Surveillance of Nuclear Pore Complex Assembly by ESCRT-III ...
The ESCRT machinery regulates the secretion and long-range activity of Hedgehog
Transcript of The ESCRT machinery regulates the secretion and long-range activity of Hedgehog

LETTERdoi:10.1038/nature13847
The ESCRT machinery regulates the secretion andlong-range activity of HedgehogTamas Matusek1,2,3*, Franz Wendler1,2,3*, Sophie Poles1,2,3, Sandrine Pizette1,2,3, Gisela D’Angelo1,2,3, Maximilian Furthauer1,2,3
& Pascal P. Therond1,2,3
The conserved family of Hedgehog (Hh) proteins acts as short- andlong-range secreted morphogens, controlling tissue patterning anddifferentiation during embryonic development1. Mature Hh carrieshydrophobic palmitic acid and cholesterol modifications essentialfor its extracellular spreading2. Various extracellular transportationmechanisms for Hh have been suggested, but the pathways actuallyused for Hh secretion and transport in vivo remain unclear. Here weshow that Hh secretion in Drosophila wing imaginal discs is dependenton the endosomal sorting complex required for transport (ESCRT)3.In vivo the reduction of ESCRT activity in cells producing Hh leadsto a retention of Hh at the external cell surface. Furthermore, we showthat ESCRT activity in Hh-producing cells is required for long-rangesignalling. We also provide evidence that pools of Hh and ESCRTproteins are secreted together into the extracellular space in vivo andcan subsequently be detected together at the surface of receiving cells.These findings uncover a new function for ESCRT proteins in con-trolling morphogen activity and reveal a new mechanism for the trans-port of secreted Hh across the tissue by extracellular vesicles, whichis necessary for long-range target induction.
Diffusible signals, such as secreted morphogens, are key players in inter-cellular communication during development. The Hh morphogen hasa critical role in patterning the anterior–posterior axis of the developingepithelial wing imaginal discs (WIDs) in Drosophila, by specifying thepresumptive intervein domain between vein 3 and vein 4 (v3–v4) throughboth short- and long-range activity1. Mechanisms proposed to controlspreading of Hh include the formation of multimers, loading on filopo-dia, and packaging into lipoprotein particles4–7. However, the possibilityof Hh transport via membranous vesicles released by cells (exovesicles)has never been functionally explored. Exovesicles may be generated byplasma membrane budding or by fusion of multivesicular bodies (MVBs)with the plasma membrane, leading to the release of MVB intraluminalvesicles into the extracellular space8–10. Both plasma membrane buddingand the formation of MVB intraluminal vesicles require the severingof thin membrane stalks by a highly conserved specialized machinery,the ESCRT complex3.
In WIDs, Hh is produced and secreted by the epithelial cells of theposterior compartment. It induces a concentration-dependent gradedresponse in anterior cells, through activation of short-range target genes,such as the transcription factor engrailed (en) and the Hh receptor patched(ptc), and long-range target genes, such as decapentaplegic (dpp), theDrosophila homologue of TGF-b11 and iroquois (iro)12. The strong celldeath and tumorigenic phenotype induced by the lack of ESCRT functionin WIDs13–16 made it impossible for us to analyse its role in Hh secretionwith classical loss-of-function mutants. We therefore used a tempo-rally controllable RNA interference (RNAi) method restricted to Hh-producing cells and developed a ‘tester line’ (Methods) in which increasingHh production in the posterior cells of the WID induced the ectopicproduction of dpp in the most anterior cells of the WID, leading to severeoutgrowth of the anterior compartment (Fig. 1a and Extended DataFig. 1a–d). Moreover, we also observed an enlargement of the endogenous
dpp expression domain at the anterior–posterior border (10–12 cells wideinstead of 6–8 cells wide in the wild type; Fig. 1b, c), leading to an increasein the size of the v3–v4 intervein space of the adult wings (Extended
*These authors contributed equally to this work.
1Universite de Nice Sophia Antipolis, iBV, UMR 7277, 06100 Nice, France. 2CNRS, iBV, UMR 7277, 06100 Nice, France. 3INSERM, iBV, U1091, 06100 Nice, France.
TSG
101
2394
4GD
C
hmp3
2927
5GD
C
hmp3
1002
95KK
Chm
p1
2890
6TriP
Genotype
10
30
20
40
50
60
70
80
90
100 n =
Perc
en
tag
e
65 52 21 61 20 27 22 41 30 40 65
Test
er li
ne
Ci1
0562
0KK
Dis
p10
004G
D
AliX
3204
7GD
AliX
3204
9GD
AliX
EY10
638
Vps2
221
658G
D
No suppression
Partial suppression
Complete
suppression
a
Chmp3 AliXWT
LacZ LacZLacZ
Tester line
Tester line Chmp3 AliX TSG101exHhexHh exHh exHh Chmp1 exHh
Chmp3Tester line TSG101 Chmp1AliXA/P: 0.65 A/P: 0.33 A/P: 0.151 A/P: 0.45 A/P: 0.343
b c d e
h j f l n
g i k m o
65 52 21 61 20 27 22 41 30 40 65
LacZ
exHh exHh exHh exHh exHh
* *
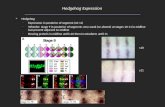
Figure 1 | ESCRT proteins are required for the long-range activity of Hh.a, Percentage of outgrown, moderately and fully rescued WIDs with respectto their genotypes. The number of dissected discs (n) is shown at the top of thecolumns. Representative photographs of each category are shown on the right.Negative control: RNAi against Cubitus interruptus (Ci), which is notexpressed in the posterior compartment. Positive control: RNAi against Disp.Superscripts refer to the transformant identity of the RNAi line. b–e, dpp-lacZexpression in wild-type (WT), tester line and tester line plus ESCRT RNAidiscs. A magnification of the dpp-lacZ stripe is shown for each genotype in thebottom-right corner. Note that not only the cental dpp stripe but also theectopic dpp expression is suppressed in tester line plus ESCRT RNAi discs(white asterisks on c and e). Owing to the low level of Dpp staining, signalintensity was increased in d. Scale bar, 50mm. f–o, Representative X–Z(f, h, j, l, n) and X–Y–Z (g, i, k, m, o) sections showing extracellular Hh stainingin control discs and discs with tester line plus ESCRT RNAi. On X–Z sections(scale bar, 20mm), apical is towards the top. The anterior–posterior borderis labelled with a white dashed line. On X–Y–Z sections (scale bar, 15mm),the anterior–posterior (A/P) ratio of Hh staining intensity is indicated in thebottom-right corner for all genotypes. In all images anterior is to the left andin b–e dorsal is to the top. Information about optical sections shown in allfigures can be found in Supplementary Table 3. ex, extracellular.
4 D E C E M B E R 2 0 1 4 | V O L 5 1 6 | N A T U R E | 9 9
Macmillan Publishers Limited. All rights reserved©2014

