Studies of Trypanosoma melophagium (Flu 1908) Noller 1917 ...
Transcript of Studies of Trypanosoma melophagium (Flu 1908) Noller 1917 ...
University of Montana University of Montana
ScholarWorks at University of Montana ScholarWorks at University of Montana
Graduate Student Theses, Dissertations, & Professional Papers Graduate School
1974
Studies of Trypanosoma melophagium (Flu 1908) Noller 1917 Studies of Trypanosoma melophagium (Flu 1908) Noller 1917
(Protozoa Mastigophora) (Protozoa Mastigophora)
Sen-Chi Lu The University of Montana
Follow this and additional works at: https://scholarworks.umt.edu/etd
Let us know how access to this document benefits you.
Recommended Citation Recommended Citation Lu, Sen-Chi, "Studies of Trypanosoma melophagium (Flu 1908) Noller 1917 (Protozoa Mastigophora)" (1974). Graduate Student Theses, Dissertations, & Professional Papers. 6880. https://scholarworks.umt.edu/etd/6880
This Thesis is brought to you for free and open access by the Graduate School at ScholarWorks at University of Montana. It has been accepted for inclusion in Graduate Student Theses, Dissertations, & Professional Papers by an authorized administrator of ScholarWorks at University of Montana. For more information, please contact [email protected].
STUDIES OP TRYPANOSOMA MELOFEIAGIUM (FLU, I9O8) NOLLER, 1917 (PROTOZOA: MASTIGOPHŒÎA)
BySen—Chi Lu
B.S., National Taiwan University, I96O
Presented in partial fulfillment of the requirements for the degree of
Master of Science UNIVERSITY CP MONTANA
1974
Approved hy:
Chairman, Board of Examiners
School
/ 2 ^ a i / f / yDate ^ ^ y
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
UMI Number: EP37681
All rights reserved
INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted.
In the unlikely event that the author did not send a complete manuscript and there are missing pages, these will be noted. Also, if material had to be removed,
a note will indicate the deletion.
UMI*Oi««erUtton Publishing
UMI EP37681Published by ProQuest LLC (2013). Copyright In the Dissertation held by the Author.
Microform Edition © ProQuest LLC.All rights reserved. This work Is protected against
unauthorized copying under Title 17, United States Code
ProQ st*ProQuest LLC.
789 East Eisenhower Parkway P.O. Box 1346
Ann Arbor, Ml 48106 - 1346
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
ACKNOWLEDGMENTS
The author wishes to express his sincere appreciation to Dr. Franklin Sogandares—Bernali Professor of the Department of Microbiology, University of Montana, for his advice and guidance throughout the course of this investigation. Furthermore, I wish to express my appreciation to Drs. Mitsuru Nakamura and Richard N. Ushijima of the Department of Microbiology, Dr. Carl L. Larson of the Department of Microbiology and the Director of the Stella Duncan Memorial Research Institute, and Dr. Gregory Patent of the Department of Zoology for their assistance at various phases of this work.
11
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
TABLE æ CONTENTS
PageACKNOWLEDGMENTS................................................... iiLIST ΠTABLES ...............................................LIST OP FIGURES................... vi
CHAPTERI. INTRODUCTION .............................................. 1
SYSTEMATICS . . . . . .................................. 1HISTORICAL REVIEIV........................................ 2GEOGRAPHICAL DISTRIBUTION AND H O S T S .......................5LIFE CYCLE AND MORPHOLOGY . ............................ 6Development in Sheep . . . . . . . . . . . . . . . 6Development in Sheep Ked and Transmission . . . . . . . 7
HOST-PARASITE RELATIONS............................. . 14In Sheep ................. . . . . . . . 1 4In Sheep K e d ......................................... .14Experimental Animal Infection ............ . . . . . . I4Immunology . I5
CYTOLOGICAL STUDIES .................................... 16CU L T U R E ........... 17
STATEMENT OF Pit OB L E M ..................................... I9II. MATERIALS AND M E T H O D S ..................................... 20
GENERAL ICBTHODS AJ'ID MATERIALS........................... 20Dissection of Sheep Ked G u t ........................... 20Culture Media and Their Preparations ................ .20Isolation of T. melophagium from Sheep Ked . . . . . . . 2 2Maintenance of Cultures . . . . . . .................. 22Preparation of Stained Slides ........................ 22Counting Methods ................... . . . . 23
iii
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
IV
CHAPTER PageMeasuring Methods . . .................... . . . . . . 23Statistical Methods .................................. 26
EXPERIIffiNTAL IŒTHODS AND MATERIALS..................26Growth Studies with Different Inoculum Size . . . . . . 26Growth Studies at Different Temperatures ........ . . . 2 6Growth Studies at Different pH ........................ 26Developmental Stage Composition in Culture Smear Slides 27Morphological Studies of Trypomastigotes in MÎ.ÎMTand 199—CS—5 Incuhated at 37° C on Day Pour . . . . . 27
III. R E S U L T S ............................................. 28GROWTH STUDIES OF T. MELOPHAGIUM IN CULTURE MEDIA . . . . 28
Inoculum S i z e ................................... 28Effect of Temperature on ήD\IT Cultures of T.
