THE CHEMISTRY OF LIFE CHAPTER 6. ATOMS AND THEIR INTERACTIONS.
-
Upload
kory-spencer -
Category
Documents
-
view
223 -
download
2
Transcript of THE CHEMISTRY OF LIFE CHAPTER 6. ATOMS AND THEIR INTERACTIONS.

THE CHEMISTRY OF LIFE
CHAPTER 6

ATOMS AND THEIR INTERACTIONS

ELEMENTS
Everything is made of substances called elements, no matter if it is biotic or abiotic.Elements are substances that cannot be broken down into simpler chemical substances.If we have Silver, no matter how small we break it up to be, no matter what we treat it with it is and will continue to be Silver.It is the same with every other element.

• Of all the naturally occurring elements, only about 25 are needed by living organisms.• The four most abundant are Hydrogen, Oxygen, Nitrogen, and Carbon.
(They can be remembered as HONC)• Each element can give us various information about itself based on
what we find on the periodic table….but we’ll come back to that in a few slides.

• In living organisms, some elements such as our HONC elements are very important.• Some elements, such as metals are only seen in very small
amounts and are called trace elements.


ATOMS
• An atom is the smallest particle of an element that has the characteristics of that element.• How an atom is arranged affects its’ physical and chemical
properties and behavior.• Atoms have 3 parts: protons, neutrons, and electrons.• Protons have a positive charge and a mass of 1.• Neutrons have a neutral/no charge and a mass of 1.• Electrons have a negative charge and a negligible mass.

• Atoms have a central part, called a nucleus. It contains the protons and neutrons of an atom.• Around the nucleus circulate the electrons. They are kept in a
“cloud” due to their attraction to the protons and their repulsion from other electrons. We commonly draw electrons in rings to symbolize their location.• Each element has a specific number of protons in its’ nucleus.
https://www.youtube.com/watch?v=aNK1mQfNeik

• We can find the number of protons, neutrons and electrons from the boxes on the periodic table.• The chemical symbol is one or two
letters that tell us the name of an element.
11
Na22.99

• This is the periodic table. From it we can get a lot of information.

• The lower magnitude number (usually found above the symbol) is called the atomic number. It tells us the number of protons.
11
Na22.99

• The higher magnitude number (usually found below the symbol) is called the atomic mass. It is a basic measure of the number of protons and the number of neutrons added together.
11
Na22.99

• There is also another way of representing the information. It will not look the same as the box in the periodic table, but will give us the same information. It will give us the atomic number, mass number, and charge.
23Na11

IONS
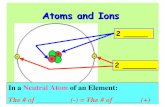
• The number of electrons in an atom usually equal the number of protons. Their charges cancel out and the atom has a charge of zero. • If the number of electrons is different
than the number of protons, then the atom will have a charge and be called an ion.
23Na11
-5

• We can tell the number of electrons by looking at the charge of the atom and working opposite.• So if an ion has a positive charge, it has more protons
than electrons.• If an ion has a negative charge, it has more electrons
than protons.• If an ion has no charge, then the two are equal.

ISOTOPES
• An isotope is when an element has different numbers of neutrons in different atoms.• The number of protons does not change. If it does,
then the element will be a different one.• Usually Carbon has 6 protons and 6 neutrons for a
mass of 12. It is referred to as Carbon-12.• Carbon-14 has 6 protons and 8 neutrons. It is very
distinctive and is used by scientists to date things.

COMPOUNDS AND BONDING• A compound is a substance that is composed of atoms of two or more
different elements that are chemically combined. (They cannot be filtered or heated apart.)• An example is NaCl (Sodium Chloride) or table salt.
• A covalent bond is when two or more atoms are combined by sharing atoms. • A molecule is a group of two or more atoms held together by covalent
bonds.• An example is water or H2O. The Hydrogens and Oxygen share electrons to
make the compound stable.