Data Fig. 1e–h). These two phenotypes were rescued by RNAi targetingthe only known regulator of Hh secretion, Dispatched17 (Disp) (Fig. 1aand Extended Data Fig. 1e, i).
Expression in Hh-producing cells of RNAi against ESCRT proteins,including AliX, TSG101, Vps22 (also known as Larsen), Chmp1 andChmp3 (members of the ESCRT complexes I, II and III; see Supplemen-tary Table 1) abolished the Hh-dependent v3–v4 intervein space enlarge-ment and WID anterior outgrowth (up to 48% of the WID showedcomplete rescue) (Fig. 1a and Extended Data Fig. 1e, j–k) without chang-ing the level of Hh protein (Extended Data Fig. 1m, n). Rescued discswith wild-type appearance did not display any sign of apoptosis orcell-architecture defects, with the exception of Vps22, investigation ofwhich was not further pursued (Extended Data Fig. 2a–k). Distribu-tion of DE-cadherin (also known as Shotgun), Disp, and the glypicanDally-like (Dlp) in cells depleted for ESCRT function were also notaffected (Extended Data Fig. 2h–s), indicating that the ESCRT deple-tion conditions used did not induce pleiotropic or indirect effects onHh secretion.
The rescue of WID morphology revealed a role of the ESCRT proteinson the regulation of expression of the Hh long-range target dpp. Indeed,
very anterior ectopic expression of dpp and the broadening of its cen-tral stripe at the anterior–posterior border were abolished (Fig. 1d, e).Surprisingly, the expression of short-range targets requiring high-levelsignalling, such as En and Ptc, was still extended over up to six cells(Extended Data Fig. 3a–l). Consistent with a role of ESCRT in the long-range activity of Hh, accumulations of Hh at the external apical side ofHh-producing cells were observed upon depletion of ESCRT in poste-rior cells whereas the extracellular apical Hh levels in the anterior receiv-ing cells were decreased compared with wild-type discs (Fig. 1f–o).
The requirement of ESCRT function for Hh long-range activityprompted us to test whether ESCRT function was also required in ani-mals in which Hh is expressed at physiological levels. We thus depletedChmp1, AliX, TSG101 and Vps32 (also known as Shrub) by RNAi (Sup-plementary Table 1) in the posterior cells of an otherwise wild-type disc.We observed a significant decrease of dpp and iro expression, whereasthe domains of Ptc and En expression were similar to those in the wildtype (Fig. 2a–e, v, x and Extended Data Fig. 4a–j, k). We obtained similarresults by using the temperature-sensitive allele of the hh gene (hhts2),which increases the sensitivity for defects in Hh secretion (ExtendedData Figs 1l, 4l–t).
LppVps28;hhG4 Ubi
TSG101/apGal4 exHh
j
exHhexHhWT
apG4/Vps4DN exHh
apG4;AliX
apG4/Vps4DN Hh
apG4;Chmp3exHh exHh
dpp-lacZ
AliX/hhG4
dpp-lacZ
Vps32/hhG4
dpp-lacZ
Chmp1/hhG4
dpp-lacZ
WT Chmp1 TSG101 AliXVps32
dpp-lacZ
LppUbi
apG4;Chmp1
TSG101/hhG4
dpp-lacZ
dpp-lacZ
WT
Distance (pixels)
Rela
tive inte
nsity
20
40
60
80
100
120
140
20 40 60 80 100 20 40 60 80
Distance (pixels) 20
40
60
80
100
120
140
Rela
tive inte
nsity
WT Vps28 30 h Vps28 48 h
D/V: 1.46
D/V: 0.91 D/V: 2.85 D/V: 2.33 D/V: 2.32 D/V: 2.04
A/P border
A/P border
a b c d e
f g h i j
k l
p q r v w
s t u
apG4/Vps4DN Actin
m n o
apG4/Vps4DN ActinapG4/Vps4DN Chmp1
x y
Vps28;hhG4
30 h
48 h
6/7 12/12 6/8 5/8
Figure 2 | Lack of ESCRT function in posterior cells leads to defects inHh secretion and target gene induction. a–e, Pattern of dpp-lacZ expressionin WIDs with 30 h of RNAi against the indicated ESCRT components.A magnification of the dpp-lacZ stripe is shown in the bottom panels.b–e, Numbers at bottom right indicate number of discs displaying reduceddpp activity per whole population analysed. f–j, Extracellular staining ofendogenous Hh in wild-type (WT) discs and in discs with the indicated ESCRTRNAi. Dorsal is to the top and anterior to the left. f–k, The dorsal–ventral (D/V)ratio of Hh staining intensity is indicated in the bottom-right corner for allgenotypes. k–m, Extracellular (exHh; k) and conventional (l–m) Hh stainingin discs expressing Vps4DN. n, o, Chmp1 accumulates in endosomal
compartments. F-actin staining is used to outline the cell cortex (m, o).Magnified views of dorsal-posterior cells are shown in the top-right corner.f–o, The dorsal–ventral boundary is marked with a white dashed line.p–u, Misexpression of Vps28 RNAi for 30 h (p–r) or 48 h (s–u) inHh-producing cells. v, Quantification of dpp-lacZ from a–e. A/P,anterior–posterior. w, Quantification of dpp-lacZ expression in Vps28RNAi discs presented in p, s. x, y, Schemes presenting the expressiondomain (red) of the compartment-specific Gal4 driver lines hedgehog-Gal4(hhGal4; x) and apterous-Gal4 (apGal4; y). Green circles indicate Hh protein.Scale bars, 30mm.
RESEARCH LETTER
1 0 0 | N A T U R E | V O L 5 1 6 | 4 D E C E M B E R 2 0 1 4
Macmillan Publishers Limited. All rights reserved©2014

