The relationship between cure and performance of single pack moisture cured urethanes
-
Upload
ian-thompson -
Category
Documents
-
view
219 -
download
3
Transcript of The relationship between cure and performance of single pack moisture cured urethanes

The relationship between cure and performance of s ingle pack moisture
cured urethanes lan Thompson, BSc., C.Chem., M.R.S.C., Colin Temple, BSc*
Abstrac t Moisture Cured Urethane (MCU) paint systems are being used increasingly for new construct/on and for maintenance painting. The popularity of these materials is partly due to their weather tolerance which allows them to be used under adverse environmental condit/ons, but also to their very short overcoating time which makes it possible to apply a complete three coat system in a single day.
A number of problems have recently been identified mainly related to intercoat adhesion weakness which, in some cases, has led to coating detachmenL This paper identifies some of the condit/ons which can contribute to adhesion failure in particular the effect of environmental conditions during application and curing, the chemical changes which occur in the paint as it ages in the container and the effect of sunlight as the material cures. Where possible, the paper indicates precautions which should be taken to reduce the incidence of adhesion problems.
Introduction For many years the paint industry has been aware of the need to provide 'all weather' paint systems which can be applied under adverse weather conditions (low temperature and high humidity) and which will provide good corrosion resistance and long term decorative properties. This type of system theoretically opens up the 'weather window' for painting and thus allows winter painting programmes to be carded out.
Moisture cured urethane (MCU) paints appear to fulfil many of the requirements of an 'all weather' paint system. 1,2-~ According to suppliers literature materials can be applied at temperatures below 5°C and are tolerant to humidities above 90%, conditions which do not favour the application of traditional paint systems.
MCU paints are single pack materials and therefore offer certain advantages over two pack systems such as epoxies and polyurethanes which need accurate metering of the two components and thorough mixing prior to application. The materials are overcoatable after 2- 3 hours, making it possible to apply a complete three coat system in a single day thus reducing scaffolding costs and downtime.
Because of their potential for fast curing and rapid overcoating, many users have applied MCU paints but a lack of appreciation of the conditions which affect their behaviour has lead to a number of problems on site, particularly intercoat adhesion weakness. The purpose of this paper is to describe the work carried out at the British Gas Engineering Research Station to understand the effects of environmental factors on film cure and the related coating performance.
• British Gas Engineering Remmrch Station, Hanmycombe, KiUingworth, Newcutle-Upon-Tyne NE99 1LE.
Chemistry of moisture cured urethanes MCU paints are supplied as primers pigmented with aluminium, zinc or red oxide, as micaceous iron oxide (MIO) buildcoats, as highbuild undercoats and as eggshell or full gloss finishes. Primers and buildcoats are formulated using aromatic poly.isocyanates, ie toluene-di.isocyanate (TDI) or diphenylmethane-4.4 di- isocyanate (MDI); these are cheaper than aliphatic isocyanates but are more susceptible to chalking and discoiouration in sunlight. Where a highly decorative finish is required, the more expensive aliphatic iso- cyanate, hexamethylene di.isocyanate (HMDI) is pre- ferred because of its improved resistance to chalking and discolouration in sunlighL
TDI, MDI and HMDI are pre.polymerised with water or various polyois to give a resinous polymer containing approximately 10% of isocyanate functional groups. Pre.polymerisation of the isocyanate monomer reduces its vapour pressure (making it safer to use) and accelerates the rate of film formation when exposed to moisture. Aromatic isocyanates react faster than aliphatic isocyanates because of the inductive effect of the aryl group.
Acceleration of the reaction between the aliphatic isocyanate and water is effected by the addition of a small quantity of a catalyst such as di-butyl tin di-laurate.
