The influence of medium composition on the maintenance of cytochrome P-450, glutathione content and...
-
Upload
patricia-watts -
Category
Documents
-
view
212 -
download
0
Transcript of The influence of medium composition on the maintenance of cytochrome P-450, glutathione content and...

Journal of Hepatology 1995; 231 605-612 Printed in Denmark AN rights reserved
Copyright 0 Journal of Heporology I995
Journal of Hepatology
ISSN 0168-8278
The influence of medium composition on the maintenance of cytqchrome P-450, glutathione content and urea synthesis: a comparison of rat and
sheep primary hepatocyte cultures
Patricia Watts’, Mark D. Smith’, Ian Edwards*, Victor Zammit3, Vivienne Brown* and Helen Grant’
‘Bioengineering Unit, University of Strathclyde, Wolfson Centre, Glasgow, ‘Biotechnology Department, Bldg 353, HarweN Laboratories, Oxford, and 3Hannah Research Institute, Ayr, UK
Rat and sheep primary hepatocytes have been cul- tured in four different medium formulations: Willi- ams’ E, Cheek, Medium 199 and Mod&d Earle’s. The total cytochrome P450 content, intracellular con- centration of reduced glutathione, rate of urea syn- thesis and total protein content of cultures of cells from both species in each medium have been deter- mined. Modified Earle’s and Chee’s medium proved to be the most favourable formulations for the culture of rat hepatocytes. After 48 h, cells cultured in Modified Earle’s had significantly more cytochrome P450 and a significantly greater rate of urea synthesis than cells in any other medium. After 6 days in culture the dif- ference in cytochrome P450 levels between rat hepato- cytes in Chee’s medium and those in Mod&d Earle’s medium was abrogated. The difference in the rate of urea synthesis between rat hepatocytes cultured in each of these two media was shown to be more de-
T” USE of hepatocytes in vitro to provide detoxi-
fication in an artificial biological liver support system depends on the maintenance of hepatocyte function in culture. However, in common with most differentiated mammalian cells, the function of liver cells deteriorates in culture. De-differentiation in pri- mary cultures of liver cells occurs particularly rapidly, with many key aspects of function being lost within 48 h (1,2). To provide effective liver support for patients in acute liver failure, it will be necessary to optimise culture conditions that prolong retention of specific liver functions in the hepatocytes.
One of the first considerations is which culture me-
Received I December 1994; revised 24 February: accepted 5 April 1995
Correspondence: Patricia Watts, Bioengineering Unit, University of Strathclyde, Wolfson Centre, 106 Rottenrow, Glasgow G4 ONW, UK.
pendent on the medium in which the cells were main- tained during the period of urea synthesis measure- ment than on the medium in which the cells had been previously cultured. Sheep hepatocytes cultured in Chee’s medium ruptured and died within 24 h. Apart from this, sheep cells were less sensitive to changes in medium formulation than were rat hepatocytes. The initial plating efficiency was lower in sheep cells. Total cytochrome P450 content was the most discriminat- ory of the four parameters for evaluating the status of rat hepatocyte cultures. However, urea synthesis may be the most useful parameter for assessment of he- patocyte function in hybrid liver devices such as bioar- tificial liver support systems where access to the cells during operation of the device is restricted.
Key words: Culture medium; Liver cell culture; Liver function; Species differences. 0 Journal of Hepatology.
dium to choose, and there are numerous options avail- able as described in the catalogues of companies such as Gibco BRL and Flow Laboratories. In this paper we have examined the stability of hepatic functions in four different media: Modified Earle’s Medium (3,4), Williams’ E Medium, Medium 199(M 199) and Chee’s Medium (5). They were chosen for the following rea- sons: Williams’ E is one of the most widely used media for the culture of liver cells; Modified Earle’s has been shown to be one of the most efficient media for cyto- chrome P450 maintenance (1,3); Chee’s is a new serum- free medium in which hepatocytes have been shown to respond to phenobarbitone induction of cytochrome P450 (5), a characteristic which has previously been rapidly lost in cultured hepatocytes (6); and Ml99 has previously been used in bioreactors for hybrid liver support systems (7,8). A second consideration is the choice of species. Rats were chosen because of the vast
605

P. Watts et al.
literature available. The number of cells required for a liver device is estimated to be 1O’O cells (equivalent to 20 rat livers); thus an animal with a larger liver was required. Sheep livers were chosen to supply the necess- ary numbers of cells for a bioreactor.