melophagium................... 31Effect of pH on MMI T Cultures of T. melophagium
Incubated at 30° C . 31Growth of T. melophagium in Medium 199“CS Plus Different Concentrations of Hemolyzed Defibrinated Rabbit Blood at 27° C ....................................... 34
Growth of T. melophagium in 199—CS—5 at 37° C . . . . . 39STAGE COMPOSITION STUDIES ............................. 39miMT at 27° C ................................... 39m m T at 30° ......................................... 44m#IT at 37° c ................................... 44199-CS-5 at 37° C ............................... 44Culture Forms of T. melophagium Observed in the Cultures $1
MORPHOLOGICAL STUDIES OF THE TRYPOMASTIGOTES IN M #TAND 199-CS-5 INCUBATED AT 37° C ..................51
IV. DISCUSSION AND CONCLUSIONS ................................ 63V. SUMl^lARY..............................................70
VI. BIBLIOGRAPHY..........................................12
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
LIST ΠTABLES
Table Page1. Mensural Ranges and Means, and Nuclear and Kinetoplastic
Indices of the Trypomastigotes Cultured in MKI-IT and 199-CS- 5 on Bay Four of Incubation at 37° C ..................... 58
2. Comparison of Blood Form Trypomastigotes of T. melophagiumwith Those Cultured in M'MT and 199“CS-5 at 3Y^ C onDay F o u r ................. • « . • 6 9
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
LIST œ FIGURES
Figure Page1. Trypanosoma (Me gat rypanum) melophagium................... 9
2. Sheep keU, Lïelophagus ovinus, and its pupa . . . . . . n3* Trypanosoma ( Me gat rypanum ) mel ophagium— Life Cycle . . . . . 13
4 . Diagram of measurements of trypanosomes . . . . . . . . . . . 2 5
5» Effect of inoculum size on the growth of T. melophagium inFiiaiT at 27° and 30° ..................................... 30
6. Effect of temperature on the growth of T. melophagium inK -2 .iT ....................................................................... " ....................................................33
7» Effect of pH on the growth of T. melophagium in I@iIvITat 30° C . . . . . . . . . . . . . . . . . . . . . . . 3 6
8. Effect of concentrations of hemolyzed defibrinated rabbitblood on the growth of T. melophagium in 199~CS at 27° 0 . 38
9 . Growth curve of T. melophagium in 199—08—5 at 37° 0 . . . . . 4 I10. Changes in proportions of stages of T. melophagium in
culture in lv]I>3'iT at 27° C ....................... 4311. Changes in proportions of stages of T. melophagium in
culture in IM'.IT at 30° C ............................... 4°12. Changes in proportions of stages of T. melophagium in
culture in MWiT at 37° 0 . . . . 48
13* Changes in proportions of stages of T. melophagium inculture in 199-08—5 at 37° 0 ............................. 5®
14. Various stages of T. melophagium from cultures in M-fflTat 27°, 30°, and"37° 0, and 199-08-5 at 37° 0 ..............53
15. Trypomastigotes from four—day MHIT cultures at 37° 0 . . . . 5516. Trypomastigotes from four—day 199-08-5 culture at 37° 0 . . . 6l17# Dice squares for trypomastigotes incubated at 37° 0 in
I®MT and I99-C8 - 5 ................................... 62
VI
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
CHAPTER I
INTRODUCTION
SYSTEMATICS
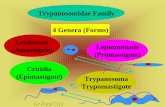
According to Hoare (1966), the classification of Trypanosoma melophagium is as follows;
Phylum PROTOZOA Goldfuss, I8 l8 j emend. Siehold, I845
Subphylum SARCŒ1ASTIG0PH0RA Honigberg & Balamuth, I963
Superclass MASTIGOFHORA Diesing, 1866 Class ZOŒÀSTIGOPHOREA Calkins, I909
Order KIHETOPLASTIDA Honigberg, I963
Suborder TRYPANOSOI-IATINA Kent, 1880Family TRYPANOSOIiATIDAE Doflein, I9OI ; emend. Grobben, 1905 Genus Trypanosoma Gruby, 1843 Subgenus Megatrypanum Hoare, I964
Species Trypanosoma (Me gatrypanum) melophagium (Flu, I90 8) Holler, 1917
The synonyms of this parasite are listed below:'Trypanosomenahnliche Flagellaten* Pfeiffer, I905
Crithidia melophagia Flu, I9O8
*Leptomonas du Melophage* Roubaud, 19O9
Leptomonas melophagi Mesnil, I909
Crithidia melophagi Swingle, I9O9
'Crithidia of Me 1 ophagus ovinus* VJenyon, 1913