To test whether impaired Hh activity was associated with defects ofHh subcellular localization, we depleted ESCRT proteins in the dorsalcompartment of the WID (Fig. 2y). This led to the accumulation of Hhin dorsal ESCRT-depleted cells compared with their ventral wild-typeneighbours. Accumulating Hh can be detected using a non-permeabilizingantibody staining protocol (see Methods), suggesting that the moleculeis retained at the outer plasma membrane surface of producing cells(Fig. 2f–j). To confirm this, we induced the expression of a dominant-negative form of the ESCRT III protein Vps4 (Vps4DN) to block the dis-assembly and recycling of the ESCRT complex18–20 in Hh-secreting cells.Surprisingly, intracellular Hh was not trapped in maturation-deficientendosomes labelled with Chmp1, but instead accumulated at the apicalcortex of Hh-producing cells (Fig. 2k–o). This suggests that the ESCRT-dependent regulation of Hh secretion might not be related to the func-tion of ESCRT proteins in MVB biogenesis. To confirm this, we analysedthe effect of depletion of ESCRT function on MVB biogenesis. Block-ing MVB function by expression of ESCRT RNAi or of Vps4DN in Hh-producing cells led to the accumulation of poly-ubiquitinated epitopes(poly-Ubi) on proteins trafficking through degradative MVBs21 (ExtendedData Fig. 5a–e, n–p). Interestingly, upon expression of Vps28 RNAi, thisenrichment was not associated with a decrease in dpp target gene expres-sion (Fig. 2p–q, s–t, w). We also showed that an increase in poly-Ubiaccumulation in Vps32-depleted discs does not correlate with a decreasein dpp-lacZ expression (Extended Data Fig. 5m–p). Conversely, in AliX-and Vps32-depleted discs showing no accumulation of ubiquitinatedproteins, expression of the long-range target dpp was reduced (Fig. 2b,c, v and Extended Data Fig. 5j, m). This indicates that defects in MVBtrafficking do not correlate with defects in Hh signalling, but suggeststhat a subset of ESCRT proteins regulate Hh secretion in a manner thatis independent of MVB biogenesis.
Hh can be associated with the Lipophorin (Lpp) lipid carrier (whichlabels lipoprotein particles) secreted from the fat body5; therefore, we won-dered whether trafficking of Lpp is affected in the absence of ESCRTs.We found that depletion of Vps28—which has no effect on Hh signalling(Fig. 2p, s, w)—affects Lpp trafficking (Fig. 2r, u), whereas depletion ofAliX and Vps32—which affects Hh secretion and signalling (Fig. 2b,c, g)—did not perturb Lpp trafficking (Extended Data Fig. 5k, l). Inaccordance with the fact that ESCRT-dependent Hh secretion is inde-pendent of Lpp, only the knockdown of ESCRT proteins but not theknockdown of Lpp led to the accumulation of Hh at the apical pole ofthe cells (Fig. 2g–l and Extended Data Fig. 5q–t). These data suggest thatperturbation of the ESCRT machinery impairs Hh trafficking and secre-tion independently of Lpp.
To investigate whether secretion of Hh relies on the ESCRT-dependentbiogenesis of exovesicle carriers, we used a cell line (Cl8 cells) derived fromthe WID and modified to express Hh in an inducible manner22. Bio-chemical fractionation by differential centrifugation of the Hh-containingconditioned medium was carried out (Methods and Fig. 3a). Westernblot analysis indicated that the bulk of the Hh protein present in the con-ditioned medium was split equally between a 120,000g supernatant (S120)and a detergent-soluble pellet (P120) (Fig. 3a). Higher-speed centrifuga-tion for a longer time gave similar fractionation (Extended Data Fig. 6a).The S120 fraction corresponds to a soluble Hh complex with a molecularweight of 160–200 kDa (ref. 22). The analysis by transmission electronmicroscopy of the P120 fraction from Hh-expressing Cl8 cells or of P120from Cl8 cells not expressing Hh showed cup-shaped vesicles of 40–200 nm in diameter (Fig. 3b). Incubation of Hh-responsive Cl8 naivecells with an equivalent relative amount of P120 Hh or S120 Hh stim-ulated phosphorylation of the Fused protein—which reflects Hh signal-ling activation1—to similar extents (Fig. 3c and Extended Data Fig. 6a, b),suggesting that these two fractions correspond to two different pools ofHh with similar capacities to induce Hh signalling. P120 Hh was also con-fined to a 35% (w/v) Optiprep step in a density gradient, correspondingto an intermediate density (between 1.184 and 1.195 g ml21), indicatingspecific membrane association (Fig. 3d). Anti-Hh staining by immuno-electron microscopy on non-permeabilized purified P120 membranes
HhNp
1 3 2 4 5
Fractions
P120 6 7 9 8 10 11 12 13 14 P
10 25 45 35
Optiprep density step gradient (%)
200 nm Hh: 10 nm gold
200 20
100 50
200 100
50 0
Loaded exovesicles (μg)
HhNp
0.2% TX-100
S120
P120
P120
S120
HhNp
P120
S120
P10 CM
HhNp
CM
Overnight
conditioning
in SFM
Wash and change medium to SFM
c
d
a b
f
e
Collection of CM
Spin: 300g, 10 min
Supernatant
Spin: 10,000g, 30 min
Supernatant
Spin: 2,000g, 20 min
Supernatant
Spin: 120,000g, 90 min
S120 (supernatant)
Pellet
Wash in PBS
Spin: 120,000g,
90 min
P120 (pellet)
P10 (pellet)
Hh-producing Cl8
cells spreading in
culture dish
+ Induction of Hh expression
Untreated
Control
P120-Hh
S120-Hh
Fused P-Fused
Elution from anti-Hh affnity column Control
Separation of S120-
Hh and P120-Hh from
Hh-producing Cl8 CM
Incubation of naive
Cl8 cells with S120-Hh
or P120-Hh
Detection of Hh
pathway activation
200 nm
500 nm
0,2
Vesic
le s
ize (μm
)
0,4
0,6
0,8
1
1,2
1,4
1,6
Absolute number of vesicles
(120 posterior cells per genotype)
20 40 60 80 100
WT (89) Chmp1 (53)
Vps4DN (53)
350 nm
WT Chmp1/hhG4 Vps4DN;hhG4
Exo
vesic
le s
ize d
istr
ibutio
n (%
)
GenotypeWT
Chm
p1/h
hG
4
Vps4
DN ;h
hG
4
(5/87) (23/53) (15/53)
20
40
60
80
100
Below 350 nm
Above 350 nm
g h i
j k
p p p l
c c
c
l l
500 nm 500 nm
Figure 3 | Hh is secreted on extracellular vesicles. a, Conditioned medium(CM) from Hh-producing Cl8 cells subjected to differential centrifugation.The 10,000g pellet (P10), 120,000g pellet (P120) and 120,000g supernatant(S120) fractions were analysed by immunoblotting with anti-Hh antibodies(middle). Bottom, the Hh-producing Cl8-cell S120 and P120 fractions weretreated with 0.2% Triton X-100 (TX-100). HhNp, mature cholesterol-modifiedHhN peptide; SFM, serum-free medium. b, Representative transmissionelectron micrograph of the Hh-producing Cl8-cell P120 fraction contraststained with 3% uranyl acetate. c, Naive Cl8 cells were treated withHh-producing Cl8-cell S120 or P120 fractions or with conditioned mediumfrom Cl8 cells (control). Cell lysates from treated cells were analysed byimmunoblotting with anti-Fused antibodies. P-Fused, phosphorylatedFused. d, Hh-producing Cl8-cell P120 was fractionated on an Optiprepdensity step gradient from 45% to 10%. e, Immunogold labelling of Hh P120purified fraction with anti-Hh antibodies. f, Anti-Hh or control antibodiesbound to Dynabeads were incubated with increasing amounts of Hh-producingCl8-cell exovesicles from P120. Bound vesicles were eluted and analysedby immunoblotting with anti-Hh antibodies. g–i, Transmission electronmicrographs of WIDs expressing Chmp1 RNAi and Vps4DN in Hh-producingcells. White letters mark the apical side of peripodial (p) and columnar (c)cells facing the lumen (l). Black arrows indicate the luminal vesicular profiles.Scale bar, 500 nm. j, k, Quantification (j) and distribution (k) based on the sizeof vesicular profiles in Chmp1 RNAi and Vps4DN compared with wild type(WT). Numbers in brackets indicate the total number of vesicular profilescounted (j), or the number of vesicular profiles sized above 350 nm per wholepopulation (k).
LETTER RESEARCH
4 D E C E M B E R 2 0 1 4 | V O L 5 1 6 | N A T U R E | 1 0 1
Macmillan Publishers Limited. All rights reserved©2014