The mechanism of curing relies on the presence of atmospheric moisture to react with the isocyanate groups according to the following equations:-
i) R.N.=C=O + H20 - [RNHCOOH] - R.NH 2 + CO 2 isocyanate water intermediate amine carbon dioxide
ii)R.N.=C=O + R.NH 2 - R.NHCONHR isocyanate amine substituted
Lir~a
CONSTRUCTION & BUILDING MATERIALS Vol. 4 No. 3 SEPTEMBER 1990 0950 - 0618/90/030119 - 08 © 1990 Butterworth-Heinemann Lid 119

One molecule of isocyanate reacts with one molecule of water giving a primary amine and liberating carbon dioxide gas as a by-product. The amine then goes on to react with a further molecule of isocyanate to allow the urethane reaction to go to completion.
P r o b l e m s exper ienced on s i te with moisture cured urethanes Intercoat adhesion MCU systems applied to British Gas structures at a number of locations have shown occasional instances of intercoat adhesion weaknesses. The system consists of the following:-
i) Primer (zinc or aluminium) i i) Micaceous Iron Oxide (MIO) buildcoat. i i i ) Gloss finish.
The problem generally occurs between the MIO buildcoat and finish and also between finish coats.
The first incidence of adhesion weakness was observed on a structure that was undergoing routine maintenance painting. In this particular case the finishcoat could be peeled away from the buildcoat simply by pressing a strip of adhesive tape onto its surface and removing it with a snap action.
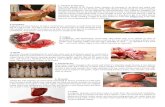
Examination of a second structure, where the MCU
system had been ageing for 18 months, revealed several areas where actual coating detachment had taken place (Fig 1). In other areas, where the coating was not already detached, it could be peeled simply by lifting the edge of the tip of a knife blade. This was assumed to be representative of the 'as applied' coating condition. Although actual breakdown (detachment) was minimal, poor adhesion extended over approximately 25% of the area examined. Whilst it was not possisble at the time to explain why some areas and not others displayed poor adhesion, subsequent work (to be discussed later) has indicated possible reasons.
F a c t o r s w h i c h i n f l u e n c e t h e m o i s t u r e c u r i n g p r o c e s s The amount of moisture in the air affects the rate of reaction of MCU paints, 4 and is a function of temper- ature and relative humidity.
As the air temperature rises the amount of moisture required to saturate it increases. The mass of water vapour in a specific volume of air is termed the absolute humidity. The effect of temperature on absolute humidity is shown in Fig 2, Figs 3 and 4 show typical seasonal variations for 1988, indicating a low of 3 g/m 3 in March and a high of 18.5 g/m s in July. Monthly
Fig I Intercoat adhesion failure
50 45 40
E ~, 35
o ;, ,'2 ,; 2; Ternperoture (° C)
×
/
X
I
The effect of temperature on absolute humidity at saturation Fig 4
25.0 22.5 20.0 17.5
~ 15.0
~ 12.5 I0.0 I .
{ 7.51 5.0 2.51
0 0
, i i i I I I I
75 150 225 500 375 450 525 600 Time (hours)
Fig 3 Water concentration in air - March 1988
25.0 ~-'22.5 E20.O
=" 17.5 ~5.0
§ 12.5 ~, I0.0
, o -
o 7.5 5.0 2.5
I I i I I i l I
0 75 150 225 500 1575 450 525 600 Time (hours)
Water concentration in air - July 1988
120 CONSTRUCTION & BUILDING MATERIALS Vol. 4 No. 3 SEPTEMBER 1990

averages for 1988 range from 6.5 to 12.5 g/m s res- pectively measured at a particular location.
It is assumed by MCU suppliers that there will be sufficient water in the air, under all atmospheric conditions, to cure MCU paints. Although a valid assumption under the conditions which prevail in the United Kingdom, it takes no account of the kinetics of the system. It would apear from the results shown in Figs 3 and 4, that a MCU applied in March will not cure as rapidly as one applied in July. It is important, therefore, to understand how environmental factors such as absolute humidity and temperature influence cure and hence material performance.