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
Leptomonas melophagia Lunkerley, 1913Trypanosoma {Cystotrypanosorna) melophagi Bxximpt, 1913Trypanosoma woodcoclci Brumpt, 1913Herpetomonas melophagia Doflein, 1916Trypanosoma (Cystotrypanosoma) melophagia Brumpt, 1922
HISTORICAL REVIEW
Pfeiffer (1905) discovered the presence of a trypanosome—like organism in the gut of sheep keds in GeiPiany. These parasites were later described by Flu (19O8) as Crithidia melophagia. Flu (I9O8 ) and many subsequent observers, including Swingle (1909)» Roubaud (I9O9 ), Porter (1910), and Bunkerley (1913)» regarded it as a specific insect parasite, transmitted from ked to ked through the ova or by cysts voided with the feces. Woodcock (1909b), on the other hand, suggested that ked—flagellates represented a phase in the life cycle of a sheep trypanosome. This view had been expressed previously by Leger (I9 0 2, I9 0 4) and by Woodcock (1909a) for the Crithidia (now Blastocrithidia) of blood—sucking insects in general. In 1910, Woodcock observed a trypanosome in the blood of an English sheep and suggested the possibility that the ked-flagellate was the invertebrate phase of this parasite. His views were severely criticized by Porter (1911a, b) who claimed to have demonstrated transmission of the ked-flagellate by means of cysts or "hereditarily" through the ova of this insect. On the other hand. Woodcock (I9 II) replied by defending his opinion on theoretical grounds, since the finding of an isolated trypanosome could not be regarded as conclusive proof of the relationship.
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
The first serious attempt to solve this question experimentally was made hy Swingle (1911a, h). He examined the hlood of numerous sheep, obtained hlood cultures from them, and inoculated other sheep with the gut contents of keds infected with melophagia. He also used newly—hatched keds for xenodiagnosis. All of his work produced negative results,
Behn (19II, 1912) observed scanty trypanosomes in the hlood of German sheep. By 1912, Chatton and Delanoe ( 1 9 1 2 ) and Cauchemez ( 1912)
had denied the occurrence of this flagellate in the ova of keds,Holler (1917) presented an important contribution to this question
hy demonstrating that the culture forms of both the sheep trypanosome and the ked-flagellate were identical. Thus, he transferred C, melophagia to the genus Trypanosoma Gruby, 1843, Holler (1919^, h) also demonstrated by hémoculture the presence of trypanosomes in a large proportion of sheep in Germany, Kleine (1919) provided experimental evidence of the connection between the sheep—trypanosome and the ked-flagellate. He demonstrated that laboratory—bred keds became infected only after feeding on sheep that harbored trypanosomes in their blood. He proved that keds were unable to transmit the flagellates to one another and also reported that the ked transmitted the trypanosome to sheep by the inoculative method through the bite, Witzky (1922) and Sprehn (1923), however, examined the proboscis of numerous keds and failed to find the infective stages of the flagellates described by Kleine (1919)*
Although there was strong evidence of the connection between the sheep—trypanosome and the ked-flagellate, there still remained the possibility that keds might harbor two kinds of tryposoraatid flagellates—
one representing the invertebrate phase of the sheep-trypanosome, the
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
other a specific insect-flagellate (Bnimptt 1922; Sluiter et al., 1922). This problem was finally settled when Hoare (1923) provided morphological and experimental evidence against the existence of "cysts" in infected keds. "Hereditary" transmission of the ked-flagellates was disproved hy the inability to find organisms in the ova and larvae of keds, as well as by the absence of infection in newborn insects (Chatton and Delanoë,1912; Cauchemez, 1912; Ndller, 1913» Sikora, I9 1 8; Hoare, I9 2 1). Moreover, the possibility of any monogenetic trypanosomatid being associated with this insect was excluded by the inability of keds to infect each other (Kleine, 1919).