We have measured three parameters as indicators of retained liver function: (i) the content of cytochrome P450, (ii) the ability to convert ammonia to urea, and (iii) the intracellular reduced glutathione (GSH) con- tent. The toxicity and carcinogenicity of many chemi- cals depends upon activation and detoxification by the liver microsomal cytochrome P450 monooxygenase system and the conjugating enzymes. Conjugating with GSH plays an important role in detoxification of elec- trophilic xenobiotics, and this tripeptide is also in- volved in the termination of free radical reactions which may otherwise cause oxidative damage to cell macromolecules. GSH takes part in metabolic func- tions that protect the cell membrane including thiol transfer reactions, whilst GSH peroxidase prevents lipid peroxidation that can lead to hepatocellular lysis (9, 10). The conversion of ammonia to urea is a vital liver function, in which the ammonia produced through amino acid deamination is detoxified via the urea cycle. This pathway is relatively stable in culture in comparison to cytochrome P450 activity (11). In liver failure, plasma ammonia concentrations can rapidly reach toxic levels. During treatment of a pa- tient with a bioartificial liver support system, ammonia clearance is a convenient parameter of liver function that could. be monitored, via discrete plasma sampling, to give an indication of device performance.
Materials and Methods Preparation and culture of rat hepatocytes Hepatocytes were isolated from male Sprague-Dawley rats (190-220 g) by collagenase (Type IV collagenase from Clostridium histolyticum, Sigma Chemical Co.) perfusion (12). The viability of the hepatocytes deter- mined by Trypan blue exclusion was 80-95%. One- hundred-millimetre tissue culture dishes (Falcon) coat- ed with type 1 collagen (1.5 mg/dish) isolated from rat tail tendons as described by Elsdale & Bard (13) were seeded with 5X106 cells in 10 ml of medium. The cul- tures were incubated in a 5%COz/air atmosphere at 37°C. For 48-h cultures the medium was changed at 24 h. Six-day cultures had additional medium changes on day 3 and day 5.
Preparation and culture of sheep hepatocytes Sheep hepatocytes were prepared from 6-month-old wethers by collagenase (Sigma Type 1) perfusion of the caudate lobe of the liver, as described previously (14).
Briefly, the caudate lobe was immediately cleared of blood upon removal by perfusion with Ca*+-free buf- fer containing 140 mM NaCl, 6.7 mM KCI, 10 mM Hepes, 2.5 mM glucose and 0.5 mM EDTA, pH 7.4. This was followed by a recirculating collagenase per- fusion with buffer containing 30 mg of collagenase/lOO ml, 2.5 mg of trypsin inhibitor/ml, 140 mM NaCl, 6.7 mM KCl, 30 mM-Hepes, 2.5 mM glucose and 5 mM CaC12. Digestion was continued for various periods (30-70 min), depending on the size of the lobe. The cell yields were in the range of 1.34.5X lo8 cells per perfusion. The determination of viability (87-98%) and the preparation of cultures were the same as for rat hepatocyte cultures. Sheep hepatocytes were cul- tured for 24 h. Medium was changed on the cultures 6 h before harvesting.
Media tested Four media were tested: Modified Earle’s Medium (3, 4), M199, Williams’ E Medium, and Chee’s Medium (5). All media were suplied by Gibco BRL, Paisley. Chee’s is a serum-free formulation, the other media were supplemented with 5% (v/v) foetal calf serum (Seralab). Penicillin (100 U/ml), streptomycin (100 pg/ ml) and fungizone (2.5 ,&ml) were added to all cul- tures.