Deve lopment of a method for measur ing the degree of cure of M C ( / p a i n t s Methods considered Chemical cure can be monitored by determining some physical property of the film or by following changes in its composition. The latter approach was considered to be most appropriate and two analytical techniques were initially considered viz Differential Scanning Calorimetry (DSC) and Infra-red spectroscopy (IR).
Although DSC has been successfully used to monitor the cure of epoxy powder coatings (by following t h e change in the glass transition temperature), a similar approach to MCU coatings proved to be unsuccessful. It was decided, therefore, to concentrate on IR spectro. scopy. Various functional groups within molecules, such as -OH and CHs, absorb infra-red radiation at charac- teristic frequencies, the level of absorbtion depending upon their concentration. The isocyanate (NCO) group absorbs IR at a frequency of 2265 cm -I in a region where few other groups absorb, which makes it relatively easy to monitor. As a MCU film cures the number of unreacted isocyanate groups decreases, hence by
following this decrease with time (Fig 5) the extent of cure can be determined.
Experimental data Conventional IR spectroscopy, which requires that the material transmits IR, was used to carry out preliminary work on clear, unpigmented pre-polymers. Films were applied directly onto sodium chloride plates and spectra obtained throughout the period of cure.
According to the Beer-Lambert Law, concentration can be related to absorbance by the equation:
A = E c l where: -
A = Absorbance E = Extinction coefficient c = Concentration 1 = Path length
During curing of a MCU film, the extinction co- efficient and film thickness remain constant and hence absorbance is proportional to concentration. It is possible, therefore, to relate the intensity of the isocyanate peak to the degree of cure. The relationship between peak height and area under the peak has been assumed to be constant, allowing peak heights to be measured rather than peak areas.
Absorbance values can be converted into estimates of percentage cure by assuming that the initial con- centration of isocyanate groups within the MCU film represents 0% cure and that the isocyanates concen- tration will fall to zero at 100% cure. In practice the MCU pre-polymer may partially cure during manufacture and storage, varying the starting concentration of NCO groups. However, because the aim of this work is to show the effect of certain parameters on the rate of cure,
0
Isocyonote concentrotion offer curing
o~
==
0
Isocyo note concentrotion before curing
r I
,oooL 4000
I I I I I
3500 5000 2500 2000 1500 Frequency (cm - I )
Fig 5 Sirnpl~ed IR Spectrum of moisture cured urethane
I
1 0 0 0
CONSTRUCTION & BUILDING MATERIALS Vol. 4 No. 3 SEPTEMBER 1990 121

absolute values of percentage cure are unnecessary. The results obtained using unpigmented films
demonstrated the potential of IR spectroscopy for monitoring cure. However, as pigmented MCU paints do not.transmit IR it was necessary to develop a reflectance method based on a technique known as Attenuated Total Reflectance (ATR) ~ which allows IR spectra to be obtained from opaque materials such as paint films.
To obtain an ATR spectrum, samples are placed in contact with a crystal made from a high refractive index material such as KRS5 (a mixture of thallium iodide and bromide). IR radiation is directed onto the crystal and is made to move along the crystal length by a series of twelve internal reflections (Fig 6). At each reflection the IR beam penetrates slightly beyond the crystal, ie into the sample before returning to the crystal. During this process the beam loses energy at particular wavelengths corresponding to the absorption bands of the sample. If the wavelength and intensity of the attenuated beam are plotted, then the absorption spectrum of the sample is obtained.
Preliminary ATR determinations on paint films mechanically held to the surface of the crystal gave poor results. Much improved results were obtained, when the coatings were applied directly to the crystal. After appli- cation, the crystal was conditioned at a particular temperature and humidity using an environmental cabinet and spectra were obtained at intervals throughout the period of cure. Internal referencing was used to correct for variations in instrument response, by competing the isocyanate peak height with the height of a peak which remained constant throughout the test eg the methylene group absorption at 2950 cm -] . Because MCC! resins contain an aliphatic solvent which absorbs in this region, it was necessary to force dry films prior to testing. Concern that the drying process might be advancing cure resulted in this correction procedure being discontinued. Duplicate determinations without internal referencing have proven to be repeatable to within 2%.
