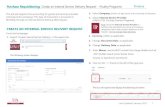
Test Request Form For Internal Tracking Use Request Form Avista Pharma Solutions Attn: Sample...
-
Upload
truongkhuong -
Category
Documents
-
view
216 -
download
2
Transcript of Test Request Form For Internal Tracking Use Request Form Avista Pharma Solutions Attn: Sample...

Test Request FormAvista Pharma Solutions
Attn: Sample Management 104 Gold St.
Agawam, MA 01001 Email: [email protected]
For Internal Tracking Use
Receipt Date/Time
Login
Page _____ of _____
Please fill out form completely Requestor Invoicing (if different from Requestor) PO # Contact Contact Quote # Company Company
Turnaround Time (TAT) Address ☐ Standard
See quote for TATs and
associated fees.
City City ☐ Tier 1 STAT State/Zip State/Zip ☐ Tier 2 STAT Phone Phone ☐ Tier 3 STAT E-mail E-mail
Sample Handling, Safety, and Disposition - Please include SDS in shipment – Complete ALL Sections Shipping
Condition (to Avista)
☐ Ambient
Sample Disposition
☐ Discard all samples Hazard Information (check all that apply) ☐ Freezer Pack ☐ Return all samples* ☐ Non-hazardous☐ Dry Ice ☐ Return unused portions only* ☐ Biological
Storage Condition (at Avista)
☐ Ambient *Return via: ☐ FedEx ☐ UPS ☐ BSL 1 Avista cannot receive and doesnot test BSL 3 materials.☐ 2-8°C *Account #: ☐ BSL 2
☐ -20°C (Additional fees may apply, see quote.) ☐ Chemical☐ -70°C Controlled Substance? ☐ No ☐ Yes Radioactive? ☐ No ☐ Yes
If yes, contact Avista for approval and to coordinate shipment/receipt of the material.
Unless noted, temperature data/ recorder returned to Requestor.
Schedule: ☐ II ☐ III ☐ IV ☐ V ☐ List DEA #:
Sample Information and Requested Testing
Quantity Lot # Material Name / Sample Description (Will be reflected on final report)
CLIENT SOP #
Test Code (See quote)
Specification (Required)
Do you want copies of the raw data? ☐ No ☐ Yes ($75 fee applies)
For the testing requested above, are microbial IDs required? ☐ No ☐ Yes
Turnaround Times Comments: (If lot number or material name/sample description listed above purposely differs from the sample label, please provide comment) MicroSeq
(MID.1161) VITEK
(MID.1162) If yes, which morphologies should be identified?
☐ 10 Days ☐ 4 Days☐ 4 Days ☐ 2 Days☐ 2 DaysTurnaround time clock begins upon attainment of an isolated culture.
☐ AllTop predominant
☐ Other (list in Comments)
☐ Do NOT transfer to MicroSeqVITEK does not identify molds. Molds and isolates which were not identified via VITEK will be automatically tested via MicroSeq unless the box above is checked. Additional fees will apply, see quote.
Submission of this executed form and the associated samples for testing signifies your acceptance of the terms and conditions incorporated in the Quote or Avista’s general terms and conditions covering these services (http://Avista.Link/TRF_Terms). A completed Request Form and Purchase Order (PO) must be submitted with your sample(s) to initiate login. Providing incomplete information may delay timeline for receiving results.
Address