Analytical methods Measurements of cytochrome P450 were carried out by a modified method of Omura & Sato (15). Fresh cells (5 X 106/ml) were homogenised in 0.1 M sodium phos- phate buffer, pH 7.6, containing 1 mM dithiotreitol, 1 mM ethylene glycol-bis(beta-amino-ethyl ether) N,N,N’N’-tetra-acetic acid (EGTA), 20% (v/v) gly- cerol and 0.02% (v/v) Nonidet P-40 (Sigma Chemical Co.) (P450 buffer) using a motor-driven teflon-glass homogeniser and stored at -70°C. Cultures of hepato- cytes were rinsed with phosphate-buffered saline, pH 7.4 , (PBS) to dislodge dead cells and cellular debris. The cells were scraped with a rubber policeman in 2 ml P450 buffer, then homogenised and stored at -70°C. Carbon monoxide-treated homogenates were com- pared to CO-treated and sodium-dithionite-reduced homogenates with a Shimadzu UV-2 10 1 PC two-chan- nel spectrophotometer, using 1 ml quartz cuvettes.
The concentration of GSH was measured as de- scribed by Hissin & Hilf (16). Freshly isolated cells were suspended in 10% (w/v) trichloroacetic acid (TCA) at a concentration of lo6 viable cells per 500 ,ul TCA and stored at -70°C. Prior to measurement, the cell/TCA suspension was centrifuged to pellet the cellular debris at 13 000 rpm for 1 min. Hepatocyte culture plates were rinsed with PBS and 2 ml lO%TCA was added to each
606

Effect of medium on hepatocyte cultures
plate. After 15 min at room temperature the acidic GSH extracts were stored at -70°C until analysis.
The rate of urea synthesis from ammonia was deter- mined with the Sigma Urea Kit No. 535-B. Freshly iso- lated cells (5x lo6110 ml) were incubated, in rotating 50- ml round-bottomed flasks under an atmosphere of 95% 02/5%C02, in each of the media with 10 mM am- monium chloride. Hepatocytes were cultured as mono- layers in each of the media for 24 h (sheep), 48 h (rat) or 6 days (rat). After this time the appropriate fresh medium containing 10 mM NH&l was added to the monolayers on the Petri dishes. The rate of urea synthesis was meas- ured over 2 h in both freshly prepared and cultured cells. Additional measurements were taken from cultures supplemented with 10 mM glucose, 2 mM lactate, and 5 mM ornithine during the assay period (17).
Plating efficiency was determined by comparing the amount of protein present in the incubated cultures to the amount in freshly seeded cultures. The amount of cellular protein present was measured by the method of Lowry and co-workers (18) with bovine serum albu- min as a standard. Protein values were corrected for the collagen coating on the dishes. Freshly isolated cells were suspended in 0.5 M NaOH (106/ml). Cul- tured cells were rinsed with PBS and 4 ml 0.5 M NaOH was added to each plate for 30 min before samples were stored at -70°C until analysis.
Statistical analyses
Data were analysed statistically using one-way analy- sis of variance and Fisher’s pair-wise comparisons. The four media were compared pairwise for each species. All data are reported as mean+SEM with nZ4.
Results Plating efJiciency
Rat hepatocytes. The plating efficiencies, defined as the protein content as a percentage of the initial value for the number of fresh cells seeded, for the cells cultured for 48 h in Modified Earle’s, Chee’s and Ml99 media were 62%, 76% and 66%, respectively; while in cells cultured in Williams’ E medium the plating efficiency was significantly lower (pcO.05) at 39% (Fig. 1). After 6 days’ incubation the amount of protein remaining in cultures in the Modified Earle’s medium was reduced to only 42% of the original, whereas the protein in the cell cultures in Chee’s medium had decreased only slightly to 71% of the original (Fig. 2). Sheep hepatocytes. After 24 h, plating efficiencies of 42%, 45%, and 44% were attained by cells cultured in Modified Earle’s, Williams’ E, and M199, respectively; a significantly lower ($0.05) plating efficiency of 10% was achieved in Chee’s medium (Fig. 3)
200
160
Value 120
60
40
0 GSH Protein Urea p450
Parameter
Fig. I. Rat hepatocyte function after 48 h culture in Modi- fied Earle’s, Chee’s, Ml99 and Williams E media. All values represent the means?SEM of at least 4 experiments (*=signi$cant at 95% confidence level). Cytochrome P450 is expressed as pmobmg protein; GSH as nmoUmg protein; urea synthesis as nmoUh per mg protein and protein content as the % of the initial value for the number of fresh cells seeded.