IR Spectroscopy has been used to study the influence of the following factors on MCU cure:
i) Absolute humidity (at constant temperature) ii) Temperature (at constant absolute humidity) iii) Curing time.
The effect of absolute humidity, temperature and t ime on the cure of MCU f i lms Effect o f absolute humidity The vastly differing water concentrations experienced during winter and summer will inevitably influence the reaction of MCU paints. The effect of these two extremes of absolute humidity on the cure rate of a typical finish coat are shown in Fig 7.
Fig 8 shows the effect of increasing absolute humidity (at 20°C) on the cure attained by an MIO buildcoat and a finish coat after 4 hours ageing. Increasing humidity advances the film cure, by 1% and 3% for build and finish coats respectively, for each 1 g/m 3 rise in absolute humidity.
MIO buiidcoats are less affected by increasing humidity than finish coats, possibly due to : -
i) The increased reaction rate of the catalysed
Emergent beam Sample beam
/' ~" Sample film KRS 5 crystal
Fig 6 Diagramatic representation of attenuated total reflectance
8 5o
90 /x 13.8 om water/m 3 3
80
70
60
~, 5o
2O
IO
0 I I I i I i 0 30 60 90 120 I50 180
Time (hours)
Fig 7 Effect of water content in air on cure rate
I O0 I
90!
8O
7 0
6ol
40!
ol 0
F g8
+ Top coat
x Build coat
j + /
2 4 6 8 I0 I 14 I 18 20 Water content (g/m 3 )
Effect of absolute humidity on the cure rate of a MCU finish and buildcoat at constant temperature (20 ° C)
aliphatic isocyanate in the finish coat. ii) The greater coating thickness of the buiidcoat
compared with the finish coat (125 microns compared with 40 microns) which, may restrict the permeation of moisture through the film.
iii) The barrier effect imposed by the iamellar MIO pigment making the passage of moisture through the coating more tortuous.
122 CONSTRUCTION & BUILDING MATERIALS Vol. 4 No. 3 SEPTEMBER 1990

Preliminary work on unpigmented, aromatic and catalysed aliphatic MCU films has shown the increased reactivity of the latter (Fig 9). Coating thickness within the range 40 - 150 microns does not appear to influence the cure rate of MIO buildcoats (see Fig 10), tending to disprove the thickness and barrier effects suggested in (ii) and (iii)~
Effect of temperature Temperature will fluctuate throughout the year and may, therefore, influence reaction rate.
Fig ] 1 shows the effect of temperature (at a constant absolute humidity of 10g/m 3) on the cure level achieved after 24 hours by a typical finish coat. The graph shows a general upward trend as temperature rises; however, it appears to be much less influential than water concentration in advancing cure.
The MIO buildcoat shows a reverse trend with cure decreasing as the temperature rises from 10°C to 30°C. One possible explanation for this behaviour may be the effect of surface curing/skinning of the film as the temperature rises which reduces moisture permeation through the coating, thus inhibiting the cure of the underside of the film (where cure measurements are made).
Effect of time Cure curves generated from IR data indicate that certain atmospheric conditions retard the curing process. The consequence being that films may not reach a very advanced state of cure before being overcoated, particularly if the manufacturers minimum recom. mended overcoating times have been followed, eg 2 - 3 hours. The effects of premature overcoating are twofold:-
i) Once a film has been overcoated, moisture may be prevented from entering it thus retarding its cure.
ii) If moisture is allowed to pass through the film and advance the cure, it may result in the generation of carbon dioxide gas and subsequent film blistering.