Finally, Holler (192O) demonstrated the true nature of the ked- flagellates by cultivating them at 37° C, and observing the transformation of epimastigote forms into typical blood forms of T. melophagium. Thus, the presence of a trypanosome in the blood of sheep had been definitely established and its connection with ked-flagellates clearly demonstrated.To that date, nothing was known regarding the exact method of its transmission from ked to sheep, nor had its complete life cycle been studied until Hoare (I9 2I, 1922, 1923) carried out experiments on all stages of development of T. melophagium in its final and intermediate hosts.
After Hoare's (1923) contribution to the morphology and life cycle studies of T. melophagium, eleven papers appeared (Section III) reporting the presence of this parasite in sheep from several countries.
Turner and Murnane (1930) reported that they were able to produce appreciable parasitaemia in a splenectomized lamb from Australia.
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
Bogdanov and Baldicina (1940) reported a case of intrauterine infection of a Still-Born lamb from Kazakhstan in which numerous trypanosomes were present in the blood.
Nelson (195^) studied the mortality of sheep keds infected with T. melophagium. Nelson (1958) also reported the study of the transfer of sheep keds from ewes to their lambs. He suggested that the infective forms present in ked feces may be airborne and may enter the sheep via the respiratory mucosa as well as by the oral route (196I).
Rodriguez Gomez (1964) reported the complement—fixation test for Trypanosomiasis melophagia of sheep in Columbia.
Herbert (19&5) reported the cytoplasmic inclusions and organelles of iji vitro cultured T. melophagium and speculated on their function.
Stewart and Beck (19&7) found the absence of the DNA-histone antigens in T. melophagium.
GEOGRAPHICAL DISTRIBUTION AND HOSTS
Little has been published on T. melophagium in recent years. Its current prevalence in sheep and its geographical distribution are not clearly delineated. The reason for this uncertainty is because of its harmless nature, non—pathogenicity and its infrequent detection by direct examination of the blood.
Scanty numbers of trypanosomes usually appear in the peripheral blood of sheep. Most authors (Woodcock, I9 IO; Behn, I9 1 2; Douwes, 1920; Dios, 1928) have detected only single trypanosomes after careful examination of numerous samples of blood, both in fresh preparations and in stained thick smears, Hoare (1923) found only several trypanosomes in
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
over a thousand blood smears (fresh and stained) taken from five sheep known infected and thoroughly examined for periods varying from several months to a year. Thus, to date the only reliable method of establishing its presence in sheep is by blood culture.
The prevalence of infection with T. melophagium is known for a few countries. In Germany, Noller (1919), and in England, Hoare (1923), demonstrated upwards of 80 per cent of the sheep examined to be infected. Probably the percentage was near to 100, since the majority of negative cultures were contaminated and were discarded. Few data are available for other countries. These were reported from Southeastern Russia (Bozhenko and Zeiss, 1928), Kazakhstan (Bogdanov and Baldicina, 1940; CeliS&ev, I94 6), North Africa (Colas—Belcour, 1931)» Canada (Nelson, 195^» 19^1)1 Argentina (Bios, 1928), Australia (Turner and Murnane, 1930;Macke rras, 1959)»
T, melophagium can be safely assumed to occur in all parts of the world where domestic sheep are kept and where there is evidence that they are infected with keds.
Nothing is known regarding the occurrence of T. melophagium in other ovine or non—ovine hosts.
Sheep keds are the only known intermediate host of T. melophagium.
LIFE CYCLE ANB MORPHOLOGY
Bevelopment in SheepIn sheep, T. melophagium is known from less than a dozen reports
of adult blood forms described by several authors (Behn, 1912; Hoare,1923; Turner and Murnane, 1930). These authors were unsuccessful in
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
discovering stages of multiplication of this trypanosome in the hlood and various organs of sheep.