p450 GSH Protein
Parameter
Urea
Fig. 2. Rat hepatocyte function after 6 days culture in Modified Earle S and Cheek media. All values represent the meanskSEA4 of at least 4 experiments. The units are the same as in Fig. I.
Concentration of GSH
Rat hepatocytes. Freshly isolated cells had an average concentration of intracellular GSH of 13.9k2.5 nmoll mg protein. The amounts of GSH present in the cells cultured for 48 h in Williams’ E and Ml99 media were
607

P. Watts et al.
P450 GSH Protein
Parameter
Urea
Fig. 3. Sheep hepatocyte function after 24 h culture in each medium. All the values represent the means?SEM of at least 4 experiments. The units are the same as in Fig. 1.
similar to fresh cell values, 14.821.4 and 15.6k2.5 nmol/mg protein, respectively (106% and 112% of fresh cell values). In comparison, the amounts in the cells cultured in Modified Earle’s and Chee’s media were nearly two-fold greater, 26.6k5.1 and 24.322.2 nmol/ mg, respectively, (191% and 175% of fresh cell values). Although these differences are marked they are not statistically significant, p=O. 153. These data are shown in Fig. 1. After 6 days culture in Modified Earle’s me- dium and Chee’s medium, the GSH levels had returned to levels found in freshly isolated cells, 11.75 1 and 16.424.1 nmollmg, respectively, (84% and 118% of fresh cell values) (Fig. 2).
Sheep hepatocytes. Freshly isolated sheep hepato- cytes had a concentration of GSH of 13.2+2 nmol/mg protein. The concentrations after 24 h in culture were not significantly different from fresh cell values in Modified Earle’s, Williams’ E or M199, 1422.6, 19.321.3, and 19.821.7, respectively, (106%, 146% and 150% of fresh cell values). No measurable amount of GSH was detected in cells cultured in Chee’s (Fig. 3).
Cytochrome P450 content
Rat hepatocytes. Freshly isolated cells contained 2162 16 pmol/mg protein of cytochrome P450. After 48 h in culture the cells in Modified Earle’s medium, Chee’s, M199, and Williams’ E contained 80.628, 66.025, 49.3+7 and 30.62 13 pmol/mg, respectively, (37%, 31%, 23% and 14% of fresh cell values). Hepato- cytes cultured in Modified Earle’s medium retained sig- nificantly more (~~0.005) cytochrome P450 than cells
cultured in any of the other three media. After 6 days in culture there was no difference in the cytochrome P450 levels of cells cultured in either Modified Earle’s medium or Chee’s medium; the respective values were 5 126 and 492 18 pmol/mg protein (24% and 23% of fresh cell values). The 6-day cultures in Modified Earle’s medium and Chee’s medium retained as much P450 as did the 48-h cultures in Williams’ E or Ml99 medium. These data are shown in Fig. 1 and 2.
Sheep hepatocytes. Freshly isolated sheep hepato- cytes contained 198.6224 pmol/mg protein of cyto- chrome P450. The levels of cytochrome P450 in cells cultured in Modified Earle’s, Williams’ E and Ml99 did not differ significantly from each other: 117.Ok19.5, 112.6214.4, 106.829.4, respectively, (59%, 57%, and 54% of fresh values). No cytochrome P450 was found in cells cultured in Chee’s (Fig. 3).