IR work (Fig 12) carried out on primer and buiidcoats overcoatecl in the early stages of cure, indicates firstly that moisture will pass through the film and allow the reaction to approach completion, and secondly that the carbon dioxide gas which is generated does not appear to present a problem, indicated by the consolidated nature of the film shown in Figure 13.
investigation into intercoat adhesion failures Effect of absolute humidity on overcoating time Under favourable reaction conditions, such as high temperature and humidity, coatings may cure to such an advanced state that intercoat adhesion problems may develop due to the highly crosslinked nature of the overcoated film and result in:-
i) A reduction in the available number of sites for reaction to take place between coats.
ii) The film developing greater resistance to attack by solvents contained in the overcoating system.
I00
8 0
6O
u
4O
2 0
~ 9
f
/y + Aliphotic
z~ Aromotic
I I I [ I
Time ( hours )
Comparison of the cure rates of unpigmented aliphatic and aromatic isocyanates
o
I 0 0
9 0
8 0
7O
6O
5 0
4 O
0 I I I I I
30 60 90 120 150 180 Cooting thickness (microns)
Fig I0 Effect of fllm thickness on the cure of an MIO buildcoat
I00
90
80
7 0 x
6 0 -
5O
4O
3 0
2O
I0
0 I0
_ _ x / x
I I I 15 20 25 30
Temperature (=C)
Fig 11 Effect of temperature on the cure rate of a MCU finish at constant absolute humidity (10g water/m s )
Fig 14 shows the effect of increasing absolute humidity on the critical overcoating time (COT) of a typical finish coat. The COT/failure time is the time within which films must be overcoated to avoid intercoat adhesion failure.
Although adhesion failures were experienced in laboratory tests, when finish coats were applied to
CONSTRUCTION & BUILDING MATERIALS Vol. 4 No. 3 SEPTEMBER 1990 123

IO0 .E 90 ~ 8o
0 ~ 60 0 ~ 50 ~ 40 ~ 20 !
x 0vercoated build u 0vercooted primer
J I J J L J ~ J J - -
I0 20 30 40 50 60 70 80 90 I00 Ti me after overcoating (hours)
Fig 12 Ability of partially cured build and primer coats to continue curing once overcoated
i 7
5 Q
4 \
2
I
! 0 l0
I I
20 30 40 Water content (g/m 5)
Fig 14 Effect of water content in air on overcoatabt~ty
I h
I & __qNl== ~ I
Finish
MI0 builclcoot
Z inc p r imer
Steel
Fig 13 Micrograph
J (x200) showing consolidated nature of HCU fllm
buildcoats, these failures only occurred under the most extreme absolute humidity, and after long overcoating periods. It would appear, therefore, that the intercoat adhesion problems experienced between build and finish coats on site may not be entirely due to absolute humidity and temperature effects.
No failures were experienced between primer and buildcoats under the environmental conditions con- sidered in this paper.
in the course of evaluating systems based on MCU paints it became apparent that there were inconsisten. cies in COTs recorded on different batches of finish received at different times. Two possible explanations were suggested:-
i) That the amount of accelerator added to the aliphatic isocyanate may have varied from batch to batch and
ii) that the material may be changing in the container during storage.
Effect of accelerator level Fig 15 shows the effect of increasing accelerator concentration on the cure rate of an unpigmented aliphatic isocyanate. As the concentration of accelerator increases the material cure rate increases.
The addition Of extra accelerator to a number of proprietary aliphatic finishes produces a significant decrease in their COT and may account for the batch to batch variation mentioned above.
Effect of chemical/physical changes which occur during storage and application Storage stability is known to be a problem with MCU systems, particularly finishes, which contain pigments with higher levels of retained water than those generally used to pigment primers and MIO buildcoats. The generation of carbon dioxide during storage (indicated by a build-up of pressure and distortion of the container) and the fact that materials often gel in unopened
124 CONSTRUCTION & BUILDING MATERIALS Vol. 4 No. 3 SEPTEMBER 1990

IOO
90
80
70
® 60
c~ "50
40
30
20
IO
O
+ O.00% Accelerator D 0 .05% Accelerator o O. IO% Accelerotor
o
o E3
0 I 2 3 4 5 Time (hours)
Fig 15 Effect of accelerator concentration on the cure o f an aliphatic isocyanate pre.polymer
containers, also confirm the potential instability of these materials.