revealed that Hh was associated with the surface of vesicles (Fig. 3e) butnot on vesicles purified from non-Hh-expressing cells (Extended DataFig. 6c). Moreover, when Hh-containing vesicles were loaded onto a Hhimmunoaffinity column we found that Hh was detectable in fractionseluted from the column, confirming the presence of Hh epitopes on thesurface of vesicles (Fig. 3f).
We next identified the proteins present in the Hh-containing vesiclesby performing mass spectrometry on the P120 Hh fraction. We identifiedabout 160 proteins, including the Hh amino-terminal peptide, whichcontains all the signalling activity of Hh (Supplementary Table 2). Theseproteins included nine ESCRT protein members, five of them (AliX,Chmp1, Chmp3, Vps32 and Vps4) being the ones that affect Hh secre-tion in vivo.
We further investigated ESCRT function using electron microscopy.Careful examination of the plasma membrane and luminal space betweenthe columnar and peripodial cells revealed the presence of extracel-lular vesicular profiles in the luminal space, while MVB morphologywas not significantly affected (Fig. 3g–k and data not shown). Interest-ingly, these structures are bigger in size and their number is decreasedin Chmp1 RNAi- and in Vps4DN-expressing discs (Fig. 3g–k). The accu-mulation of Hh at the surface of producing cells (Fig. 2g–l), combinedwith the results of our electron microscopy study, suggest that, as forexovesicle release from HEK293T cells8 and in Caenorhabditis elegans9,Hh may be released through plasma membrane budding into the extra-cellular space. We therefore analysed the in vivo association of extra-cellular Hh with ESCRT proteins in more detail. In WIDs expressinggreen fluorescent protein (GFP)-tagged Vps32 in Hh-producing cellswe observed dynamic Vps32–GFP-positive particles in the luminalspace between columnar epithelial and squamous peripodial cells, ata distance from producing cells (Fig. 4a, b and Extended Data Fig. 7a,b). Interestingly, extracellular Hh partly colocalized with Vps32–GFP,at the apical external side of both posterior producing and anteriorreceiving Hh cells (Fig. 4c–e and Extended Data Fig. 8a–i). No suchcolocalization was observed between external Hh and an inner plasmamembrane marker (GAP43–GFP) (Extended Data Fig. 8j–l). Moreover,endogenous Chmp1-positive external particles were observed at variousplaces in the luminal space (Fig. 4f–h and Extended Data Figs 7c–f, 9).Importantly, extracellular Hh–GFP was found to be colocalized withendogenous Chmp1 in the luminal space (Fig. 4f–h and Extended DataFig. 7c–f).
It has been previously shown that Hh is present in the fly haemolymph23,which we therefore used as another in vivo model to analyse Hh secre-tion. In this tissue, we found similar overlap between Vps32–GFP andendogeneous Hh on secreted particles (Fig. 4i–k). Such overlap was alsofound with endogeneous AliX (for validation of the anti-AliX antibodysee Extended Data Fig. 5i), but not with GAP43–GFP or Vps36–GFP(Extended Data Fig. 10), suggesting that, in this tissue also, Hh is asso-ciated with particles that are positive for a subset of ESCRT proteins.
If Hh is secreted together with ESCRT proteins, then misexpressionof the Hh-receptor Ptc should trap both Hh and ESCRT proteins at thecell surface. We investigated this possibility by expressing a mutant formof Ptc, Ptc(1130X), in the Hh-receiving cells at the anterior–posteriorborder. Ptc(1130X) can bind and sequester external Hh but cannot beinternalized. Consistent with a potential role of Chmp1-positive exo-vesicles in the delivery of Hh to its target tissue, Ptc(1130X) expressionled to the trapping of endogenous Chmp1 and Hh at the surface of recip-ient cells in the anterior compartment (Fig. 4l–n). By contrast, Ptc(1130X)misexpression did not cause any detectable trapping of Lpp (Fig. 4o–q),indicating that ESCRT/Hh-positive exovesicles are distinct from previ-ously described Lpp-positive Hh carriers5.
Our findings provide evidence for a previously unidentified mechanismfor Hh release and intercellular communication (see SupplementaryDiscussion). Overall, our results suggest a new mechanism for morpho-gen secretion, in which a pool of signalling molecules follows an ESCRT-dependent route in morphogen-producing cells, contributing to theestablishment of a morphogen gradient. We believe that this mechanism
of Hh secretion may be conserved, as the vertebrate counterpart of Hh,Sonic hedgehog (Shh), has been observed on exovesicles in the mouseventral node24. However, it remains to be determined whether mam-malian ESCRTs are involved in this process and whether functionallyactive vesicular Shh spreads through the extracellular space.
Y
Y
Y
Y
Y
Y
Y
Y
Y
Y
Y
Y
Y
Native GFP
exHh
FM4-64
native GFP
b
c d e
Vps32–GFP;
hhGal4<UAS-Hh
Ptc(1130X)/PtcGal4
Chmp1 l
FM4-64 Native GFP
t1 t2
t3
t4
n
l c
p
HhChmp1
GFP exHh
YY
YY
YY
Y
YY
YYY
YY
YYYY
YYY
Hh–GFP;hhGal4 GFP Chmp1 GFP
Chmp1
f g h
c
l p
Hh
p
c
a l
Vps32–GFP;hhGal4
Ptc(1130X)/PtcGal4
HhHhHhHhHhHHhHhHhHhHhHhHhHhHhhhhhh
Lpp
YY
YY
YY
YY
Y
YY
YY
YYY
YYYY
Y
Hh
m
o qp
Hh
Vps32–GFP;hhGal4 GFP DAPI GFP
Hh
i j k
HhLpp
FM4-64 Native GFP
Figure 4 | ESCRT-positive exovesicles transport Hh in the extracellularspace of Drosophila wing discs and haemolymph. a, X–Z section of aliving WID in which Vps32–GFP is expressed in the Hh-secreting posteriorcompartment (green cells on the right). The arrow indicates a Vps32-positiveparticle in the lumen. Scale bar, 20mm. Plasma membrane was labelled withthe vital membrane dye FM4-64 [(N-(3-triethylammoniumpropyl)-4-(6-(4-(diethylamino) phenyl) hexatrienyl) pyridinium dibromide)]. b, Temporalprojection of a time-lapse video of a Vps32–GFP particle moving through theluminal space. Single time points (acquisition time: 0.77 s per frame) areindicated in the bottom right corner (t1–t4). Scale bar, 10mm. c–e, X–Z sectionof a disc showing partial colocalization of extracellular Hh (exHh andVps32–GFP in Hh-receiving cells. Apical is to the top, anterior to the left.The anterior–posterior border is labelled with a white dashed line. Scale bar,10mm. Pearson coefficient: 0.576; M1M2 coefficients: 0.308 (Hh) and 0.593(Vps32–GFP). f–h, Colocalization of Hh–GFP and endogenous Chmp1protein in particles in the luminal space (see also Extended Data Fig. 7).Scale bar, 10mm. i–k, Colocalization of Hh and Vps32–GFP in non-cellularparticles within the haemolymph. White circles mark 49,6-diamidino-2-phenylindole (DAPI)-positive blood cells, white arrows indicate examples ofcolocalization. Scale bar, 20mm. l–q, Trapping of Hh and Chmp1 but not Lpp atPtc(1130X)-expressing cells. Scale bar, 20mm. c, columnar cells; l, lumen;p, peripodial cells. Magnification is shown in the bottom-right corner of c–e,l–n and o–q. Schemes on the right column indicate position of columnar cells(in red) that express the drivers used in a–q. Green circles indicate Hh, andblack branches indicate Ptc(1130X) proteins.
RESEARCH LETTER
1 0 2 | N A T U R E | V O L 5 1 6 | 4 D E C E M B E R 2 0 1 4
Macmillan Publishers Limited. All rights reserved©2014

Online Content Methods, along with any additional Extended Data display itemsandSourceData, are available in the online version of the paper; references uniqueto these sections appear only in the online paper.
Received 27 September 2013; accepted 8 September 2014.
1. Briscoe, J.&Therond, P.P. ThemechanismsofHedgehogsignalling and its roles indevelopment and disease. Nature Rev. Mol. Cell Biol. 14, 416–429 (2013).
2. Mann, R. K. & Beachy, P. A. Novel lipid modifications of secreted protein signals.Annu. Rev. Biochem. 73, 891–923 (2004).
3. Rusten, T. E., Vaccari, T. & Stenmark, H. Shaping development with ESCRTs. NatureCell Biol. 14, 38–45 (2012).
4. Zeng, X. et al. A freely diffusible form of Sonic hedgehog mediates long-rangesignalling. Nature 411, 716–720 (2001).
5. Panakova, D., Sprong, H., Marois, E., Thiele, C. & Eaton, S. Lipoprotein particles arerequired for Hedgehog and Wingless signalling. Nature 435, 58–65 (2005).
6. Sanders, T. A., Llagostera, E. & Barna, M. Specialized filopodia direct long-rangetransportofSHHduringvertebrate tissuepatterning.Nature497,628–632 (2013).
7. Bischoff, M. et al. Cytonemes are required for the establishment of a normalHedgehog morphogen gradient in Drosophila epithelia. Nature Cell Biol. 15,1269–1281 (2013).
8. Nabhan, J. F.,Hu,R.,Oh,R.S.,Cohen,S.N.&Lu,Q. Formationandreleaseofarrestindomain-containing protein 1-mediated microvesicles (ARMMs) at plasmamembrane by recruitment of TSG101 protein. Proc. Natl Acad. Sci. USA 109,4146–4151 (2012).
9. Wehman, A. M., Poggioli, C., Schweinsberg, P., Grant, B. D. & Nance, J. TheP4-ATPase TAT-5 inhibits the budding of extracellular vesicles in C. elegansembryos. Curr. Biol. 21, 1951–1959 (2011).
10. Raposo, G. & Stoorvogel, W. Extracellular vesicles: exosomes, microvesicles, andfriends. J. Cell Biol. 200, 373–383 (2013).
11. Torroja, C., Gorfinkiel, N. & Guerrero, I. Mechanisms of Hedgehog gradientformation and interpretation. J. Neurobiol. 64, 334–356 (2005).
12. Gomez-Skarmeta, J. L.&Modolell, J.araucanandcaupolicanprovidea linkbetweencompartment subdivisions and patterning of sensory organs and veins in theDrosophila wing. Genes Dev. 10, 2935–2945 (1996).
13. Vaccari, T. & Bilder, D. The Drosophila tumor suppressor vps25 preventsnonautonomous overproliferation by regulating notch trafficking. Dev. Cell 9,687–698 (2005).
14. Herz, H.-M. et al. vps25 mosaics display non-autonomous cell survival andovergrowth, and autonomous apoptosis. Development 133, 1871–1880 (2006).
15. Thompson, B. J. et al. Tumor suppressor properties of the ESCRT-II complexcomponent Vps25 in Drosophila. Dev. Cell 9, 711–720 (2005).
16. Vaccari, T. et al. Comparative analysis of ESCRT-I, ESCRT-II and ESCRT-III functionin Drosophila by efficient isolation of ESCRT mutants. J. Cell Sci. 122, 2413–2423(2009).
17. Burke, R. et al. Dispatched, a novel sterol-sensing domain protein dedicated to therelease of cholesterol-modified hedgehog from signaling cells. Cell 99, 803–815(1999).
18. Babst, M., Sato, T. K., Banta, L. M. & Emr, S. D. Endosomal transport functionin yeast requires a novel AAA-type ATPase, Vps4p. EMBO J. 16, 1820–1831(1997).
19. Fujita, H. et al. A dominant negative form of the AAA ATPase SKD1/VPS4 impairsmembrane trafficking out of endosomal/lysosomal compartments: class E vpsphenotype in mammalian cells. J. Cell Sci. 116, 401–414 (2003).
20. Rusten, T. E. et al. ESCRTs and Fab1 regulate distinct stepsof autophagy. Curr. Biol.17, 1817–1825 (2007).
21. Raiborg, C. & Stenmark, H. The ESCRT machinery in endosomal sorting ofubiquitylated membrane proteins. Nature 458, 445–452 (2009).
22. Gallet, A., Ruel, L., Staccini-Lavenant, L. & Therond, P. P. Cholesterol modification isnecessary for controlled planar long-range activity of Hedgehog in Drosophilaepithelia. Development 133, 407–418 (2006).
23. Palm, W. et al. Secretion and signaling activities of lipoprotein-associatedHedgehog and non-sterol-modified Hedgehog in flies and mammals. PLoS Biol.11, e1001505 (2013).
24. Tanaka, Y., Okada, Y. & Hirokawa, N. FGF-induced vesicular release of Sonichedgehog and retinoic acid in leftward nodal flow is critical for left–rightdetermination. Nature 435, 172–177 (2005).
Supplementary Information is available in the online version of the paper.
Acknowledgements This work was supported by the French Government (NationalResearch Agency, ANR) through the ‘‘Investments for the Future’’ LABEX SIGNALIFE(program reference number ANR-11-LABX-0028-01) and by the Fondation pour laRecherche Medicale (reference number DEQ20110421324). T.M. and F.W. weresupported by the Fondation Association pour la Recherche Contre le Cancer (ARC) andLigue Nationale Contre leCancer, respectively.M.F. was supportedbyATIP/Avenir, ARCand the Human Frontier Science Program. The authors acknowledge S. Pagnotta fortechnical support. We thank K. Haglund for sharing the anti-AliX antibody beforepublication. We thank L. Staccini-Lavenant and M.-A. Derieppe for technical help.
Author Contributions P.P.T. conceived the project and supervised the study. P.P.T. andT.M. designed the genetic studies. T.M. performed all the genetic tests and most of theimmunohistochemistry on WIDs. F.W. and P.P.T. designed the biochemical studies.F.W. performed the biochemistry/molecular biology experiments. M.F. designed andperformed the live imaging experiments and generated the Chmp1b antibody. M.F.and S.Po. analysed the localization of Hh, Chmp1 and Lpp in wild-type animals and indiscs expressing Vps4DN, Ptc(1130X) and Lpp RNAi. G.D’A. produced reagents andcontributed to the experimental design of extracellular labelling of Hh. S.Pi. performedthe electron microscopy and immunoelectron microscopy analysis, and performed thecounting on the electron microscopy data. P.P.T., M.F., F.W., S.Pi. and T.M. analysed theindividual collected data sets. P.P.T. and T.M. assembled and edited the figures. P.P.T.wrote the manuscript. All authors commented on the manuscript versions. S.Pi. andS.Po. contributed equally to this work.
Author Information Reprints and permissions information is available atwww.nature.com/reprints. The authors declare no competing financial interests.Readers are welcome to comment on the online version of the paper. Correspondenceand requests for materials should be addressed to P.P.T. ([email protected]).
LETTER RESEARCH
4 D E C E M B E R 2 0 1 4 | V O L 5 1 6 | N A T U R E | 1 0 3
Macmillan Publishers Limited. All rights reserved©2014