Urea synthesis
Rat hepatocytes. Freshly isolated rat hepatocytes were assayed in Modified Earle’s synthesised urea at the rate of 1712 18 nmol/h per mg protein. The ability of cul- tured hepatocytes to convert ammonia into urea was significantly greater (~~0.001) for cells cultured for 48 h in Modified Earle’s (1842 19 nmolh per mg protein) than in cells cultured in Williams’ E, Ml99 or Chee’s (84?24, 84+8, 3126, respectively) (Fig. 1). Further- more, the rate of urea synthesis of cells cultured in Chee’s medium was significantly less than in cells cul- tured in Williams’ E or Ml99 (~~0.001). After 6 days, the rate of urea synthesis in cells cultured in Modified Earle’s medium had diminished to approximately 40% of that recorded at 48 h, while cells cultured in Chee’s medium maintained the level of urea synthesis found at 48 h (Fig. 2). However, when cells cultured in Modified Earle’s medium were subsequently assayed for urea synthesis in Chee’s medium, and likewise when cells cultured in Chee’s medium for 48 h were assayed in Modified Earle’s medium, the rate of urea synthesis was found to be more dependent upon the assay me- dium (~~0.0001) than on the culture medium (p= 0.014) (Fig. 4). The addition of supplements to each medium, as described in Methods, resulted in the rate of urea synthesis being independent of the type of cul- ture medium used (Fig. 5).
Sheep hepatocytes. Freshly isolated sheep hepato- cytes assayed in Modified Earle’s medium synthesised urea at the rate of 13622 nmol/h per mg protein. In 24-h cultures, cells cultured in Modified Earle’s con- verted significantly more (~K0.05) ammonia to urea than those cultured in the other three media (Williams’ E, 51? 18; M199, 70?7; Chee’s, 0). It should be noted
608

Effect of medium on hepatocyte cultures
nmol/h per mg protein that Chee’s medium did not support retention of urea synthesis in cultures (Fig. 3).
120 •j ChW’S
[7 Mod. Earles
100 r-l
60
Cheek Mod. Earles
Medium
Fig. 4 The effect of assay medium on urea sythesis in 48 h cultures of rat hepatocytes. AN values represent the meankSEA4 of at least 4 experiments. Urea synthesis is shown as nmol/h per mg protein. Filled bars represent values from cells cultured in Chee ‘s medium while empty bars indi- cate values from cells cultured in Modtfied Earle’s medium. The cells were then assayed for urea synthesis in either Chee ‘s medium or Modtf?ed Earle S medium as indicated on the X-axis.
nmol/h per mg protein
250,
200-
150-
loo-
50 -
0-r WmE Ml99 Chee’s Mod.Esrle’s
Medium
Fig. 5. Effect of addition of 10 mM glucose, 2 mM lactate and 5 mM ornithine to each medium on the rate of urea synthesis in rat hepatocytes. Units are expressed as nmobh per mg pro tein. All values represent mean 2 SEA4 of at least 4 experiments.
Hepatocyte morphology
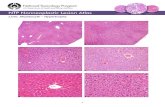
Rat cells cultured in Modified Earle’s or Chee’s media had more canaliculae present and were more flattened at 48 h than cells cultured in either Williams’ E or M199. At 6 days the cells cultured in Chee’s medium looked healthier, were less granular and contained more canaliculae than those cultured in Modified Earle’s (Fig. 6).
Sheep cells were different from rat hepatocytes in terms of both morphology and behaviour in culture. They looked smaller, rounder and took longer to flat- ten and attach after seeding. In contrast to rat hepato- cytes, sheep hepatoytes could not survive in Chee’s me- dium; they formed blebs, ruptured and died within 24 h.
