagi Ph....
Transcript of Thomson’s Model of Atom - :: Vishwa Bharti Public … and...1 Atom and Nucleus by Umesh Tyagi Ph....
1 Atom and Nucleus by Umesh Tyagi Ph. No-9811156956
ATOM AND NUCLEUS
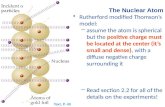
Thomson’s Model of Atom
Thomson was the first to discover that every atom has negatively
charged particles called electrons.
An atom is a sphere of positive charge and mass is distributed uniformly
over the entire body of the atom with negative electrons embedded in this
continuous positive charge. The radius of atom is of the order of 10-10 m.
This was called Thomson’s plum pudding model because the negatively
charged particles (the "plums") were embedded in a sphere of uniform
positive charge (the "pudding"). Although this model was later on found to be highly incorrect.
α -Particle Scattering Experiment (Geiger-Marsden Experiment)
On the suggestion of Rutherford, his two associates H. Geiger and Marsden performed a classic
experiment to investigate the structure of the atom. Following figure shows the apparatus used to
investigate the scattering of α-particles.
A narrow beam of high-energy α-particles (helium nuclei)
from a radioactive source (radon) was incident on a thin
sheet of gold (about 0.2 micrometer thick). The alpha-
particles scattered in different directions were detected by
α-particle detector.
The angle θ of the deviation of α-particle from its original
direction is called scattering angle.
Observations:
The following important observations were made by
Geiger and Marsden
(i) Most of the α-particles passed straight through the gold foil and
only about 0.14% were scattered by more than 10.
(ii) A very few alpha-particles were deflected through the angle
90º of more
(ii) Rarely, about 1 in 8000 α-particle bounced straight back i.e.
suffers a deflection of 180°.
The graph between the total number of α—particles scattered at angle θ and the scattering angle θ is
shown in Fig.3. Note that very few α-particles are scattered through large angles (more than 90°).
4
1( )
sin ( / 2)N
Explanation: Rutherford made the following conclusions from the scattering of α-particles:
2 Atom and Nucleus by Umesh Tyagi Ph. No-9811156956
(i) As most of the α-particles pass straight through the gold foil, so most of the space within the
atoms should be empty.
(ii) Since α-particles are about 7000 times heavier than an electron so these can be deflected only
by a heavy & positively charged particle hence for explaining the large angle scattering of α-
particles, Rutherford suggested that all the positive charge and almost total mass of the atom
should be concentrated in a very small region (which is called as nucleus of the atom)
(iii) The nucleus is surrounded by electrons, some distance away and the electrons should move in
circular orbits because if they were at rest, they would fall into the nucleus due to electrical
attraction.
(iv) As the atom is electrically neutral so the total positive charge on the nucleus should be equal to
the total negative charge of the electrons in the atom.
Rutherford Atomic Model:
(i) Maximum space within the atoms is empty.
(ii) All the positive charge and almost total mass of the atom is concentrated in a very small region
called as nucleus of the atom.
(iii) The electrons are moving in circular orbits about the nucleus and the necessary centripetal force
is provided by the electrostatic force of attraction between nucleus and electrons.
(iv) Atom is electrically neutral as the total positive charge on the nucleus is equal to the total
negative charge of the electrons in the atom.
DISTANCE OF CLOSEST APPROACH (SIZE OF NUCLEUS)-
The smallest distance between the nucleus and α—particle fired for head on collision towards the
nucleus is called the distance of closest approach (ro).
Suppose a α-particle is directed towards the centre of the nucleus of an atom as shown in figure. The α-
particle slows down due to repulsive force. As the α-particle slows down, its kinetic energy starts
transferring into electric potential energy of the system. The transfer is complete when the α-particle
stops momentarily. At this point, the distance of the α-particle from the nucleus is ro and its entire kinetic
energy is transferred to the electric potential energy of the system. The α-particle now retraces its path.
Mathematical expression for ro
Let m= mass of the α- particle
V = velocity of the α- particle
+2e = charge on α- particle
+Ze = charge on nucleus
Initial K. E. of α- particle, 21
2K mv
Electric Potential energy of the system when the α- particle is a distance r0 from the nucleus is given by-
3 Atom and Nucleus by Umesh Tyagi Ph. No-9811156956
0 0
1 (2 ) ( )
4
e ZeU
r
According to law of conservation of energy, K=U
2
2 2
0
0 0 0
1 1 (2 ) ( ) 1 2 1 { K= }
2 4 4 2
e Ze Zemv r mv
r K
The following points may be noted carefully:
(i) The radius of the nucleus will be less than ro.