METHODSFly stocks and genetics. Fly stocks were grown under standard conditions. Forinvestigations of the effect of ESCRT RNAi in Hh gain-of-function conditions, thew;tubGal80ts;hhGal4, UAS-Hh/TM6b,Tb ‘tester line’ was crossed with either con-trol or ESCRT RNAi lines. Starter crosses were flipped every 12 h at 25 uC, to syn-chronize the progeny, and vials with L2 instar larvae were transferred to 29 uC. Theinduction of upstream activating sequence (UAS)-Hh expression in this setup leadsto strong pharate adult lethality, the amount of eclosed escaper flies in the controlw;tubGal80ts/1; hhGal4, UAS-Hh /1 genotype is below 1%. For the WID mor-phology assay, non-Tb larvae were dissected at the L3 larval instar stage. As a con-trol for suppression of Hh-dependent outgrowth, we used the Disp10004GD RNAiline, which gives no phenotype in otherwise wild-type animals (data not shown),confirming the greater sensitivity of our experimental set-up for defects in Hh secre-tion. In Hh loss-of-function experiments we used the hhGal4 (enhancer trap for thehh locus, weakly hypomorphic by itself)/hhts2 (conditional null allele25) heteroalleliccombination, which is viable at permissive temperature (18 uC) and semi-lethal(up to 60% of the flies die as pharate adults) after incubation at restrictive temper-ature (29 uC) for 3 days. For simplicity, we refer in the text to hhts2 for hhts2/hhGal4.ESCRT RNAi;hhts2/SM6bTM6b,Tb males were crossed with w;dpp-nuc-lacZP10638;hhGal4/SM6bTM6b,Tb virgin females at 18 uC. Crosses were kept at 18 uC and flippeddaily. Vials containing L1 larvae were shifted to 29 uC, and non-Tb L3 larvae weredissected after 4 days of induction. For visualization of the changes in the levels ofendogenous extracellular Hh, wild-type control or ESCRT RNAi lines were crossedwith apGal4, dpp-nuc-lacZP10638;MKRS/SM6bTM6b,Tb. Crosses were kept at 25 uC,flipped every 12 h, and non-Tb L3 larvae from the progeny were dissected for fur-ther analysis.
The RNAi lines used in this study were: Ci105620KK (VDRC), Disp10004GD (VDRC).Detailed description about the usage of ESCRT RNAi-s and further ESCRT propertiescan be found in Supplementary Table 1. The other alleles used were w1118 as a wild-type control; hhts2 (Bloomington #1684), iro-lacZ26, the nuclear dpp-nuc-lacZP10638
reporter (provided by L. Xinhua), UAS-Ptc1130X (Bloomington #44612)27.Live imaging of Vps32–GFP in Drosophila WIDs. For visualization of thedistribution of Vps32–GFP in living WIDs, animals of the UAS-Vps32-GFP/1;hhGal4,tubGal80ts/1 genotype were kept at room temperature until the late thirdlarval instar stage and were then shifted to 29 uC the evening (or for 6 h when look-ing at both Hh and Vps32–GFP distribution in fixed discs) before dissection.
Dissected WIDs were mounted in Drosophila Shields & Sang M3 medium (Sigma)supplemented with FM4-64 (1:2,000 from 10mgml21 stock, Invitrogen F34653) and0.7% low melting point agarose.Adult wing preparation. Adult flies were collected in glycerol:ethanol (3:1) andstored for at least 24 h before dissection. Adult wings were removed with forceps attheir hinge and briefly rinsed in 96% ethanol before mounting in Euparal, as pre-viously described28. Preparations were kept at 60 uC on a slide-heating plate over-night before image acquisition. Bright-field images were taken with a Leica Axioplan2microscope (objective, 35; Plan NEOFLUAR; NA 5 0.15) equipped with a LeicaDFC490 digital camera. For image acquisition, we used LAS v.2.5.0 R1 (build 975).Dissections and immunohistochemistry. Conventional staining was performedas described previously29. For imaging, WIDs were mounted in VECTASHIELDmounting medium (Vector Laboratories).
For extracellular Hh and GFP staining, L3 larvae were collected and cleaned inice-cold PBS. Larvae were then dissected in ice-cold Schneider’s medium (Sigma)and incubated with primary antibodies in Schneider’s medium at 4 uC for 1 h. Fol-lowing this step, samples were rinsed three times with ice-cold PBS and fixed for15 min in PBS plus 2% formaldehyde at 4 uC. Fixed tissue then was washed withPBS (three times for 10 min each in PBS at room temperature) and blocked by incu-bation for 30 min in PBS plus 2% bovine serum albumin (BSA). Secondary anti-bodies in PBS plus 2% BSA were added and the samples were incubated for 1 h atroom temperature and then washed in PBS and mounted as described earlier.
For the visualization of exovesicles containing Hh–GFP and endogenous Chmp1protein in WIDs, animals of the hhGal4,tubGal80ts/UAS-Hh-GFP genotype werekept at room temperature until the late third larval instar stage and were then shiftedto 29 uC on the evening before dissection.
For analysis of the effect of Vps4DN on the localization of Hh and Chmp1, animalsof the UAS-Vps4DN/tubGal80ts;hhGal4,UAS-Hh/1 and UAS-Vps4DN/tubGal80ts;hhGal4/1 genotypes were kept at 18 uC until the late third larval instar stage andwere then shifted to 29 uC for the last 8 h before dissection.
We induced Ptc(1130X) expression in Hh-receiving cells, by keeping animals ofthe ptcGal4,tubGal80ts/UAS-ptc1130X genotype at room temperature for 24 h afteregg laying, after which they were shifted to 29 uC until dissection.
For haemolymph immunohistochemistry, ten larvae were bled on ice for 1 minand 1ml of the haemolymph was dispersed on poly-L-lysine-coated 8-well tissuechamber slides (MAT-TEK). The preparation was then dried in vacuum for 5 minand immediately fixed in PBS plus 2% formaldehyde for 20 min at room temperature.
The fixed haemolymph was then washed with PBS three times for 5 min andincubated with PBS plus 5% FCS for 1 h. Following this step, slides were incubatedwith primary antibodies overnight at 4 uC, and afterwards washed three times for10 min with PBS. Secondary antibodies were diluted in PBS plus 5% FCS and addedto the slides for 1 h. Finally, before mounting in VECTASHIELD supplementedwith DAPI, slides were washed again three times for 10 min with PBS.
Mounted WID preparations and haemolymph were imaged with a Leica TCSSP5 confocal microscope (objective, 340; HCX PLAN APO; NA 5 1.3) and the LASAF 2.6.3.8173 program, a Zeiss LSM510 (objective, 340; PLAN APO; NA 5 1.3) or aZEISS LSM780 (objective, 363; PLAN APO; NA 5 1.4). Bright-field WID imageswere taken with a Leica Axioplan2 microscope (objective, 310; Plan NEOFLUAR;NA 5 0.3).Antibodies used in this study. Primary antibodies used were as follows. Rabbitanti-Hh30 (1:500 from crude serum for conventional, 1:100 from purified serum forextracellular), rat anti-Hh (1:250, this study), mouse anti-GFP (1:1,000 for conven-tional, 1:50 for extracellular; Roche #1814460), mouse anti-Dlg (1:50; DSHB 4F3),rat anti-DE-cadherin (1:50; DSHB DCAD2), chicken anti-b-gal (1:1,000; GeneTEXGTX77365), rat anti-Ci 2A1 (provided by B. Holmgren; 1:10), mouse anti-Ptc 5E10(provided by P. Ingham; 1:400), rabbit anti-En (1:1,000; Santa Cruz sc-28640), rabbitanti-Caspase III (1:500;Cell Signaling #9661), mouse-anti-Ubi (clone FK2, 1:1,000;ENZO Life Sciences, BML-PW8810-0100), guinea-pig-anti-AliX (1:2,000; providedby K. Haglund before publication), rabbit-anti-Lpp and guinea-pig-anti-Lpp (both1:500; provided by S. Eaton).
Chmp1 protein was detected using a custom-made antibody (used at a dilutionof 1:300) generated through immunization with an antigenic peptide (QDELSQRLAKLRDQV) corresponding to a carboxy-terminal epitope of zebrafish Chmp1bthat is largely conserved in Drosophila melanogaster (Eurogentec).
Secondary antibodies used were as follows. Goat anti-rabbit Alexa488 (1:500; Invi-trogen A11034), goat anti-rabbit Alexa546 (1:500; Invitrogen A11035), goat anti-rabbit DyLight649 (1:500; Jackson Immunoresearch Laboratories 111-605-003),goat anti-rabbit Cy5 (1:200; Jackson Immunoresearch Laboratories 111-175-144),donkey anti-mouse Alexa488 (1:500; Invitrogen A21202), donkey anti-mouse Alexa546(1:500; Invitrogen A10036), donkey anti-rat Alexa488 (Invitrogen A21208), goatanti-rat Alexa546 (1:500; Invitrogen A11081), goat anti-chicken Alexa488 (1:500;Invitrogen A11039) and TRITC-phalloidin (1:100; Sigma P1951-1MG) were used.Image analysis. Bright-field and confocal images were edited in Adobe PhotoshopCS5 Extended (x64, v.12) or Fiji 1.48a. For intensity measurements, intensity pro-files were originally determined with Fiji. Raw intensity values were then furtherprocessed in Microsoft Excel 2010. To calculate Pearson and M1M2 coefficientsthe Fiji JACoP plugin was used31.Hh exovesicle isolation and partial purification. Flasks of confluent cells (from20 to 60 75-cm2 flasks) were washed the day before the experiment, with 10 ml PBS.They were then incubated overnight with 4 ml serum-free Schneider’s medium sup-plemented with 100mM CuSO4 for Hh expression induction. Conditioned mediumwas then subjected to centrifugation at 300g for 10 min and the supernatant wascentrifuged a second time at 2,000g for 20 min. The supernatant was then clearedby centrifugation at 10,000g for 30 min and then at 120,000g (a single centrifuga-tion at 160,000g gave similar results) for 90 min. The P120 pellet was resuspendedin PBS and centrifuged at 120,000g for 90 min. All steps were performed at 4 uC.The resuspended pellet was then immediately frozen in 300–500ml aliquots inliquid nitrogen, giving a typical protein concentration of 5–10mgml21, and storedfor up to 3 months at 270 uC.
Typically, we loaded 25% of the P120 obtained from 1 ml of conditioned mediumper lane. An equal amount of the S120 pellet was loaded after TCA precipitation andresuspension in 13 loading buffer. A similar protocol was followed for the isolationof exovesicles from cells not producing Hh. When indicated, vesicle preparationswere subjected to equilibrium centrifugation. In such cases, the resuspended P120was adjusted to 400ml of 45% (w/v) Optiprep (60% iodixanol stock; Axis-Shield)and layered on top of a step gradient consisting of 400ml 35%, 400ml 25% and200ml 10% Optiprep (w/v). After centrifugation for 16–18 h at 120,000g, 100mlaliquots were collected, starting from the top of the tubes, and snap-frozen in liquidN2. Immunoblotting was performed as previously described32.Immunoelectron microscopy. Fractions (7 to 9 from the Optiprep gradient) purifiedfrom Hh-expressing or non-expressing cells were fixed in 2% paraformaldehyde,deposited on Formvar carbon-coated electron microscopy grids and immunola-belled with rabbit anti-Hh TK antibody (a gift from T. Kornberg) and protein A-goldconjugates, as described previously33. Exovesicles were observed at 80 kV with a120 kV JEOL JEM-1400 electron microscope and digital acquisitions were obtainedwith a MORADA digital camera (Olympus SIS). Protein A-gold conjugates (10 nm)were purchased from the Cell Microscopy Center, Department of Cell Biology,Utrecht University.Transmission electron microscopy. WIDs were fixed in 1.5% glutaraldehyde in0.075 M cacodylate buffer and embedded in Epon according to standard procedures.
RESEARCH LETTER
Macmillan Publishers Limited. All rights reserved©2014