Discussion It has been shown here that the choice of medium for successful culture of primary hepatocytes depends upon the species of hepatocyte origin, and to some ex- tent upon the differentiated function that is being in- vestigated. The phenotype of rat hepatocytes was more sensitive than that of sheep to the different media, with the exception of Chee’s medium which was unique in that the sheep cells in this medium ruptured and died within 24 h of culture. The inability of Chee’s medium to support sheep hepatocyte cultures was not abro- gated by the addition of 5% FCS (Watts, unpublished data). Rat hepatocytes cultured in the four media showed significant differences in plating efficiency, cytochrome P450 content, and rate of urea synthesis, but not in intracellular concentration of GSH. No- tably, Williams’ E, which is widely used for rat hepato- cyte culture, performed rather badly in our tests. Sheep hepatocytes cultured in William’s E, Ml99 and Modi- fied Earle’s media showed a significant difference only in the rate of urea synthesis.
Although GSH concentration per se, does not repre- sent a specific hepatocyte function, the presence of GSH in the 6-day rat hepatocyte cultures in Modified Earle’s and Chee’s media does present evidence of maintained differentiation. In most cells GSH is syn- thesised from three substrates: L-glutamate, L-cysteine and L-glycine; however, hepatocytes are one of only a few cell types able to utilise L-methionine for GSH synthesis in place of L-cysteine via the cystathionine pathway (19). However, the activity of the cystathion- ine pathway is rapidly lost in de-differentiated liver cells (20-23). In the Modified Earle’s and Chee’s media there is no L-cysteine or L-cystine; therefore hepato-
609

P. Watts et al.
Fig. 6. Hepatocyte morphology: a) sheep, 24 h in Williams’ E; b) rat, 24 h in Modi- fied Earle ‘s; c) rat, 48 h in Chee’s medium d) rat, 6-day cultures in ModiJied Earle’s and e) rat, 6-day cultures in Chee’s me- dium (magniJication a-c: x2.50, d-e:
cytes cultured in these media can synthesise GSH only through the cystathionine pathway using L-methion- ine. GSH levels in rat hepatocytes are well maintained in these two media for at least 6 days in culture, indi- cating that these cells have retained this differentiated function.
The hepatic microsomal cytochrome P450 system plays an important role in the detoxification function of hepatocytes. In rat hepatocytes, P450 levels were sig- nificantly higher at 48 h in cells cultured with Modified Earle’s medium than in those cultured with any of the other media. This may be explained by increased pro- duction and/or reduced degradation of P450 in Modi- fied Earle’s medium. This is the only medium which contains 5-amino-laevulinic acid (ALA) which stimu- lates P450 production in cultured hepatocytes (24). In addition, it has been shown that the presence of L- cysteine and L-cystine in media results in the loss of P450 due to enhanced degradation (3). Modified Earle’s contains neither L-cysteine nor L-cystine. The two media in which the loss of P450 was greatest, Wil- liams’ E and M199, contain both L-cysteine and L-
610
cystine. After 6 days in culture the cells in Modified Earle’s and Chee’s media contained less P450 than at 48 h, but these 6-day levels were comparable with the 48-h levels in cells cultured in Williams’ E and Ml99 media. Sheep hepatocytes, in contrast, show no sig- nificant difference in cytochrome P450 content in the three media which supported growth. Recent evidence suggests that the loss of total P450 in cultured hepato- cytes is concomitant with a reduction in mRNAs cod- ing for P450 isoenzymes (25,26), and that substantial amounts of this loss may even occur during the col- lagenase perfusion before culture has been initiated. If this is the case, then the protection offered by L-cys- teine-free media will be less important.