(ii) The value of ro depends upon the initial kinetic energy K of α —particle. If the value of K is very
large, then α -particle will reach extremely close to the nucleus. Under this condition, the nuclear
forces will become active and the α -particles will penetrate into the nucleus.
Therefore, α -particles with kinetic energy greater than certain value cannot be scattered back by the
nucleus.
IMPACT PARAMETER -
When the α-particle is far away from the nucleus then the
perpendicular distance of the velocity vector (v) of the α-
particle from the centre of the nucleus is called impact
parameter. It is denoted by b. Rutherford found
mathematically the relation Nucleus between impact parameter b and the scattering angle
Drawbacks: Rutherford’s atomic model presented the following two main
difficulties:
(i) According to Maxwell’s theory of electro magnetism, a charge that is
accelerating radiates energy as electromagnetic waves. The electron
moving around the nucleus is under constant acceleration and, therefore,
it should continuously lose energy. Due to this continuous loss of energy, the electrons in Rutherford’s
model were bound to spiral towards the nucleus and fall into it when all of their rotational energy was
radiated.
Hence, Rutherford’s atomic model cannot be stable while in actual practice, an atom is stable. This
shows that Ruthe1ford’s model is not correct.
(ii) During inward spiraling, electrons will radiate electromagnetic waves of all frequencies i.e. the
spectrum should be continuous in nature. But, experiments show that an atom emits line spectra. So
Rutherford’s model fails to explain the line spectra.
4 Atom and Nucleus by Umesh Tyagi Ph. No-9811156956
Thus the Rutherford model failed to account for the stability of the atom. It was also unable to explain the
emission of line spectra.
Bohr’s Model-This model is also called planetary model of the atom and based upon the following
postulates-
(i) Nuclear Concept-Every atom consists of a small and massive central core, called nucleus
around which electrons revolve on circular path. The centripetal force required for rotational
motion is provided by the electrostatic force between the electrons and nucleus. i.e.
2 2
2
mv kZe
r r
(ii) Quantum Condition-Electrons are permitted to circulate only in those orbits in which the
angular momentum of the electron is an integral multiple of h/2π. These orbits are called
stationary orbits. Therefore for any permitted orbit,
2
nhL mvr
(iii) Stationary Orbit and frequency Condition-While revolving in stationary orbit an electron
does not radiate energy. An atom can emit or absorb radiation in the form of EMW only when an
electron jumps from higher to lower orbit or from lower to a higher orbit, respectively.
If E1 and E2 are the energies associated with these permitted orbits, then the frequency ‘ν’ of the
emitted or absorbed radiation is given by
hν = E2 - E1
Bohr’s Theory for Hydrogen Atom-
(i) Radii of permitted orbits- According to Bohr’s theory, For circular motion of electron around the
nucleus in an atom, centripetal force is provided by the electrostatic force between nucleus (+Ze)
and electron carrying charge ( –e). Therefore
2 2
2
2
2
(1)
mv kZe
r r
kZemv
r
From the quantum condition for angular momentum, we have
2
nhL mvr
(2)2
nhv
mr
From equation (1) and (2),
2 2
2
nh kZem
mr r
2 2
2 2
4
n hr
mkZe
Since,2
r n the radii of the permitted orbits increases in the ratio of 1:4:9… 2 2 2 34 2
2 2 2 31 9 19 2
(6.63 10 )
4 4(3.14) (9.1 10 ) (9 10 ) (1.6 10 )
n h nr
mkZe Z
5 Atom and Nucleus by Umesh Tyagi Ph. No-9811156956
2
0.529
nr
Z
The radius of the inner most orbit of hydrogen atom is called as Bohr’s Radius (r0) which can be
determined by putting Z=1 and n=1 in the above equation.
00.529r
Speed of an electron in nth orbit of H-atom- According to Bohr’s theory of hydrogen atom,
Centripetal Force = Electrostatic Force
2 2
2
mv kZe
r r
2
. (1)kZe
mvr vr
From the quantum condition for angular momentum-
2
nhmvr
Therefor the equation (1) becomes
2
.2
nh kZev
r
22
kZe
vnh
22
.ke cZ cZ
vch n n
Here α is called as fine structure constant. Its value is 2 9 19 2
8 34
2 2 3.14 (9 10 ) (1.6 10 ) 1=
3 10 (6.63 10 ) 137
ke
ch
Hence 1
.137
cZv
n
For hydrogen atom, Z=1 ∴ 1
.137
cv
n
For first orbit (n=1), ∴ 8
63 102.2 10 /
137 137
cv m s
Thus the speed of the electron in the innermost orbit of H-atom is 1/137 times the speed of light in
vacuum.