Seventy-nanometre sections were observed at 100 kV with a 120 kV JEOL JEM-1400electron microscope and digital acquisitions were obtained with a MORADA digitalcamera (Olympus SIS).
Both immuno- and transmission electron microscopy were performed on theCentre Commun de Microscopie Appliquee electron microscopy core facility (Uni-versite Nice Sophia Antipolis).Immunoisolation. Dynabead (Life Technologies) magnetic beads already coupledto protein A were saturated with rabbit serum containing either anti-Hh or controlIgGs. The prepared beads were then incubated with Hh vesicles from enriched frac-tions. The beads were washed according to the manufacturer’s protocol and boundmaterial was eluted with 0.1 mM glycine pH 2 and subjected to western blotting.Hh response assay. On the day before the assay, Cl8 cells were used to seed six-well plates at 50% confluence. The following day, the cells were washed in PBS andincubated with P120 or S120 fractions (from the equivalent of 3 ml of Hh-producingCl8-cell conditioned medium) for 1 day. Cells were then scraped off the plates, washedtwice and lysed. The lysate was centrifuged at 12,000g and the supernatant wasanalysed by western blotting for Fused mobility shift (anti-Fu34 1:1,000). Equalrelative amounts of P120 and S120 were analysed, to determine the amount of Hhbefore and after incubation with Cl8 cells, by western blotting (anti-Hh: 1:1,000;data not shown).Western blot on rescued WIDs. One-hundred rescued discs of ESCRT RNAi/tubGal80ts;hhGal4, UAS-Hh/1 genotype or controls (wild type, Disp10004GD) werecollected into ice-cold PBS, and extracted in Laemmli buffer. The lysate was ana-lysed on 12% SDS–PAGE and developed with anti-Hh and anti-Tubulin. Banddensity was measured with Fiji and raw data were analysed in Microsoft Excel.Hh-P120 analysis by combined liquid chromatography with tandem mass spec-trometry. Mass spectrometry analysis was performed on the Hh-containing P120fraction obtained from Hh-producing Cl8-cell conditioned medium. Processed sam-ples were sent to the European Molecular Biology Laboratory (EMBL) mass spectro-metry core facility centre (http://www.embl.de/proteomics/proteomics_services) andwere treated according to the facility protocol (detailed protocol can be providedupon request). In brief, peptides resulting from either in-gel or in-solution digestion
with trypsin were analysed by combined liquid chromatography with tandemmass spectrometry (LC-MS/MS) on a nano-high-performance liquid chromato-graphy (HPLC) system (Proxeon) coupled directly to either an LTQ Orbitrap Velos(Thermo Fisher Scientific) or MaXis Q-Tof (Bruker). Data were processed usingeither MaxQuant (v.1.0.13.13) or within Hystar (Bruker) to generate files that couldbe submitted to Mascot (Matrix Science) for database searching against a UniProtspecies specific database (D. melanogaster).
25. Ma, C., Zhou, Y., Beachy, P. A. & Moses, K. The segment polarity gene hedgehog isrequired for progression of the morphogenetic furrow in the developingDrosophilaeye. Cell 75, 927–938 (1993).
26. Letizia, A., Barrio, R. & Campuzano, S. Antagonistic and cooperative actions of theEGFR and Dpp pathways on the iroquois genes regulate Drosophila mesothoraxspecification and patterning. Development 134, 1337–1346 (2007).
27. Johnson, R. L., Milenkovic, L. & Scott, M. P. In vivo functions of the patched protein:requirement of the C terminus for target gene inactivation but not Hedgehogsequestration. Mol. Cell 6, 467–478 (2000).
28. Adler, P. N., Charlton, J. & Vinson, C. Allelic variation at the frizzled locus ofDrosophila. Dev. Genet. 8, 99–119 (1987).
29. Gallet, A., Ruel, L., Staccini-Lavenant, L. & Therond, P. P. Cholesterol modification isnecessary for controlled planar long-range activity of Hedgehog in Drosophilaepithelia. Development 133, 407–418 (2006).
30. Gallet, A., Rodriguez, R., Ruel, L. & Therond, P. P. Cholesterol modification ofHedgehog is required for trafficking and movement, revealing an asymmetriccellular response to Hedgehog. Dev. Cell 4, 191–204 (2003).
31. Bolte, S. & Cordelieres, F. P. A guided tour into subcellular colocalization analysis inlight microscopy. J. Microsc. 224, 213–232 (2006).
32. Ruel, L., Rodriguez, R., Gallet, A., Lavenant-Staccini, L. & Therond, P. P. Stability andassociation of Smoothened, Costal2 and Fused with Cubitus interruptus areregulated by Hedgehog. Nature Cell Biol. 5, 907–913 (2003).
33. Thery, C., Amigorena, S., Raposo, G. & Clayton, A. Isolation and characterization ofexosomes from cell culture supernatants and biological fluids. Curr. Protoc. CellBiol. Chapter 3, Unit 3.22 (2006).
34. Therond, P. P., Knight, J. D., Kornberg, T. B. & Bishop, J. M. Phosphorylation of thefused protein kinase in response to signaling from hedgehog. Proc. Natl Acad. Sci.USA 93, 4224–4228 (1996).
LETTER RESEARCH
Macmillan Publishers Limited. All rights reserved©2014

