[Substrate] affects rate and it changes during reaction Can measure just initial rate, V o , when...
description
Transcript of [Substrate] affects rate and it changes during reaction Can measure just initial rate, V o , when...
![Page 1: [Substrate] affects rate and it changes during reaction Can measure just initial rate, V o , when [S]>>[E] E + SESE + P](https://reader035.fdocuments.in/reader035/viewer/2022070500/56816847550346895dde27fe/html5/thumbnails/1.jpg)
[Substrate] affects rate and it changes during reactionCan measure just initial rate, Vo, when [S]>>[E]
E + S ES E + Pk1
k-1
k2
k-2
Slow stepRate-limiting
Enzyme Kinetics
maximum velocity
Velocity (V) = k [S]
![Page 2: [Substrate] affects rate and it changes during reaction Can measure just initial rate, V o , when [S]>>[E] E + SESE + P](https://reader035.fdocuments.in/reader035/viewer/2022070500/56816847550346895dde27fe/html5/thumbnails/2.jpg)
E + S ES E + P
Enzyme KineticsMichaelis-Menten
k1
k-1
k2
k-2
Michaelis-Menten equation
Derive:Assume that [P] low at start and that k-2 is very small
V0 = k2[ES][ES] hard to measure, so [Et] used[Et] = [E] + [ES], [E] = [Et] - [ES]
[ES] small because [S] so large, [Et] = [E]
Formation of ES = k1([Et] - [ES])[S]Breakdown of ES = k-1[ES] + k2[ES]
Assume steady state [ES] ~ constant, sok1([Et] - [ES])[S] = k-1[ES] + k2[ES]
Rearrange:k1[Et][S] = (k1[S] + k-1 + k2) [ES]
k1[Et][S] (k1[S] + k-1 + k2)[ES] =
![Page 3: [Substrate] affects rate and it changes during reaction Can measure just initial rate, V o , when [S]>>[E] E + SESE + P](https://reader035.fdocuments.in/reader035/viewer/2022070500/56816847550346895dde27fe/html5/thumbnails/3.jpg)
k1[Et][S]
Enzyme KineticsMichaelis-Menten
(k1[S] + k-1 + k2)[ES] =
[Et][S]
[S] + (k-1 + k2) / k1
[ES] = Km = (k-1 + k2) / k1
[Et][S]
[S] + Km [ES] =
RememberV0 = k2[ES]
k2 [Et][S]
[S] + Km V0 =
Vmax occurs when [ES] = [Et]Vmax = k2[Et]
Vmax[S]
[S] + Km V0 = Michaelis-Menten equation!!
Rate equation for 1 substrate, enzyme-catalyzed reactionKm has units of concentration
Km = Michaelis constant
![Page 4: [Substrate] affects rate and it changes during reaction Can measure just initial rate, V o , when [S]>>[E] E + SESE + P](https://reader035.fdocuments.in/reader035/viewer/2022070500/56816847550346895dde27fe/html5/thumbnails/4.jpg)
Enzyme KineticsMichaelis-Menten
Low [S], Km >> [S]Vmax[S]
Km V0 =
High [S], [S] >> Km VmaxV0 =
Vmax[S]
[S] + Km Vmax / 2 =
When V0 = 1/2 Vmax
[S]
[S] + Km 1 / 2 =
Km = [S]
Vmax[S]
[S] + Km V0 =
Michaelis-Menten
![Page 5: [Substrate] affects rate and it changes during reaction Can measure just initial rate, V o , when [S]>>[E] E + SESE + P](https://reader035.fdocuments.in/reader035/viewer/2022070500/56816847550346895dde27fe/html5/thumbnails/5.jpg)
Enzyme KineticsTransform Michaelis-Menten Equation
Double reciprocal or Lineweaver-Burk plotplot 1/V vs. 1/[S]
Straight line --> slope, y-intercept, x-interceptMore accurate determination of Vmax
Vmax[S]
[S] + Km V0 = V0 =
1 Km
Vmax[S] +
Vmax
1
![Page 6: [Substrate] affects rate and it changes during reaction Can measure just initial rate, V o , when [S]>>[E] E + SESE + P](https://reader035.fdocuments.in/reader035/viewer/2022070500/56816847550346895dde27fe/html5/thumbnails/6.jpg)
Enzyme KineticsKm and kcat
kcat = Vmax/[E]T
kcat is catalytic constant or turnover number (first order rate constant, s-1)
Km =k2 + k-1
k1
Km measurement --> affinity of enzyme for its substrate
CATALYTIC EFFICIENCY
![Page 7: [Substrate] affects rate and it changes during reaction Can measure just initial rate, V o , when [S]>>[E] E + SESE + P](https://reader035.fdocuments.in/reader035/viewer/2022070500/56816847550346895dde27fe/html5/thumbnails/7.jpg)
Enzymes can be Inhibited
Competitive inhibitor competes with substrate for active site
Noncompetitive inhibitor binds elsewhere, influencing binding at active site