The trypanosome in the blood of the sheep is of a very large size (Figure 1, a). The measurements (Hoare, 1972) of eight specimens from stained blood smears were as follows : L - 41 to 6 0 ,5 urn, P = 2.5 to7*5 urn, body width at the level of the nucleus = 2.3 to 4 urn, KN = 2.4 to 9 "um, PK = 13 to 21 um, kinetoplastic index KI = Pn/KN = 3.3 to 6.0, and the rod—shaped kinetoplast is 1 ,4 um in length. 0"or abbreviations, see Figure 4 .)
According to Hoare (1972), the prepatent period in experimentally infected sheep is from six to seven days, while the duration of infection varies considerably, usually about three months. In some cases, infection may last from one week to a month. Infection often terminates in spontaneous recovery, after which time no trypanosomes can be detected in the blood.
Development in Sheep ked and TransmissionThe entire life cycle of T. melophagium in the intermediate host,
sheep ked (Figure 2), takes place in its alimentary tract (Hoare, 1923).The complete life cycle of T. melophagium is represented diagram—
matically in Figure 3.Hoare (1923) proved the infection with T. melophagium is trans
mitted to sheep by the contaminative method, per os. He used lambs, bred from ewes that had been freed of keds and disinfected before parturition, for the experiment. Nelson (196I) recently suggested that the infective forms present in ked feces may be airborne and may enter the sheep via the respiratory mucosa as well as by the oral route.
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
8
Figure 1. Trypanosoma (Me gat rypanum) melophagium (I6OO x). a, From sheep's blood, b-e, Bpimastigotes in midgut. f and g, Promastigotes in gut. h, Amastigotes in hindgut. i, Meta- trypanosomes in hindgut. j and k, Trypomastigotes in primary hémoculture. 1 and m, Large and small epimastigotes in culture, n and o, Immature me tat rypanos ome s in old culture. (From Hoare,1923.)
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
10
Figure 2. Sheep ked, Melophagus ovinus (left), and its pupa (right). About 137 X.
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
f
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
12
Figure 3» Trypanosoma (Me gat rypanum) melophagium. Life cycle in sheep ked: 1, Trypanosome ingested with sheep's hlood.2, Large epimastigote, giving rise hy division, 3» to smaller epimastigotes, 4 and 5i in midgut. 6, Transitional epimastigote in hindgut which is transformed into small one, 8, hy division, 7* 9i 9a» 10, Two modes of division of epimastigotes, 8, leading to formation of metatrypanosomes, 10a, in hindgut, lOh, Amastigotes. (From Hoare, 1923, 1972.)
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
13
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
14
HCB7VPARASITE RELATIONS
In SheepHoare (1923) detected no signs of discomfort in naturally and ex
perimentally infected sheep kept under observation for long periods. Thus» no manifestations of disease were produced in his experimental lambs of different ages (from fourteen days to one year) and adult sheep experimentally infected with large doses of flagellates* In addition, there was no evidence that trypanosomiasis in the dam was the cause of abortion (Bogdanov and Balcidina, I94O).
In Sheep KedNelson (195^) reported that large numbers of keds have been ob
served to die on sheep as a result of blockage of the posterior midgut by large masses of epimastigotes of T. melophagium. In 1958, he also observed that only newly emerged keds are transferred to the lambs. The older keds on the ewes gradually die as a result of infection with T. melophagium.
Experimental Animal InfectionLaveran et al. (1914) found amastigotes in mice fed on the sheep
ked infected with T. melophagium. In 1919* these investigators also inoculated six mice intraperitoneally with pure cultures of this parasite.One of the mice remained uninfected, two acquired a light infection, but three were heavily infected. In these mice, intraerythrocytic forms were found in the blood and amastigotes in different organs. All the infected animals showed symptoms of a diseased condition. Galli—Valerio (1923) also claimed to have produced a similar infection in a rat.
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
15
On the contrary, Hoare (1921) failed to infect five mice inoculated intraperitoneally with the contents of the infected ked gut. In 19231 he extended the experiments to introduce the stages from the ked gut and cultures both from the ked gut and the sheep blood into laboratory animals (mice, rats, and guinea pigs) per os, intravenously, and intraperitoneally. All the results were again negative. Similar results were obtained by Buchner (1922) who inoculated the contents of ked gut into mice, per os, intraperitoneally, and subcutaneously. Becker (1923) also failed to produce any infection in rats, mice, a rabbit, and a guinea pig inoculated intraperitoneally with T. melophagium from the ked. In addition, Bogdanov and Baldicina (1940) inoculated various rodents, two rabbits, three guinea pigs, and four mice, with the blood of a heavily infected lamb, but likewise failed to detect any trypanosomes in the experimental animals.