Extended Data Figure 1 | ESCRT depletion in posterior cells rescues the Hhgain-of-function phenotype in adult Drosophila wings. a–d, Morphologyand dpp-lacZ expression pattern of wild-type (WT; a, b) and Hh-overexpressing WIDs (c, d; tester line). Scale bar, 100mm. Dorsal is towards thetop and anterior is to the left. The white arrow in c indicates anterior outgrowth,whereas in d it points to cells expressing ectopic dpp-lacZ. e, Quantification(mean of the ratios of absolute values) of the v3–v4 intervein space (shorter blueline on f) relative to the whole wing span (longer blue line on f) normalizedto the tester line. Sample numbers (indicating the number of wings examined)
are shown at the top of each column. f–k, Representative examples ofcontrols (f–i) and wings of tester line plus ESCRT RNAi (j, k) escapers. Theamount of eclosed escaper flies in the control w;tubGal80ts/1;hhGal4,UAS-Hh/1 genotype is below 1%. l, hhGal4/hhts2 mutant wing from animals kept atrestrictive temperature for 4 days from L1 larval stage. m, Representativewestern blot of WID lysates from rescued discs (ESCRT RNAi, UAS-Hh;hhGal4). n, Quantification of band density from m. HhNp values werenormalized to tubulin values in each individual case.
RESEARCH LETTER
Macmillan Publishers Limited. All rights reserved©2014

Extended Data Figure 2 | Mild ESCRT RNAi knockdown does not leadto apoptosis or cell polarity changes. a–g, Control (a, b) and ESCRTRNAi (c–g) discs stained with antibodies against the apoptotic marker CaspaseIII (green). Peripodial nuclei are labelled with DAPI (red). Rescued discswith wild-type appearance did not display any sign of apoptosis or cell-architecture defects with the exception of Vps22, investigation of which was notfurther pursued. Scale bar, 100mm. h–k, X–Z section of WIDs expressingESCRT RNAi in the dorsal compartment (D) with apGal4 driver and labelledfor the polarity markers DE-cadherin (DCAD2; green) and Disc-large
(Dlg; red). The ventral domain (V) is wild type. Scale bar, 20mm. l–o, Secretionof Dlp is not affected in discs expressing ESCRT RNAi in the dorsalcompartment for 12–30 h. ECDlp, for extracellular Dlp protein. p–s, DispHA
subcellular localization is not affected in Hh-producing cells simultaneouslyexpressing ESCRT RNAi for 30 h. In all X–Z images, apical is towards thetop and dorsal is to the left. The dorsal–ventral boundary is indicated by adashed white line. White arrowheads in p–s indicate the apical side of thecolumnar cells.
LETTER RESEARCH
Macmillan Publishers Limited. All rights reserved©2014

Extended Data Figure 3 | The anterior expression of Engrailed and Patchedis not affected by ESCRT RNAi in conditions of Hh gain of function.a–l, In all images, dorsal is towards the top and anterior is to the left.
En (a–f) and Ptc (g–l) expression in control discs (a–c, g–i) and discs with testerline plus ESCRT RNAi (d–f, j–l) in the posterior compartment. White trianglesindicate the anterior–posterior border. Scale bar, 50mm.
RESEARCH LETTER
Macmillan Publishers Limited. All rights reserved©2014