Energy of the electron-Total energy of an electron is equal to the sum of K.E and P.E.
En = K.E. + P.E.
2 2
2 2
Kinetic energy of the electro in n orbit is
1 . . (as )
2 2
th
kZe kZeK E mv mv
r r
6 Atom and Nucleus by Umesh Tyagi Ph. No-9811156956
2
1 2. .( )
. .q q k Ze e kZe
P E kr r r
2 2 2
Hence Total energy of the electron in n orbit is
E . . . .2 2
th
n
kZe kZe kZeK E P E
r r r
2 2 2
2 2
2 2 4
2 2
4 E .
2
2 E
n
n
kZe mkZe
n h
mkZ e
n h
The negative sign shows that the electron is bound with nucleus and it can’s leave its orbit itself.
2 31 9 2 19 4
34 2 2
2 (3.14) (9.1 10 )(9 10 ) (1.6 10 )E .
(6.63 10 )n
Z
n
19
2E 21.76 10 .
n
Zjoules
n
19
19 2 2
21.76 10E . 13.6
1.6 10n
Z ZeV eV
n n
For hydrogen atom Z=1 ∴ 2
13.6E
neV
n
Energy Level diagram for an atom- It is the diagram in which energies of the different stationary
states of an atom are represented by parallel horizontal lines, drawn according to the some suitable
scale. From Bohr’s theory, the total energy of
an electron in nth orbit is given by
2
13.6E
neV
n
Putting n = 1, 2, 3, 4…………,
We get the energies of different orbits as
follows:
1 2
13.613.6 ;
1E eV
2 2
13.63.4 ;
2E eV
3 2
13.61.51 ;
3E eV
n=5 -0.54eV n=4
n=3
n=2
n=1
n=6
Ground
State
Excited
States
-13.6eV
-3.4eV
-1.51eV
-0.85eV
-0.37eV 0 n=∞
Unbound (ionized) atom
7 Atom and Nucleus by Umesh Tyagi Ph. No-9811156956
4 2
13.60.85 ;
4E eV 5 2
13.60.54 ;
5E eV 6 2
13.60.37 ;
6E eV and 0E
Clearly, an electron can have only certain definite values of energy while revolving in different orbits.
This is called energy quantization.
The energy state corresponding to n-=1 has the lowest energy equal to -13.6 eV and is called as
ground state.
Origin of Spectral Lines of Hydrogen Atom-
When an electron jumps from higher energy level to some lower energy level the spectral line are
observed. Suppose an electron jumps from higher energy level n2 to lower energy level n1. So from
Bohr’s frequency condition-
2 1
2 2 4 2 2 4
2 2 2 2
2 1
2 2n n
mk e mk eh E E
n h n h
2 2 4
2 2 2
1 2
2 1 1hc mk e
h n n
2 2 4
3 2 2 2 2
1 2 1 2
1 2 1 1 1 1mk eR
ch n n n n
2 2
1 2
1 1 1R
n n
Where
2 2 4
7 1
3
2R= 1.0973 10
mk em
ch
is known as Rydberg Constant.
The origin of various spectral series in hydrogen spectrum can be explained as follows-
1. Lyman series- When an electron jumps from any higher orbit to first orbit then Lyman series is
obtained. This series lies in UV region and is given by-
22 2
2
1 1 1, 2,3, 4,........
1R n
n
2. Balmer Series- When an electron jumps from any higher orbit to second orbit then Balmer series is
obtained. This series lies in visible region and is given by-
22 2
2
1 1 1, 3, 4,........
2R n
n
3. Paschen Series When an electron jumps from any higher orbit to third orbit then Paschen series is
obtained. This series lies in IR region and is given by-
8 Atom and Nucleus by Umesh Tyagi Ph. No-9811156956
22 2
2
1 1 1, 4,5, 6, 7........
3R n
n
4. Brackett Series- When an electron jumps from any higher orbit to fourth orbit then Brackett series
is obtained. This series lies in IR region and is given by
22 2
2
1 1 1, 5, 6, 7........
4R n
n
5. Pfund Series-When an electron jumps from any higher orbit to fifth orbit then Pfund series is
obtained. This series lies in IR region and is given by
22 2
2
1 1 1, 6, 7........
5R n
n
Limitation of Bohr’s atomic model-
1. This model is applicable only for hydrogen and hydrogen like atoms.
2. This model does not explain the fine structure of spectral lines.
3. This model is unable to explain the relative intensity of spectral lines.
4. It does not explain why only circular orbits should be chosen when elliptical orbits are also possible.
5. In this model wave nature of electron is not considered.
De-Broglie’s Explanation of Bohr’s Second Postulate-
According to Bohr’s second postulate, angular momentum of electron orbiting around the nucleus is
quantized i.e, 2
nhmvr
.