No other animal experiments have been made since 1940. It is the opinion of Hoare (1972), however, that T. melophagium is restricted to sheep as hosts.
ImmunologyHoare (1972) believed that numbers of T. melophagium in peripheral
sheep blood are always kept at a minimum, reflecting upon a certain degree of natural immunity against infection with the flagellates. Turner and Murnane (1930) were able to produce appreciable parasitaemia only in a splenectomized lamb, supporting the views of Hoare (1972). Moreover, the low numbers of trypanosomes normally present in infections are apparently insufficient to elicit a lasting immunity. Hoare (1923) demonstrated that sheep, which had recovered from an infection and were free of
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
16
trypanosomes for up to eight months, could be readily reinfected with the second infection lasting two to four weeks. He showed also that as long as sheep are infected with keds, trypanosomes are present in the blood.If the keds are removed, however, the infection diminishes in two to three months, but reappears when sheep are again exposed to keds. The immunity in ovine trypanosomes is, therefore, due to typical premunition (Hoare,1972),
The immunological test for trypanosomiasis melophagia of sheep so far reported is the complement—fixation test by Rodriguez G&nez ( 1964) *
The only other immunological study was reported by Stewart et al, (1967), They found the absence of DNA—histone antigens in T, melophagium and suggested that this is perhaps linked with the inability of the species to survive in a free—living environment,
CYTOLOGICAL STUDIES
Herbert (19^5) studied cytoplasmic inclusions and organelles of T, melophagium cultured vitro in an enriched N, N. N. medium at 28° 0. Prom electron microscope-histochemistry studies he demonstrated a complex system of organelles (mitochondria, Golgi apparatus, endoplasmic reticulum, ribosomes, and lysosomes) and inclusions (lipid) in T, melophagium. He reported that his study did not leave one certain that structures seen in electron photomicrographs can be accurately matched with the chemical reactivities seen in the histochemical tests. However, analogy with studies on vertebrate cells permit a number of speculations which he felt might have some basis in fact.
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
17
The electron photomicrographs and histochemical studies showed increased granulation or globule formation of trypanosomes as the culture medium ceases to provide a favorable environment. He attributed this granulation in some measure to the accumulation of toxic metabolic products and to the aging of individual cells.
From his studies, he suggested that the volutin granules in trypanosomatidae are either several different inclusions or organelles, or inclusions or organelles at differing stages of development and activity.
CULTURE
NSller (1917) was the first to culture T. melophagium from both sheep blood and ked gut. The culture medium used was a modified N. N. N. (Nicolle's modification of Kovy and MacHeal's) medium.
Hoare (1923) used defibrinated sheep blood and slightly alkaline nutritive broth to establish primary culture of T. melophagium. Either Holler's or Wenyon—Noguchi's medium was used for his subcultures. The latter medium is also a modified N. N. N. medium. For his cultures from ked gut, only Holler's and Wenyon—Noguchi's media were used both for primary and subsequent cultures.
Holler (1919) observed that cultures require a minimum temperature of about 30° C, and will not grow at lower temperatures. For this reason, Hoare kept his cultures at 30° C, On the other hand, Herbert (196I) isolated and maintained T. melophagium from ked guts in an enriched H. N. H. medium incubated at 28° C. Hoare (1923) stated that in experiments using Holler's medium, the flagellates grew rapidly. Maximum development was reached at about nine or ten days then rapidly declined in numbers. On
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
18
the contrary» in Wenyon—Noguchi'8 medium the flagellates were reported to grow very slowly, sometimes taking a month to attain their full development and were found in cultures after two months.
primary cultures of T. melophagium from sheep blood, blood trypomastigotes are transformed into culture trypomastigote forms (Figure l). These culture trypomastigotes develop into a variety of epimastigotes, which in turn give rise to metacyclic trypomastigote forms. These begin to appear in old Noller's cultures after about one month. Their numbers increase both with the age of the individual culture and with the period during which the strain was under cultivation. In NGller's culture medium,T. melophagium from ked gut exhibits similar growth patterns as in the ked gut. Primary Noller's cultures from sheep blood show the same patterns as those from ked gut growing from epimastigotes, the main stages in ked gut, whereas the blood forms begin as blood trypomastigotes. Development of trypanosomes in culture may imitate normal development in the intermediate host.