The liver is the major site of ammonia detoxifica- tion, and one of the main features of this metabolism is the conversion of ammonia to urea. A measure of the function of hepatocytes can be made by determin- ing the rate of synthesis of urea from ammonia, and this is a convenient parameter that could be used for monitoring the function of hybrid artificial liver de- vices. Of the four media tested, the rate of urea syn-

Effect of medium on hepatocyte cultures
thesis was significantly greater in Modified Earle’s, while cells cultured in Chee’s medium gave the slowest rate of urea synthesis. This emergence of urea synthesis as the most discriminatory parameter of liver function in the study was unexpected because the expression in culture of the enzymes of urea synthesis has been found to be more stable than those of the cytochrome P450 system (11). However, the medium used during the urea analysis proved to be more significant than the medium in which the cells had been cultured prior to the assay. Furthermore, no significant differences in the rate of urea synthesis were found between cells cul- tured in the different media when glucose, lactate and ornithine were added to each. It appears that all four media under investigation support expression of the urea enzymes in culture and that the rate of urea syn- thesis is mainly a function of the composition of the assay medium, rather than of the differentiated status of the hepatocytes at this time point.
We conclude that Chee’s medium does not support sheep hepatocytes and that Williams’ E and Ml99 are the least hospitable of the media tested in which to culture rat hepatocytes. Modified Earle’s supported the P450 levels of rat hepatocytes better than any of the other media in the first 48 h of culture, but this differ- ence was reduced to parity with the Chee’s medium over 6 days. It is of particular importance to note that Chee’s is a serum-free formulation and thus a com- pletely defined medium. Serum-free conditions have previously been demonstrated to increase the activity of cytochrome P450 in cultured rat hepatocytes (27). The same authors demonstrated that GSH content was not affected by the absence of serum. In addition, MacDonald et al. (23) have also reported improved ex- pression of bilirubin UDP-glucuronosyltransferase ac- tivity, but not ghttathione-S-transferase activities, in immortalised rat hepatocytes cultured in serum-free conditions. Clearly these serum-free conditions do not improve retention of all liver specific functions in hepatocytes.
We have shown that there are significant differences in retained hepatocyte functions in rat and sheep cell cultures in the four media investigated, and that there are startling species differences. In addition to the ob- servation that Chee’s medium, one of the best for rat hepatocytes, did not support sheep hepatocytes, we have demonstrated that rat hepatocytes retained func- tion significantly better in Modified Earle’s, while sheep hepatocyte function was maintained equally well in Williams’ E and M199. We therefore recommend careful choice of media for artificial liver bioreactor cultures dependent on the source of the cells used.
Acknowledgements This work was supported by Science and Engineering Research Council Link Scheme Grant No. GR- H25522.
References 1. Grant MH, Melvin MAL, Shaw P Melvin WT, Burke MD.
Studies on the maintenance of cytochromes P-450 and monooxygenase and cytochrome reductases in primary cul- tures of rat hepatocytes. FEBS Lett 1985; 190: 99-103.
2. Paine AJ. The maintenance of cytochrome P450 in rat he- patocyte culture: some applications of liver cell cultures to the study of drug metabolism, toxicity and the induction of the P450 system. Chem Biol Interact 1990; 74: 1-31.
3. Paine AJ, Hockin LJ. Nutrient imbalance causes the loss of cytochrome P-450 in liver cell culture: formulation of culture media which maintain cytochrome P-450 at in vivo concen- trations. Biochem Pharmacol 1980; 29: 3215-8.
4. Morrison MH, Hammarskiold V, Jernstrom B. Status of re- duced glutathione in primary cultures of rat hepatocytes and the effect on conjugation of benzo[a]pymne-7,8-dihydrodiol- 9,10-oxide. Chem Biol Interact 1983; 45: 235-42.
5. Waxman DJ, Morrissey JJ, Naik S, Jauregui HO. Phenobar- bital induction of cytochromes P450. Biochem J 1990; 271: 113-9.
6. Guzelian PS, Li D, Schuetz EG. Induction of cytochromes P450b/e by phenobarbital in primary culture of adult rat hepatocytes: test of differentiated liver gene expression. Drug Metab Rev 1989; 20: 793-809.
7. Gerlach J, Kloppel K, Schauwecker HH, Tauber R, Muller C, Bucherl ES. Use of hepatocytes in adhesion and suspen- sion cultures for liver support bioreactor. Int J Artific Organs 1989; 12: 785-91.