De Broglie postulated that the electron in its circular orbit must be seen as a particle wave.
Hence according to de Broglie, a stationary orbit is that which contains an integral number of de
Broglie waves associated with revolving electron.
We know that in a string, standing waves form when total distance travelled by a wave down the string
and back is an integral number of wavelengths.
Therefore for an electron in nth orbit of radius rn
Total distance covered = circumference of the orbit = 2 π rn
∴ For a permissible orbit, 2 π rn = n λ
According to de Broglie, n
h
mv
∴2
2 or ( )2
hn n n
n
nh nhr mv r n
mv
2 or ( )22
n n n
n
nh nh hr mv r nmv
Thus the angular momentum of electron in nth orbit must be an integral multiple of h/2π, which is the
quantum condition proposed by Bohr in second postulate.
9 Atom and Nucleus by Umesh Tyagi Ph. No-9811156956
Excitation Energy- The minimum amount of energy required by an electron to jump from the ground
state to any one of the excited state, is called excitation energy.
First excitation energy of hydrogen =E2-E1= -3.4-(13.6) =10.2eV
Ionization Energy- The energy required to knock an electron completely out of atom is called
ionization energy.
Ionization Energy of hydrogen = E∞-E1=0-(-13.6) =13.6eV
Excitation Potential-It is that accelerating potential which gives to a bombarding electron, sufficient
energy to excite the target atom by raising one of its electrons from inner to an outer orbit.
First excitation potential of hydrogen atom = -3.4-(-13.6) =10.2 Volt
Second excitation potential of hydrogen atom = -1.51-(-13.6) =12.09 Volts
Ionization Potential- It is that accelerating potential which gives to a bombarding electron, sufficient
energy to ionize the target atom by knocking one of its electrons completely out of the atom.
Ionization Potential of hydrogen atom = 0-(-13.6) =13.6Volt
Composition of Nucleus- Proton-Neutron Model:
According to this model, each nucleus is formed by proton and neutron. The nucleus contains Z protons and (A - Z) neutrons. The nucleus therefore has a net positive charge of + Ze; where e is the charge on the electron.
Nuclear Size and Nuclear Density:
The experimental measurements show size of nucleus is proportional to the mass number. If R is the radius of nuclei then,
3/10
3
3
4
ARR
AR
Where R0 is constant and its value is 1.1 x 10-15 m and nuclear density is the ratio of mass of nucleus to its volume. If the mass number of substance is A, the mass of nucleus is = m A. Where m=1.66x10-27 kg is the mass of each nucleon.
317
30
28
33/10
/1097.2
3
4
106.1
)(3
4
mkg
AR
ADensityNuclear
ARnucleusofVolume
The result shows that the magnitude of nuclear density is very large and is independent of the mass number.
10 Atom and Nucleus by Umesh Tyagi Ph. No-9811156956
Atomic Mass Unit:
It is defined as the mass of 1/12th the mass of one carbon-12 atom. Using molar theory,
6.023 x 1023 carbon atoms has mass = 12 gm
gm
atomcarbonofmasstheth
masshasatomCarbon
24
23
23
1066.1
10023.6
1
12
1
10023.6
121
Energy corresponding to 1 a.m.u. can be calculated using Einstein’s mass energy equivalence relation, E = Δm c2
MeV
JoulesE
5.931106.1
1091066.1
1091066.1
13
1627
1627
Isotopes: These are the substances having same atomic number but different atomic weights. The two isotopes thus always have same atomic numbers but different number of neutrons in it. As chemical properties of substance depends on the atomic number, therefore, isotopes have identical chemical properties and cannot be separated using chemical means. Therefore, to separate two isotopes we take the help of device like centrifuge. The common example of isotopes are (1H1, 1H2, 1H3) or (17Cl35, 17Cl37) or (92U235, 92U238)
Isobars: These are the substances having different atomic number but same atomic weight. In isobars, the number of proton, electron and neutron all are different, only the total number of nucleon or mass number is same. The chemical properties of isobars are different; therefore, they can be easily separated using chemical means. For example, (11Na22, 10Ne22) or (20Ca40, 18Ar40)
Isotones: These are the substances having same number of neutrons in them. For e.g. 17Cl37 and 19K39 or 1H3 and 2He4 etc.
Nuclear forces- The attractive forces which are responsible to hold the nucleons together inside the nucleus are called nuclear forces.