The works cited above are the only ones known for the culture of T. melophagium outside of its hosts. Most of these investigators have not dealt with their data in an interpretable manner. Furthermore, the differentiation of different morphs, pH, and varied temperatures have not been studied. The only primary culture media used previously have an N. N. N. base for ked and blood forms; whereas, broth culture medium has only been used for primary cultures of blood forms.
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
19
STATEMENT CF PRCBLEI-I
Trypanosoma melophagitun seems to exhibit a high prevalence and world—wide distribution in domestic sheep and the common sheep ked, Melophagus ovinus. In spite of the fact that T# melophagium is a common parasite I very little is known of its cultural characteristics. Its diagnosis in sheep is almost entirely dependent upon its culture from infected blood. For this reason, it was decided to study the growth of T, melophagium at different inoculum sizes, temperatures, and pH in Modified Monophasic Medium for Trypanosomes (MI'MT) and Medium 199 with ten per cent calf serum containing different percentages of hemolyzed defibrinated rabbit blood. The stage composition of T, melophagium at different temperatures in media as well as the morphological studies of trypomastigote forms in î#D>îT and Medium 199 with ten per cent calf serum containing five per cent hemolyzed defibrinated rabbit blood (199—CS—5) were studied statistically.
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
CHAPTER II
MATERIAIS AND METHODS
GENERAL IŒTHCCDS AND MATERIAIS
Dissection of Sheep Ked GutSheep keds were placed on their hacks on a slide under the dis
secting microscope. The living ked was cut into two parts along the transverse suture just anterior to the second pair of legs. The portion with the head was discarded. The lateral abdominal margins of both sides of the body were cut carefully with a razor blade to avoid damaging theinternal organs. The ventral abdominal wall was then separated with a pairof scissors. One drop of antibiotic—saline (0.7 per cent NaCl withpenicillin G, 100 units/ml and streptomycin sulfate, 100 ugm/ml) wasplaced on the preparation. The ked was held fast with a needle and the abdominal wall was reflexed to the rear with a forceps. The end of the rectum was then held by the forceps and pulled to the rear, causing removal of the entire gastrointestinal tract. The midgut was then cut off for inoculating into the medium (Section III).
Culture Media and Their PreparationsOne diphasic and two monophasic media were used for the studies.
In Nicolle's modification of Novy and KacNeal's (n . N. N.) Medium, 0.7 per cent NaCl with penicillin G, 100 units/ml and streptomycin sulfate,100 ugm/ml, was used for overlay. Medium 199—08 was made by adding
20
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
21
10 per cent inactivated ($6° C for 30 minutes) calf serum into Medium 199*It was kept at 4^ C in a cold room as a stock medium. Before using, 5i 10, and 15 per cent hemolyzed defibrinated rabbit blood were added to make media 199-08—5 * 199—CS-10, and 199—08—15* Another primary monophasic medium used was Modified Monophasic Medium for Trypanosomes (M'5MT)*
The preparations of N. N. N. medium and Medium 199 are as indicated by Novy et al. (1904) and Morgan et al. (1950), respectively.
The medium I.0ÆMT described by Wang et al. (1973) was modified inone respect: calf serum replaced the horse serum. The composition andpreparation of this medium were as follows:
Tryptlcase soy broth . . ........................ . . I 6 gmDistilled water . . . . . . . . . . . . . . added to 5OO mlThe above mixture was heated gently and then autoclaved at I5 lbs
pressure at 121° C for I5 minutes. After cooling, the following materials were added.
Inactivated (56° C for 30 minutes) and filtrated calfserum ................................... 30 ml
Hemolyzed defibrinated rabbit blood (1 vol blood in3 vols distilled w a t e r ) ...................... 120 ml
Penicillin G . ................... . . . . . . 200 units/mlStreptomycin sulfate . . . .................... 200 ugm/mlThe pH values of KP.1I4T were adjusted by adding 10 per cent sodium
bicarbonate or 2 N hydrogen chloride to the trypticase soy broth beforeautoclaving.
The media were prepared and dispensed in such a manner that each culture tube ( 16 mm X I5O mm, with screw cap) contained a final volume of 5 ml*
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
2 2
Isolation of T* melophaprium from Sheep KedThe midgut was dissected from the sheep keds. Only the posterior
two—thirds of the midgut was used for inoculation into the medium. The isolated portion of midgut was then teased with two needles and washed continuously in three drops of ântihiotic—saline on the slide before inoculation into screw capped tubes containing a N. N. N. medium. The culture tubes were kept in the incubator at 27° C. Seven days after inoculation the tubes were examined for the presence of organisms by removing a bacteriological loop of medium antiseptically. If flagellates were present, they were transferred into I#IMT after an additional three days for maintenance of the flagellates.