Extended Data Figure 4 | Ptc production is maintained in conditions inwhich iro-lacZ and dpp-lacZ expression is reduced by ESCRT RNAi.a–d, The long-range target iro-lacZ expression is reduced in discs expressingESCRT RNAi in the posterior compartment. The red dashed lines indicate theareas used for the quantification in e, f. e, f, Quantification of the expressionarea (ratio of the area of iro-expressing cells to the whole WID pouch) (e)and intensity (where absolute average intensity values of iro-lacZ-expressingcells in the WID pouch were normalized to the background) (f) of iro-lacZexpression in ESCRT RNAi discs compared with wild type. g–j, Ptc (g, i)and En (h, j) expression in wild type and conditions of Chmp1 RNAi inposterior cells. White triangles indicate the anterior–posterior (A/P) border.k, Quantification of Ptc protein expression in discs expressing ESCRT RNAi inthe posterior compartment. l–o, Representative examples of the decrease indpp-lacZ expression in hhts2 discs with RNAi against ESCRT in posterior cells(ESCRT RNAi;hhts2) grown at restrictive temperature for 4 days from L1
onwards. At restrictive temperature, the hhts2 allele is semi-lethal, with upto 60% of the flies dying as pharate adults (Methods). The surviving fliesdisplayed a 50% decrease in the v3–v4 intervein space (Extended Data Fig. 1e, l).dpp-lacZ expression, which was mildly affected in the hhts2 WID (l, p), becamestrongly reduced in all ESCRT RNAi discs in the hhts2 background analysed(m–p). A magnification of the dpp-lacZ stripe is shown for each genotype belowthe panels. p, Quantification of dpp-lacZ expression in wild-type, hhts2 andESCRT RNAi;hhts2 discs. q–t, Ptc expression in conditions of ESCRT RNAi;hhts2
discs. ESCRT RNAis had no effect on the decrease of Ptc expression observed inhhts2 WIDs, suggesting that Hh long-range activity is sensitive to ESCRTfunction, but not short-range Hh signalling (q–t). On all fluorescence images,dorsal is shown to the top and anterior is to the left. Scale bar, 50mm.b–d, l–o, Numbers on bottom right indicate number of discs displayingreduced iro or dpp activity per whole population analysed.
LETTER RESEARCH
Macmillan Publishers Limited. All rights reserved©2014

Extended Data Figure 5 | Specificity controls for ESCRT RNAi.a–e, Expression of Vps4DN (b) or ESCRT RNAi (c–e, and see also in n–p) in theposterior compartment results in the accumulation of the poly-Ubi epitopecompared with wild type (a). f–h, Chmp1 RNAi in the Hh-producing cellsleads to the depletion of Chmp1 epitopes (f), whereas overexpression ofUAS-Chmp1 in the Ptc domain results in the accumulation of the Chmp1signal (g, h). i, AliX RNAi in the Hh-producing cells leads to the depletion ofAliX epitopes. j, k, AliX RNAi in Hh-producing cells does not result in theaccumulation of either poly-Ubi or Lpp epitopes. This disc is the same as theone in Fig. 2b. l–p, Panels show four examples of discs expressing RNAi againstVps32 in the posterior compartment stained for Lpp (l), poly-Ubi (m–p,top row) and dpp-lacZ (m–p, bottom row). Note that dpp-lacZ expression can
be reduced (m, bottom panel) even in a disc with no change in poly-Ubi(m, top panel) and Lpp distribution (l). Increased poly-Ubi accumulation isnot correlated with a further decrease in dpp-lacZ expression. l, m, The discshown is the same as the one in Fig. 2c. q–t, Expression of an Lpp RNAiconstruct in the fat body using the CgGal4 (CgG4) driver decreases Lpp proteinlevels in the WID (r, t), but does not cause Hh accumulation at the apical cellsurface (q, s). Shown is a close-up of the central disc quadrant overlying theanterior-posterior compartment boundary. In all images dorsal is at the top andanterior is to the left. s, t, Note that because of general depletion of Lpp thesize of the disc is considerably reduced. a, q, s, Scale bars, 30mm. In all imagesdorsal is at the top and anterior is to the left.
RESEARCH LETTER
Macmillan Publishers Limited. All rights reserved©2014

Extended Data Figure 6 | Control experiments for exovesiclecharacterization. a, The S10 fraction was run at 160,000g for 24 h. Hh proteinsplit equally between the 160,000g supernatant and the 160,000g pellet.These two fractions induced Fu phosphorylation (P-Fused) with the sameintensity. b, The relative amount of S120-Hh and P120-Hh before and after
incubation of cells in Fig. 3c. c, Immunogold labelling of P120 fraction isolatedfrom non-Hh-expressing Cl8 cells. In this experimental condition the Hhepitope is not detectable on the surface of exovesicles. This is a control forFig. 3e.
LETTER RESEARCH
Macmillan Publishers Limited. All rights reserved©2014

Extended Data Figure 7 | Visualization of ESCRT-positive exovesicles in theluminal space of Drosophila WIDs. a, b, Live imaging of Vps32–GFP in aWID. Cell membranes are stained with FM4-64 (red). Scale bar, 20mm. a, X–Ysection through the WID pouch showing the posterior Vps32–GFP-producingcells on the right (strong green signal) and the anterior luminal space on theleft. The white square indicates the region shown at a higher magnification inFig. 4b. b, X–Z section showing the Vps32–GFP-expressing columnar cellson the right (bright green) and a Vps32–GFP-positive vesicle in the anteriorlumen. c–f, Colocalization of Hh–GFP and endogenous Chmp1 protein in theWID lumen. c–e, Transverse section through the ventral part of the wing pouch
parallel to the dorsal–ventral axis. Scale bars, 20mm. d, e, Higher magnificationviews of the region indicated by the white box in c. Note the presence ofHh- and Chmp1-positive exovesicles in the luminal space in c–f. Cell outlinesare visualized by the staining of cortical filamentous actin (phalloidin, blue).f, Transverse section through the WID across the dorsal–ventral axis. The whitesquare indicates the region shown at a higher magnification in Fig. 4f–h.Scale bar, 50mm. Schematic representations of the positions of the opticalsections (dashed yellow lines) used in c and f are shown in the bottom-rightcorner of the corresponding images.
RESEARCH LETTER
Macmillan Publishers Limited. All rights reserved©2014

Extended Data Figure 8 | Controls and additional examples for theextracellular colocalization of Hh and Vps32–GFP. a–i, Partialcolocalization of extracellular Hh and Vps32–GFP on the apical side of both theproducing (a–c) and recipient cells (d–i). Scale bar, 10mm. Pearson coefficients:0.426 (d–f) and 0.542 (g–i). M1M2 coefficients: 0.156 for Hh and 0.146 forVps32–GFP (d–f); 0.284 for Hh and 0.382 for Vps32–GFP (g–i). j–l, GAP43–GFP, an inner plasma membrane marker, was expressed in the posterior
compartment. No colocalization with Hh was observed in the anteriorcompartment (Pearson coefficient: 0.009; M1M2 coefficients: 0.001for Hh and0.142 for GAP43–GFP). Note that GAP43–GFP-labelled peripodial membraneabove the columnar cells. Scale bar, 20mm. Apical is towards the top andanterior is to the left. The anterior–posterior border is marked with a dashedwhite line. Magnification of Hh punctae is shown in the bottom-right corner.
LETTER RESEARCH
Macmillan Publishers Limited. All rights reserved©2014

Extended Data Figure 9 | Endogeneous Chmp1 is secreted into the luminalspace of WID. a–d, Transverse section through the ventral part of the wingpouch across the anterior–posterior axis. Scale bars, 20mm. b–d, Highermagnification views of the region indicated by the white box in a. Note the
presence of Chmp1-positive exovesicles in the luminal space. Cell outlinesare visualized by the staining of cortical filamentous actin (phalloidin, red).Schematic representation of the position of the optical section (dashed yellowline) used in a–d is shown in the bottom-right corner of a.
RESEARCH LETTER
Macmillan Publishers Limited. All rights reserved©2014

Extended Data Figure 10 | Specificity controls for Drosophila haemolymphstaining. a–d, Endogenous Hh colocalizes with AliX on non-cellular(DAPI-negative) particles in the Drosophila haemolymph. White arrowsindicate examples of colocalization. e–h, Most of the Hh epitope is depletedfrom the circulating particles in the hhts2 homozygous mutant, which was kept
at a restrictive temperature for 4 days. i–l, GAP43–GFP expressed in allposterior cells is not secreted into the Drosophila circulation. m–p, The ESCRTII member Vps36–GFP expressed in all posterior cells does not colocalizewith endogeneous Hh in the fly blood, but is secreted as different particlesinstead. Scale bar, 20mm. White circles mark DAPI-positive blood cells.
LETTER RESEARCH
Macmillan Publishers Limited. All rights reserved©2014