The Refined Electrolyte-NRTL Model applied to CO2...
Transcript of The Refined Electrolyte-NRTL Model applied to CO2...

1
The Refined Electrolyte-NRTL Model applied to CO2-H2O-alkanolamine systems
- Equilibrium model predictions- Implementation into the CO2SIM simulator.
Erik Troøien Hessen, Finn Andrew Tobiesen*, Mehdi Karimi, Xiao Luo, Tore Haug-Warberg and Hallvard F. Svendsen
Norwegian University of Science and Technology (NTNU)Department of Chemical Engineering
*SINTEF Materials and Chemistry
Trondheim, Norway
Erik Troøien Hessen, TCCS-9

2
Outline
• Equilibrium problem statement
• The refined electrolyte-NRTL model– Theory– Predictions of partial pressures, speciation and heat of absorption
• Implementation into CO2SIM
Erik Troøien Hessen

3
Equilibrium: Problem statement
Vapour – liquid equilibria (VLE):
Chemical equilibria (liquid phase):
Erik Troøien Hessen

4
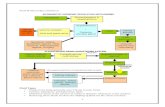
Model structure
General model structure:
Erik Troøien Hessen
Helmholtz energy formulation (EOS) :A = ASR + ALR
e.g.A = Aclassical eos + ADH/MSA + (ABorn)
Excess Gibbs energy formulation (γ)GE = GE,SR + GE,LR
e.g.GE = GE,NRTL + GE,DH/MSA + (GE,Born)
The latter structure is adopted in the e-NRTL framework
SRShort range interactions
• GE: E-NRTL, UNIQUAC etc. • EOS: cubic, CPA etc.• Parameter estimation
LRLong range interactions
• Coulombic interactions• Stronger interactions
• Great deviations from ideality• Debye-Hückel/MSA term
• Consistency issues• Weak theoretical basis

5
Model requirements (rigorous models)
1. Thermodynamic consistency2. Must be able to deal with mixed solvents and molecular solutes3. Reasonable computational effort4. Reasonable number of parameters to be estimated5. Versatility (easily applied to different systems) 6. Accurate predictions for variable loadings and process
conditions1. Partial pressure of CO2 and amine2. Speciation (distribution of species)3. Heat of absorption (important for energy consumption in stripper)
Erik Troøien Hessen

6
Models found in literature
EOS based models (φ -φ)
Few models found – weaker, but growing basis.
• Fürst - Renon (Cubic EOS + MSA)
• CPA models (cubic + association)
• Versatile models (+)• Complex models (-)• Mixing rules (-)• Less experience and know-how (-)• Many parameters to be estimated (-)
GE models (γ-φ)
• E-NRTL, Pitzer, UNIFAC, UNIQUAC, etc.• More complex (-)• Often many parameters (-)• Better predictability (+)• Parameter databases (+)• Much experience and know-how (+)• Especially e-NRTL is much used
Most work found in literature is done in this field.
Numerous models found in literature. Most are GE based.
Erik Troøien Hessen
Non-rigorous (empiric) models• Kent-Eisenberg, (Desmukh-Mather)• Simple structure (+)• Few parameters (+)• Limited usefulness (-)
Not speciation, γH2O, γamine and ΔHabs• May be suited for early-phase studies

7
The Electrolyte-NRTL model1
• Maybe the most used (rigorous) model for industrial electrolyte systems
• Simplifying assumption made in differentiation to yield the activity coefficients
• Inconsistent for multi-ionic solutions,– Multi-ionic: Multiple cations and/or anions
• Recent paper by Bollas et al.2 presented a corrected e-NRTL model The refined electrolyte NRTL model
Erik Troøien Hessen
[2] G.M. Bollas, C.-C Chen and P.I. Barton, AIChE J.,54 (2008) 1608.
lnE
ii
GRTn
g ¶=¶lnE
i ii
G RT n g"
¹ å
[1] C.-C. Chen and L. B. Evans, AIChE J., 32 (1986) 444

8
Refined vs. unrefined e-NRTL
Erik Troøien Hessen
Refined e-NRTL:- Almost no applications so far- No parameters in literature- Complex model equations- (Complex parameter definitions)
+ Consistent
Original e-NRTL:- Inconsistent- (Complex parameter definitions)
+ Less complex model equations+ Parameters in literature+ Implemented in Aspen Plus