Properties-
1. It is a charge independent force and its magnitude is same for p-p, p-n or n-n.
2. They are very short range forces and are effective only up to a distance of few fermi.
3. It is strongest force in nature and its magnitude is about 100 times stronger than electromagnetic interaction.
4. Nuclear force is a saturation force as given nucleon can interact only with its immediate neighbors and not all the nucleon present in nucleus.
11 Atom and Nucleus by Umesh Tyagi Ph. No-9811156956
5. Nuclear forces are non central forces and do not acts only the line joining the two particles.
6. It is a spin dependent force. Nucleons having parallel spin are more strongly bound to each other than nucleons having antiparallel spin.
7. These are the exchange forces and don’t obey inverse square law.
8. Although these forces are attractive forces but becomes repulsive if the distance between two nucleons becomes less than 0.8 Fermi. This property stops the nucleus to be collapsed.
Yukawa Theory of Nuclear Forces:
According to Yukawa theory the particles intermediate in mass between electrons and nucleons is responsible for nuclear forces. Today these particles are called pions or p-measons.
p-measons can be positively charged or negatively charged or they can be neutral also. According to Yukawa theory, every nucleon continuously emits and reabsorbs pions.
00
00
measons- ofEmission
meason- of Absorption
nnpp
np
pn
nnpp
np
pn
Binding Energy:
It was observed that whenever number of nucleons join together to form nucleus, the mass of nucleus is always less than the mass of individual nucleons. The difference in mass of nucleons and the nucleus is called the mass defect. The energy corresponding to mass defect is called its binding energy. OR The energy required to split a nucleus into it constituents particles, is called
Distance between Two nucleons
Repulsive Force
Attractive Force
0.8 fr
12 Atom and Nucleus by Umesh Tyagi Ph. No-9811156956
A
mFractionPacking
binding energy. This energy helps in keeping the nucleons bound together and is equivalent to the energy which is to be supplied to bread the nucleons. For nucleus of mass number A and atomic number Z, the mass defect is,
Δm = [ Zmp + (A - Z) mn - MN]
Where mp, mn and MN denotes the mass of proton neutron and nucleus respectively. The energy corresponding to this mass defect Dm will be,
Binding energy = [Zmp + (A - Z) mn - MN] c2
If all the mass are taken in atomic mass units then binding energy in million electron volt will be,
Binding energy = Δm (in a.m.u.) x 931 MeV Packing Fraction- The packing fraction for a nucleus is defined as the mass defect per nucleon.
Similarly, we can define binding energy per nucleon as the total binding energy divided by the total number of nucleons.
Binding Energy Curve:
Figure shows binding energy per nucleon plotted against the number of nucleons in various atomic nuclei. The greater the binding energy per nucleon, the more stable the nucleon is. The binding energy per nucleon is maximum for number of nucleons 56, with value of 8.8 MeV/nucleon.
1. As first approximation, the binding energy per nucleon starts with low value for deuteron and then increases rapidly.
2. Some nuclei like 4 8 12 16 20
2 4 6 8 10, , , and He Be C O Ne have exceptionally high values as compared with
their neighbors so these nuclei are more stable.
3. As the number of nucleons increases beyond 56, the binding energy slowly begins to decrease due to the electrostatic repulsion between the protons.
13 Atom and Nucleus by Umesh Tyagi Ph. No-9811156956
Importance of B. E. Curve- With the help of binding energy curve, the evolution of energy can be explain in process of nuclear fission and fusion.
Explanation of Nuclear Fission-Two conclusions can be drawn from it, that if we split the heavy nucleus into two medium sized ones, each of the new nuclei will have more binding energy per nucleon than the original nucleus did. The extra energy can be given off. For example if uranium breaks up into two smaller nuclei, binding energy difference per nucleon is about 0.8 MeV. The total energy given off is therefore, (0.8 MeV/Nucleon)(235) = 188 MeV.
Explanation of Nuclear Fusion- In the nuclear fusion two or more light nuclei combine together to form a single nucleus of medium size for which the binding energy per nucleon is more than the lighter nuclei. Thus, the energy is evolved in nuclear fusion.
Radioactivity:
It is the property by virtue of which a heavy nucleus disintegrates itself without the help of any external agent. Radioactivity was found to be independent of the physical conditions like pressure, temperature etc. Therefore, electrons revolving around nucleus were not responsible for radioactivity. The radiations emitted by radioactive substance are of three types which indicate three difference decay modes:
Alpha Decay:
Alpha decay takes placed when size of nucleus is too large and emission of alpha particle reduces the size of nucleus. Mass number reduces by 4 amu and charge by 2 units.