When a MCU paint is exposed to water which may be present in the sealed container during storage, and to atmospheric moisture once the container is opened for application purposes, it can undergo a number of changes: -
i) It becomes more viscous and consequently has poorer surface wetting properties.
ii) Its molecular weight increases and the number of reactive species available for chemical crosslinking decrease.
It may be reasonable to assume, therefore, that the chemical/physical nature of the material may be changing during the process of application. This phen- omenon may explain why some areas, but not others display poor intercoat adhesion on site.
In order to investigate the ageing process small quantities of material were continuously stirred under laboratory conditions, using a rotational viscometer, at a rate just sufficient to keep the surface of the material turning over and in contact with moisture in the air. This test was intended to simulate conditions which might exist in the can during brush application. At regular intervals, viscosity readings were taken and samples were moved from the container to overcoat films which had previously been prepared from unaged material. Fig 16 shows how viscosity increases with time for a typical gloss finish, and the viscosity and time when intercoat adhesion failures start to occur. Viscosity rise is expected to take place more rapidly as the absolute humidity increases.
As with absolute humidity, the effects of viscosity on intercoat adhesion were found to be most detrimental on finish coats, failure occurring with some suppliers materials when the viscosity of the overcoating material approached 8-9 poise.
The precise conditions responsible for adhesion problems (on site) between buildcoats and finishes are complex and involve combinations of several environ. mental conditions.
Most adhesion problems on site have occurred on large horizontal surfaces which have been subjected to
direct sunlight for a substantial part of the day. Aromatic isocyanates, on which buildcoats are formulated, are known to deteriorate in sunlight over long periods of time. Recent work, however, has shown that certain buildcoats deteriorate over much shorter times, eg 6-24 hours. The work involved placing coated panels, back to back, on a south facing natural weathering rack for various periods prior to overcoating. Peel adhesion tests resulted in failures only on those panels which had been subjected to direct sunlight. Failures appear to take place from an interference layer formed on the paint surface during exposure to sunlight. If this layer is removed by overcoating and then stripping the overcoated film from the panel subsequent coats exhibited excellent adhesion. Supporting laboratory work using selective (.IV wavelengths has been used to provide more under- standing of material behaviour under UV/sunlight conditions. However, this work is at an early stage and will not be discussed further.
General d i scuss ion A number of factors have been found to influence the rate of cure of MCU paints and their susceptibility to intercoat adhesion weaknesses:
Environmental conditions High absolute humidities accelerate the cure of MCU paints and increase their susceptibility to intercoat adhesion weakness. This is less probable if films are overcoated as soon as is practicable after application. lntercoat adhesion problems are most prevalent when high gloss finish coats are applied over one another.
Material condition As materials age their viscosity rises and their surface wetting properties deteriorate. Overcoating aged materials or using aged materials to overcoat, may lead to intercoat adhesion problems. It may be possible to avoid failures of this type by using fresh material of a specified viscosity, by discarding material which has gassed during storage, by disposing of partially depleted containers and by preventing material standing in open tins for long periods of time eg 2 - 3 hours prior to, and during breaks in application.
UV effects Materials based on aromatic pre-polymers deteriorate
18
16
14
12
I0
8
6 ~
4
2
0 0
Fig 16
Point at which / odhesion problems J develop X ~ . x /
~ _ ~ , < I ~ ' ~ "
I I I I ! 20 40 60 80 100 120
Time (mins)
Viscosity - time curve for a typical finish coat
CONSTRUCTION & BUILDING MATERIALS VoL 4 No. 3 SEPTEMBER 1990 125

in sunlight producing a surface layer which contributes to poor intercoat adhesion. Application of MCLI paints to large horizontal surfaces which are exposed for prolonged periods to direct sunlight, prior to over- coating, may present problems of this type.