• An ionic bond is when two atoms are bonded by having opposite charges that attract. • An example is NaCl. The Chlorine takes an electron from Sodium.
This results in the Sodium becoming positive and the Chlorine becoming negative. The opposite charges then attract and form a bond.• An ionic bond is weaker and therefore is less prevalent in living
things than a covalent bond.

CHEMICAL REACTIONS
• A chemical reaction occurs when bonds between atoms are formed or broken, which releases energy or requires energy.• This causes the atoms to rearrange and form new substances.• Therefore all of the chemical reactions that occur within an
organism compose that organism’s metabolism.

2H2 + O2 2H2O C6H12O6
• A chemical equation is a visual representation of a chemical reaction. • The initial substances are called the reactants. • The final substances are called the products. • An arrow shows the direction of the reaction.
• Subscripts show the number of atoms of an element in a compound/molecule.• For example look at sugar, or glucose below. It has 6 carbons,
12 Hydrogens, and 6 Oxygens.

MIXTURES AND SOLUTIONS
• A mixture is when two or more substances which are only bound together physically and each part retains its’ properties and is not chemically rearranged.• It can be homogeneous which means that it looks like one thing. Ex.
Chocolate milk or Kool Aid.• It can be heterogeneous which means that it looks like many things. Ex. Sand
or Trail Mix• It can be a colloid dispersion, which looks like one thing, but over time
degrades into two. Ex. Heavy Cream breaks down into butter and water.

• A solution is a mixture in which one or more substances are dissolved and distributed in another, liquid substance.• A solute is the part that is dissolved.• A solvent is what it is dissolved in.
• Water is known as the universal solvent because it so often plays that role, especially in living organisms.• If a solution is not the right concentration (parts of solute per
volume of solvent) it can be harmful or not beneficial enough to an organism.

• Another important consideration of a substance is its’ pH. pH is the measure of how acidic or basic a substance is.• The pH scale runs from 0-14. • Below 7 is considered an acid. It forms Hydrogen
ions in solution (H+).• Above 7 is considered basic. It forms Hydroxide
ions in solution {OH-)• 7 is considered neutral. It has an equal number of
Hydrogen and Hydroxide ions in solution.

WATER AND DIFFUSION

WATER IS IMPORTANT!
• Water is perhaps the most important substance on earth with regards to life. It is so important because of several characteristics:• Polarity• Cohesion• Adhesion• Density• High Specific Heat

POLARITY AND HYDROGEN BONDING
• Polarity is when atoms in a covalent bond do not share electrons equally. Part of the water molecule is partially positive and part of it is partially negative.• This allows water to form hydrogen bonds which are when the
partially positive hydrogen and partially negative oxygen form a temporary bond. This is what causes cohesion.
http://programs.northlandcollege.edu/biology/biology1111/animations/hydrogenbonds.html

COHESION
• Cohesion is the tendency to of water to hold together. This is what allows for surface tension to occur.
https://www.youtube.com/watch?v=QK9mcn0Bnfg

ADHESION
• Adhesion is when water can temporarily bond to something else due to its’ hydrogen bonding. This is why water can be sucked up a straw.


HIGH SPECIFIC HEAT
• Water resists changes in temperature. This is because it has a high specific heat. This means that it takes more energy to raise the temperature of water than other substances. • Think of a pot on the stove.• This means that the earth stays within
moderate temperatures throughout the day and the year, which makes life possible.

DENSITY
• Water is the only known naturally occurring substance that is less dense as a solid than as a liquid. This is why ice floats. If ice didn’t float, it would sink to the bottom of lakes and oceans and stay there, eventually causing the entire lake or ocean to freeze.• Water is at it’s densest at about 4 degrees Celsius
and below that begins to expand again.

DIFFUSION
• Diffusion is the movement of molecules from areas of high concentration to areas of low concentration.• It can be a slow process because it requires no energy. • The speed is dependent on concentration, temperature, and pressure.
• Think of a full room versus an empty room.’• When it reaches equal concentrations in all areas it is referred to as
dynamic equilibrium. This means that there are equal concentrations everywhere, but particles are still moving.• Osmosis is the diffusion of water only.