![Page 8: [Substrate] affects rate and it changes during reaction Can measure just initial rate, V o , when [S]>>[E] E + SESE + P](https://reader035.fdocuments.in/reader035/viewer/2022070500/56816847550346895dde27fe/html5/thumbnails/8.jpg)
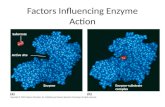
Enzymes can be Inhibited
Competitive Inhibition
Substrate
Inhibitor
Active site of enzyme
Substrate and Inhibitor can bind to active siteInhibitor prevents binding of substrate
![Page 9: [Substrate] affects rate and it changes during reaction Can measure just initial rate, V o , when [S]>>[E] E + SESE + P](https://reader035.fdocuments.in/reader035/viewer/2022070500/56816847550346895dde27fe/html5/thumbnails/9.jpg)
Enzymes can be Inhibited
Noncompetitive Inhibition
Substrate
Inhibitor
Active site of enzyme
Inhibitor binding distorts active site
Inhibitor site
Substrate can bind to active siteproduct forms
Inhibitor and substrate can bind simultaneously, rate slowed
![Page 10: [Substrate] affects rate and it changes during reaction Can measure just initial rate, V o , when [S]>>[E] E + SESE + P](https://reader035.fdocuments.in/reader035/viewer/2022070500/56816847550346895dde27fe/html5/thumbnails/10.jpg)
Enzymes can be InhibitedCompetitive Inhibition
Competitive inhibitorApparent Km will increaseNo effect on Vmax
-1/Km
Increasing concentration of inhibitor
![Page 11: [Substrate] affects rate and it changes during reaction Can measure just initial rate, V o , when [S]>>[E] E + SESE + P](https://reader035.fdocuments.in/reader035/viewer/2022070500/56816847550346895dde27fe/html5/thumbnails/11.jpg)
Enzymes can be Inhibited
Competitive Inhibition
Ingestion of methanol (gas-line antifreeze)In liver, alcohol dehydrogenase converts methanol to formaldehyde (BAD)
Ethanol competes effectively with methanol for binding to alcohol dehydrogenaseTherapy for methanol poisoning is IV with ethanol, formaldehyde not formed as readily, little tissue damage, kidneys excrete methanol
![Page 12: [Substrate] affects rate and it changes during reaction Can measure just initial rate, V o , when [S]>>[E] E + SESE + P](https://reader035.fdocuments.in/reader035/viewer/2022070500/56816847550346895dde27fe/html5/thumbnails/12.jpg)
Enzymes can be Inhibited
Noncompetitive Inhibition
0
1/V
1/[S]-1/Km
No inhibitor
+Noncompetitive inhibitor
Noncompetitive inhibitorApparent Km not affectedLowering of Vmax
![Page 13: [Substrate] affects rate and it changes during reaction Can measure just initial rate, V o , when [S]>>[E] E + SESE + P](https://reader035.fdocuments.in/reader035/viewer/2022070500/56816847550346895dde27fe/html5/thumbnails/13.jpg)
Enzymes can be Inhibited
Noncompetitive Inhibition
Also called allosteric inhibition
Example of noncompetitive inhibitor = aspirinAspirin inhibits a cyclo-oxygenase so that prostaglandins may not be synthesized, thereby reducing pain, fever, inflammation, blood clotting, etc.
Aspirin does not bind to the active site of cyclo-oxygenase but to a separate/allosteric site
![Page 14: [Substrate] affects rate and it changes during reaction Can measure just initial rate, V o , when [S]>>[E] E + SESE + P](https://reader035.fdocuments.in/reader035/viewer/2022070500/56816847550346895dde27fe/html5/thumbnails/14.jpg)
Enzymes can be Inhibited
Irreversible Inhibition - Inhibitor binds covalently to or destroys essential functional group on enzyme
Suicide inactivators - undergoes first few steps of rxn and then converts to a reactive compound that combines irreversiby with enzyme (high specificity)
INHIBITS ornithine decarboxylase (cure for African sleeping sickness)













![[Substrate] affects rate and it changes during reaction](https://static.fdocuments.in/doc/165x107/56813fce550346895daaac4e/substrate-affects-rate-and-it-changes-during-reaction.jpg)