This paper has identified three conditions which contribute to poor intercoat adhesion in/~C(.I systems, all of which can interact in practice. It has not been possible, within the timescale of this programme to combine all three effects; further work on this aspect of MCLI performance is continuing.
Concluding remarks Earlier references to MCU paints as 'All Weather Coatings' may not be as appropriate as first thought. The problems experienced within the gas industry and discussed in this paper have occurred under weather conditions which would normally be considered as ideal for painting. It would appear, therefore, that these systems lend themselves better to application under adverse winter conditions, eg low temperature/high humidity, than those conditions which prevail in summer.
MCLI paints are still considered to offer many advantages in particular situations. It is important,
however, to understand their limitations in order to avoid the type of problems discussed above. It may be that changes in formulation will enable materials to be developed which overcome these problems.
Acknowledgement The authors are pleased to acknowledge the help of various colleagues for their contribution to these studies. We are indebted to British, Gas plc for permission to publish this paper.
References 1 Jolly, A C, Painting in Inclement Weather Conditions. (./K
Corrosion '87, 8th International Conference, I Corr, S.T./ N.A.CE., Brighton
2 Sonntag, M. Moisture Curing One.Pack. Polyurethanes, Polymer, Pa/nt, Colour J. 172, Ho.408L
3 Gray, D, Thompson, !. Paint - Towards Improving Engineering Standards, Communication 1402, 126th Spring Conference of the 1GE.
4 van der Van, L G F, van Dijk, J H and de Vrlu, O T. The Curing of Polyurethane Coatings; Proc. XVIII Ch fat~oec Congress, Venice, 1989, Vol 2/A.
.5 Internal Reflection Spectroscopy, Vol 1, Willcs Scientific Corporation.
This paper was presented at the OK Corr~on '89 Con/'erence, B/ackpoot, Houember 1989.
f
il
i l i l \
S
New - bringing brick arch construction up to date
Brickwork Arch Detailing IBSTOCK • Fills an urgent need for brick constructional details not otherwise available • Describes the setting out, design, detailing and construction of minor span brick arches • Offers fully worked and calculated examples • Provides a technical up-date covering the integration of the arch into modern constructional
methods • Provides answers to the most commonly asked questions 1990 124 pages Softcooer 0 408 50047 6 £15.95 If you would Like further details complete the coupon below: . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Return to: Geraldine Hills, Butterworth Architecture, PO Box 63, Westbury House, Bury Street, Guildford, Surrey GU2 5BH, UK [] Please send me your Architecture leaflet [ ] Please send me your Construction/Civil Engineering/Building leaflet Name: Address:
Postcode:
For details of other Butterworth Arcllitecture titles, please contaCt the appropriate office. Orders should be sent to the appropriate office listed below, The UK headquarters serves all UK and overseas markets except where there is a local Buttarworth Architecture Office.
United Kingdom Buttefworth Architecture PO Box 63, Westbury House, Bury Street, Guildford. Surrey GU2 5BH, UK Australia & Papua New Guinea ButtenNorths Pry Ltd, PO Box 345 North Ryde, New South Wales 2113 Australia
Cu=tomers In ~ may orcllmr through: Butterworth & Co (Asia) Pte Ltd 30 Robinson Road. Unit 12-01 Tuan Sing Towers Singapore 0t 04, Republic of Singapore
New Zealand USA & Canada ButtenNOrths of New Zealand Ltd Butterworth Publishers 33-35 Cumberland Place 80 Montvala Avenue Wellington 1, New Zealand Stoneham, MA 02180, USA
126 CONSTRUCTION & BUILDING MATERIALS Vol. 4 No. 3 SEPTEMBER 1990