Theory of Alpha Decay: Tunneling Effect-
The α-particles emitted by different radioactive nuclei have kinetic energy ranging from 4 to 9 MeV. The nucleus of a α-emitter poses a barrier of height about 25 MeV. Therefore, classically, the emission of a α-particle cannot be explained.
In 1928, Gamow, Congdon and Gurney explain the emission of α-particle on the basis of quantum theory According to this theory:
1. A α-particle may exist as an entity (already formed) inside a nucleus before it escapes from the nucleus.
2. The α-particle is in a state of constant motion inside the nucleus with a speed of about 107 m/s.
3. Quantum mechanically, an α-particle of even insufficient kinetic energy has a small but finite probability p to cross the potential barrier.
As the size of nucleus is about 10-14m so an α-particle takes about 10-21 s to move across the nucleus. Thus the α-particle presents itself before the potential barrier 1021 times in a second. Therefore the probability P = p x 1021 becomes quite large and the α-particle can tunnel through the potential barrier. Hence α-decay occurs as a result of potential barrier tunneling.
Beta Decay: Beta decay takes place when nucleus has too many neutrons relative to proton. With each β -particle emission, the number of neutrons reduces by one and number of protons
14 Atom and Nucleus by Umesh Tyagi Ph. No-9811156956
increases by 1. In β decay neutron becomes proton and b particle. The proton remains inside the nucleus and b particles are emitted.
epn0
1 + Q
According to this relation, the Q value and thus the kinetic energy of the β particles should be constant. But experimentally it was found that the kinetic energy of emitted beta particles is not constant and it varies from minimum to certain maximum value. Most of the beta particles carry small energy and only few of the particles carry maximum energy called point energy. Moreover, the angular momentum of the reaction is also not conserved.
Thus, we make a change in the phenomenon of β decay. In addition to β particle another particle called antineutrino is also emitted along with it.
eepn
0
1 + Q
This will remove both the defects of earlier assumed reaction. As now the angular momentum of the reaction remains conserved and secondly the Q value is shared between β -particle and anti-neutrino. The kinetic energy of β- particles is not constant but the sum of kinetic energy of β particles and the anti-neutrino is constant.
Gamma Decay:
Gamma decay takes place when nucleus has excess energy and emission of gamma rays reduces the energy of nucleus. Like in an excited atom, an excited nucleus can make transition from higher energy state to the lower energy state with the emission of the particles called g rays.
Most of the radioactive substances after a or b decay leaves their daughter nuclei in the xcited state. The excited nucleus can come back to ground state in one or more than one step with emission of Υ- particles.
Properties of a, β and Υ particles:
Alpha Particles:
1. Alpha particles is a positively charged particle with two protons and two neutrons and is identical with helium nucleus.
2. As alpha particles are charged, they can suffer deflection while passing through electric or magnetic field.
3. They can produce fluorescence in materials like Zinc sulphide.
No
. of
bet
a p
arti
cles
Kinetic Energy
End Point Energy
15 Atom and Nucleus by Umesh Tyagi Ph. No-9811156956
4. Alpha particle ionizes heavily the gas through which they pass. An alpha particle produces up to about 10000 pairs of ions in its way per cm of path.
5. They are scattered while passing through metal sheets.
6. The velocity of alpha particles is characteristic of nucleus which is emitting it and is about 1/20 times the velocity of light.
7. Alpha particles are characterized by the fact that after they have proceeded a certain distance in air they are no longer able to ionize the air. This is expressed by saying that they have a well defined range.
Beta Particles:
1. Beta rays are negatively charged particles and specific charge shows that they consist of fast moving electrons.
2. Their velocity can be 33% to 99% the velocity of light.
3. They have smaller energy than a-particles of same velocity, thus they possess smaller ionizing power.
4. Beta particles are also charged rays therefore, they gets deflected in electric and magnetic field.
5. Their penetrating power is about 100 times that of the alpha particles but still they are absorbed by aluminum foil 0.5 cm thick.
6. They do not show any definite range in air and they follow an irregular path unlike alpha particles which move in straight line.
7. These particles can produce fluorescence in certain substances like barium platino cyanide and zinc sulphide.
Gamma Rays:
1. These are charge less, massless particles; therefore, they are not deflected by electric or magnetic fields.
2. The gamma rays are electromagnetic radiations travelling with the speed of light, but wavelength of g rays photon is less than the wavelength of X rays.
3. g rays have large penetrating power, about 10000 times more than a particles. Therefore, they can pass through several centimeter of iron and lead.