9
Equilibrium model development
Erik Troøien Hessen
• Stand-alone equilibrium model is developed
• Solves phase and chemical equilibrium• Speciation, partial pressures, heat of absorption
• Chemical equilibrium solved by using non-stoichiometric Gibbs energy minimization routine
• SRK or Peng-Robinson EOS for vapour phase.
• Pitzer-Debye-Hückel and Born term for long range forces
• Refined electrolyte-NRTL model implemented in the RGrad language• RGrad is a tailor made language that performs analytical differentiations3
• Verified through implementation in FORTRAN and comparison with Bollas et al.
• Electrolyte interaction parameters fitted to PCO2.• H2O-NaCl-KCl system 4 interaction parameters (τsalt,salt= 0)• H2O-MEA-CO2 system 56 parameters!• Major drawback of the e-NRTL framework
[3] B.T. Løvfall, PhD. thesis, NTNU (2008)

10Model predictions
Partial pressure of CO2 , 30wt% MEA
Electrolyte interaction parameters fitted to PCO2Molecule-Molecule interaction parameters may be retainedfrom original e-NRTL model

11
Heat of absorption and speciation, 30wt% MEA
• Minimization routine• Good predictions for major species• Problems with predicting free CO2
• Over-prediction• Revise carbamate constant• Calculated rigorously:
( )
2 2
0 .
2
,
. 2
,
ln
ln ln ln
E phys absabs i i ii
Ei
P n
Poynt ingCO COphy s abs
P n
H h h n h
h RTT
Hh RT
T T T
γ
ϕ∞
= + Δ +
∂⎛ ⎞= ⎜ ⎟∂⎝ ⎠⎛ ⎞∂ ∂ ∂ Φ= − +⎜ ⎟⎜ ⎟∂ ∂ ∂⎝ ⎠
∑

12
Evaluation of activity coefficients
• When fitted to PCO2, activity coefficients may not represent the reality• Subject to further study
• Activity coefficients for CO2 calculated from N2O solubility measurements and the N2O analogy4
[4] A. Hartono, PhD. thesis, NTNU (2009)
2 2 2
2
2 2 2
;CO N O COCO
CO N O CO
H H HH H H
γ∞ ∞ ∞= =

13
The CO2SIM simulator• Developed for simulating novel solvent systems• Possibility of building complex flowsheets for performance studiestesting and design (used for studying alternative process configurations)
• Stable solver numerical methods• Fast convergence• Graphical user interface
• The refined e-NRTL model is implemented in CO2SIM Converted to FORTRAN
• Simulations based on pilot plant runs are performed• Only absorber is studied here
Erik Troøien Hessen

The CO2SIM simulator: GUI

15
Simulation results
Erik Troøien Hessen
Example: Simulations based on NTNU/SINTEF pilot runs
Aspen simulations performed using ratebasedRadfrac in Aspen Plus v7.1
Temperature profiles (16032005-2)
0
0.5
1
1.5
2
2.5
3
3.5
4
4.5
5
59 61 63 65 67 69 71 73
T [GRC]H
eigt
h [m
]
T VAP. CO2SIM (R-ENRTL)T VAP. EXPT ASPENT VAP CO2SIM (SIMPLE)T LIQ. CO2SIM (R-ENRTL)T LIQ. CO2SIM SIMPLE
Temperature profiles (16032005-1)
0
0.5
1
1.5
2
2.5
3
3.5
4
4.5
5
48 49 50 51 52 53 54 55 56 57 58
T [GRC]
Hei
gth
[m]
T VAP. CO2SIM (R-ENRTL)T VAP. EXPT ASPENT VAP CO2SIM (SIMPLE)T LIQ. CO2SIM (R-ENRTL)T LIQ CO2SIM (SIMPLE)

16
Simulation results
Erik Troøien Hessen
Example: Simulations based on NTNU/SINTEF pilot runs
Equilibrium model will be continuously improved in order to get better predictions

17
Conclusions and further work
• Refined e-NRTL implemented in both RGRAD and FORTRAN• Good overall predictions• Problem with predicting free CO2• Work to be done on physical activity coefficients and parameterestimation
• Compare with other thermodynamic models (e.g. UNIQUAC, EOS mod.)
• CO2SIM implementation• Refined e-NRTL is implemented in CO2SIM• Results demonstrate need for rigorous thermodynamic calculations• Pilot rig at NTNU/SINTEF is simulated.• Analysis will be extended to the whole pilot, not just absorber, and to other pilot
results
Erik Troøien Hessen

18
Thank you for your attention.