8. Gerlach J, Kloppel K, Stoll P Vienken J, Muller C. Gas sup- ply across membranes in bioreactors for hepatocyte culture. Artific Organs 1990; 14: 328-33.
9. Anundi I, Hogberg J, Stead AH. Gluathione depletion in isolated hepatocytes: its relation to lipid peroxidation and cell damage. Acta Pharmacol Toxic01 1979; 45: 45-51.
10. Meister A. Glutathione. In: The Liver: Biology and Pathobi- ology. Arias IM, Jakoby WB, Popper H, Schachter D, Shaf- ritz DA, eds. New York; Raven Press, 1988: 401-17.
11. Dunn JCY, Tompkins RG, Yarmush ML. Long term in vitro function of adult hepatocytes in a collagen sandwich con- figuration. Biotechnol Prog 1991; 7: 23745.
12. Moldeus P Hogberg J, Orrenius S. Isolation and use of liver cells. Methods Enzymol 1978; 52: 60-71.
13. Elsdale T, Bard J. Collagen substrata for studies on cell be- haviour. J Cell Biol 1972; 54: 62637.
14. Emmison N, Agius L, Zammit VA. Regulation of fatty acid metabolism and gluconeogenesis by growth hormone and in- sulin in sheep hepatocytes culture: effects of lactation and pregnancy. Biochem J 1991; 274: 21-6.
15. Omura T, Sato R. Carbon-monoxide-binding pigment of liver microsomes: evidence for its haemoprotein nature. J Biol Chem 1964; 239: 2379-385.
16. Hissin PJ, Hilf R. A fluorimetric method for the determi- nation of oxidised and reduced glutathione in tissues. Anal Biochem 1976; 74: 21426.
17. Krebs HA, Lund P Stubbs M. 1n:‘Hanson RW, Mehlman MA, eds. Gluconeogenesis: Its Regulation in Mammalian Species. New York: Wiley, 1979: 269.
611

P. Watts et al.
18. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 1951; 193: 265-75.
19. Reed DJ, Beatty PW. The role of the cystathionine pathway in glutathione regulation in isolated hepatocytes. In: Sies H, Wendel A, eds. Functions of Glutathione in Liver and Kid- ney. Berlin: ‘Springer-Verlag, 1978; 13-21.
20. Duthie SJ, Coleman CS, Grant MH. Status of reduced gluta- thione in the human hepatoma cell line, Hep G2. Biochem Pharmacol 1988; 37: 3365-8.
21. Nairn A, Willett B, Grant MH, Scott A, MacDonald C. Maintenance of differentiated function in cultured hepato- cytes immortalised by transfection with viral DNA. Biochem Sot Trans 1990; 18: 1201-2.
22. Meredith MJ. Cystathionase activity and glutathione metab- olism in redifferentiating rat hepatocyte primary cultures. Cell Biol Toxic01 1987; 3: 361-77.
23. MacDonald C, Vass M, Willett B, Scott A, Grant MH. Ex-
pression of liver functions in immortalised rat hepatocyte cell lines. Hum Exp Toxic01 1994; 13: 43944.
24. Guzelian PS, Bissell DM. Effects of cobalt on synthesis of heme and cytochrome P-450 in the liver. J Biol Chem 1976; 251: 4421-7.
25. Padgham CRW, Paine AJ. Altered expression of cytochrome P-450 mRNAs, and potentially of other transcripts encoding key hepatic functions, are triggered during the isolation of rat hepatocytes. Biochem J 1993; 289: 6214.
26. Akrawi M, Rogiers V, Vandenberghe Y, Palmer CNA, Vercruysse A, Shepherd EA, Phillips IR. Maintenance and induction in co-cultured rat hepatocytes of components of the cytochrome P450-mediated mono-oxygenase. Biochem Pharmacol 1993; 45: 1583-91.
27. Hammond AH, Fry JR. Effect of serum-free medium on cytochrome WSO-dependent metabolism and toxicity in rat cultured hepatocytes. Biochem Pharmacol 1992; 44: 1461-4.
612