4. When they fall on a substance they are absorbed according to the exponential law i.e. I = I0e-Pt, where I0 is the original intensity of g rays and I is the intensity after they have passed through thickness t and P is the absorption coefficient.
16 Atom and Nucleus by Umesh Tyagi Ph. No-9811156956
5. They can produce fluorescence on substances like Willamette.
6. These rays can knock out electrons from the surface of metal on which they fall.
7. Gamma rays affect the photographic plate more than b particles.
Laws of Radioactive Disintegration:
It was experimentally observed by Rutherford and Soddy that the radioactive disintegration is a spontaneous process and is not dependent on external factors like pressure temperature etc. Also, during disintegrations no two particles are found which are emitted simultaneously. Rutherford and his co-worker Soddy gave the following laws regarding the radioactive decay-
1. When an atom emits alpha particle , then its mass number (a) decreases by 4 and its
atomic number (Z) decreases by 2.
2. When an atom emits a beta particle ( i.e. electron) then its mass number (A) does not change but atomic number increases by 1.
3. There is no change in mass number and atomic number when gamma ray photon is emitted by a nucleus.
4. The rate of disintegration at any time (i.e. number of atoms that disintegrate per second) is directly proportional to the number of radioactive atoms present in the sample at that time. This is knows as decay law.
MATHEMATICAL TREATMENT FOR DECAY LAW-
The rate at which radioactive disintegration of radioactive sample takes place depends only on the number of undecayed nuclei present in it.
If initially at t = 0 the number of undecay nuclei in radioactive sample be N0. At any other time t, this number reduces to N and then rate of disintegration at time t is given as,
Rate of disintegration =
According to decay law, the rate of disintegration is directly proportional to the number of radioactive atoms present in the sample at that time
i.e.
… (1)
17 Atom and Nucleus by Umesh Tyagi Ph. No-9811156956
Where is called as disintegration constant or decay constant. Integrating the above equation,
)2...(log CtN
dtN
dN
e
Where C is constant of integration and can be found using condition at t = 0, N = N0,
log)(
log
loglog
)3...(log
0
0
0
0
antiTakingeNN
tN
N
NtN
CN
t
e
ee
e
Thus, disintegration constant is defined as the reciprocal of time in which the number of undecayed nuclei reduces to 1/e of the initial number of nuclei.
Half Life:
Half life of radioactive sample is the time during which half the initial number of nuclei decays or it is the time during which half of the initial number of nuclei are left undecayed.
Thus, at t = t1/2, N = N0/2
693.0
log2loglog
2
2
2/1
2/1
00
2/1
2/1
t
etTaking
e
eNN
ee
t
t
Units of Radioactivity: S. I. unit of Radioactivity is Becquerel (Bq).
Other units are Curie and Rutherford.
1 Curie = 3.7 x 1010 disintegrations/sec.
1 Rutherford = 106 disintegration/sec
Relation between number of half lives and number of atoms left-
Suppose N0 are the no. of atoms in a sample at time t=0 i.e.
18 Atom and Nucleus by Umesh Tyagi Ph. No-9811156956
Average Life or Mean Life:
Average life or mean life is defined as the total life of all the atoms divided by total number of atoms. Or Reciprocal of decay constant is called mean life.
Let N0 be the number of undecayed nuclei at t = 0 and N be the number of undecayed nuclei at any time t. If dN atoms undergo disintegration in time dt then each of these dN atoms have a life span of about t. Combined age of all dN atoms is tdN.
0
0
N
Total life of all atoms tdN
0
00
( )
NtdN
Average lifeN
Also 0
( )t
dN Ndt N e dt
∴ 0
tte dt
Integrating by parts, 00
1t t
te edt
⟹
1
or 1/2
1/2 1/21.44 0.6931/ T
0.6931
TT
01
0 0 2
11
0 0 2
21
0 0 2
31
0 0 2
time t=0, N=N
time t=T, N=N / 2
time t=2T, N=N / 4
time t=3T, N=N / 8
...........................................................
.............
At N
At N
At N
At N
10 2
10 2
..............................................
time t=nT, N=
Thus the no.of atoms left after 'n' half life are given as
N=
where n=t/T
n
n
At N
N
N0
N0/2
N0/4
N0/8
N
O. O
F A
TOM
S LE
FT (
N)
T 2T 3T
TIME (t)
DECAY CURVE FOR A RADIOACTIVE
ELEMENT
19 Atom and Nucleus by Umesh Tyagi Ph. No-9811156956
Thus average life is always greater than the half life.
ACTIVITY OF a RADIOACTIVE SUBSTANCE- - The number of atom disintegrating per second of a radioactive sample, is known as activity or radioactivity.
Nuclear Fission: The splitting of heavier nucleus into smaller nuclei with the release of large amount of energy is called nuclear fission. It was discovered by Otto Hahn and Strassman. When uranium is bombarded with slow moving neutrons then the slow neutron is absorbed by U-235 and an unstable isotope of U-236 is formed which immediately breaks into barium and krypton with release of 3 neutrons and enormous amount of energy.
235 1 236 141 92 1
92 0 92 56 36 03 200U n U Ba Kr n MeV
Although the fission can result in formation of about 100 isotopes of 20 different elements [not
always barium and krypton], but the energy which is released in the fission is always around
200MeV. Most of the energy appears in the form of the kinetic
energy of the fission fragments and the remaining energy
is given to the neutrons in the form of their kinetic energy.
Chain Reaction in Nuclear Fission: When a single neutron is captured by U-235 then its fission produces three neutrons. These neutrons can split the three more U-235 nuclei, producing still more neutrons which can further cause the fission of a large number of nuclei, and so on Thus a chain reaction is set up.
The neutrons produced in the fission of Uranium nuclei have average energy of the order of 2 MeV per neutron. This energy of neutrons is very large and they will escape the system without carrying out further fission of other uranium –235 nuclei. So slow neurons (thermal energy=0.025eV) are required to sustain the chain reaction.
Requirements for sustained chain reaction:
[a] fast neutrons should be converted into slow neutrons [b] A critical mass of uranium should be taken [c] At least one thermal neutron should be available for carrying out the fission.
0
0
0
Activity
According to decay law,
Therefore we get-
t
t
t
dNA
dt
dNN
dt
A N
As N N e
A N e
A A e
20 Atom and Nucleus by Umesh Tyagi Ph. No-9811156956
Reproduction Factor and Critical Mass- Chain reaction once started will remain steady, increase or decrease depending upon the reproduction factor K. It is defined as the ratio of production of neutrons to the rate of loss of neutrons due to leakage or absorption.
The reproduction factor or multiplication factor (k) gives a measure of the growth rate of the neutrons in a fissionable mass.
If k=1, the chain reaction will be steady and the size of material is called critical
If k >1, explosive reaction takes place. The mass of fuel is called super critical
If K < 1, reaction will stop. The size of the material is called sub critical.
Thus for chain reaction to continue mass should be greater than the critical mass.
Critical Size and Critical Mass- The size of the fissionable material for which reproduction factor k=1
is called reproduction of multiplication factor and its mass is called critical mass.
Nuclear Reactor: A nuclear reactor is a device in which controlled chain reaction is achieved and the large amount of energy is released which can use for producing power.
The main parts of the nuclear reactor are
[a] Fuel: Fuel used in the nuclear reactor is some fissionable material like U-235 or Pu-239. Slow moving neutrons are made to strike the U-235 resulting in fission of nuclei. [In case the fuel is Pu-239 then fast moving neutrons strike the nuclei.]
Moderators: These are the materials used to slow down the fast moving neutrons. They are light nuclei of mass comparable to that of the neutron so that if elastic collision occurs between neutron and moderator nucleus. Thus neutron transfers its energy to the nucleus and its own energy decreases. Heavy water and graphite are generally used as the moderators. The moderators should not absorb the neutron.
Coolant: The large amount of the heat generated in the reactor must be dissipated by circulating coolant through the reactor. The coolant used are water or liquid sodium.
Control Rods: These are used for absorbing the excess neutrons from the nuclear reactor and thus controls the rate wt which the nuclear fission reaction proceeds. Boron or cadmium rods are generally used as the control rods. These materials should have large cross sectional area for neutron absorption.
Shielding: Shielding is done all around the nuclear reactor to prevent the leakage of radiation into the atmosphere. Shielding can be done by either using 0.5m thick wall of lead or 10m thick wall of lead.
21 Atom and Nucleus by Umesh Tyagi Ph. No-9811156956
Nuclear Fusion:
It is the phenomenon of joining together of two smaller nuclei to form larger nuclei with the release of large amount of energy.
The mass of the single nucleus formed is less than the mass of the two smaller nuclei and the difference in mass gets converted into energy.
If 2 deuterium nuclei fuse together to form helium nucleus then energy o around 24MEV will be released in the fusion.
MeVHeHH 244
2
2
1
2
1
The process of fusion means joining together of two similarly charged nuclei to form bigger nuclei, thus large amount of energy is required. This high kinetic energy required by the two small nuclei for fusion is provided by first carrying out fission. The fission will increase the temperature to 107K, thus providing the required energy. These reactions takes place at high temperature thus are also called thermonuclear reactions.