Maintenance of CulturesFlagellates were maintained in MI#T at 27° C for ten days by
adding 1 ml of culture medium from ten-day-old culture to 10 ml of medium newly prepared or medium thawed after freezing.
For obtaining large numbers of organisms for study, large volumes of medium were used. The medium was prepared in milk dilution bottles containing 100 ml medium or 300 ml in ^00 ml bottles. In both cases, the inoculum consisted of 10 ml ten—day—old culture per 100 ml medium.
Preparation of Stained SlidesGiemsa stain was used for tincturing culture smear slides.Culture smears were prepared by placing one drop of culture medium
on a clean slide and spreading evenly with a Pasteur pipette. The preparations were air—dried before and after fixation in absolute methanol for three minutes. The fixed preparations were then tinctured with dilute
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
23
Giemsa stain (stock Giemsa <1 drop:2 ml phosphate buffered water, pH 7.0) for 35 minutes, and washed in distilled water. Preparations were made permanent with coverslip mounted in Permount.
Counting MethodsFlagellates from four culture tubes were mixed and shaken equally,
A half or one ml of the sample was removed, fixed and diluted with a solution of 10 per cent glacial acetic acid in 10 per cent formalin in a vial. From two to twenty-one different dilutions according to the population of organisms in medium were made. The preparation was then counted in an improved Neubauer hemocytometer after fixing and diluting. All counts were performed in duplicate.
Measuring MethodsThe measurements of flagellates were made by means of a camera
lucida at a magnification of 2000 X. An outline drawing was made of the projected image of each flagellate, including nucleus and kinetoplast. A line was carefully drawn through the midline of the projected figure of organism. The distance was measured by marking with a pair of dividers and correlated with a projected scale corresponding to each drawing.Where necessary, estimates of fractions were made by eye to 0.25, 0.33, 0 .5 0 , 0.66, and 0,75 of a micrometer. This method was modified from that of Bruce, Hamenton, and Bateman (I9 0 9) by use of a transparent plastic ruler instead of dial calipers.
Figure 4 indicates the six measurements of body size where they were observed.
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
24
Figure 4* Diagram of measurements of trypanosomes, L = total length (including free flagellum) ; PK =* distance from posterior end to kinetoplast; KN = from kinetoplast to middle of nucleus; PN = from posterior end to middle of nucleus; NA = from nucleus to anterior end; F = length of free flagellum, (After Hoare, 1970.)
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
25
KN
PK
NAPN
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
26
Statistical MethodsStatistical methods were employed on the morphological studies of
trypomastigote forms cultured in IM-IT and 199—CS—5 at 37° C. The statistical constant calculated included the mean, standard deviation, standard error of the mean. In order to present the statistics for the measurements graphically. Dice squares (Dice et al., 1936) showing the mean, ranges, standard deviation, and twice standard error of the mean were made. In this system if twice the standard error of the mean areas do not overlap, the differences are statistically significant. If standard deviation areas do not overlap, separation of groups is on an 84 per cent level.
EXPERIICENTAL IŒTH0D3 AND MATERIAIS
Growth Studies with Different Inoculum Size
Two different inocula, 4*5 X 10^ organisms/ml and 8.1 X 10
organisms/ml, were used for culturing T. melophagium in Î#H‘IT at each temperature of 27° and 30° C.
Growth Studies at Different Temperatures
The temperatures of 27°, 30°, and 37° C were used for growth studies in TOIT; 27° C for 199-CS-5, 199-CS-10, and 199-CS-15; and 37° C for I99-C8-5 .
The organisms were counted every two or four days for twenty days.
Growth Studies at Different pHCultures were studied for twenty days at pH values of 6.3, 6.66,
7*25, and 7»7 in MMI-IT. Cultures at pH 5.93, 8.25, and 8.55 were studied for two to eight days.
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
27
The growth curves in different inoculum sizes, temperatures, and pH were made according to the data observed.
Developmental Stage Composition in Culture Smear Slides
A minimum of 200 organisms were counted from each culture smearslide under oil immersion (1000 x) for percentages of different stages.
Morphological Studies of Trypomastigotes ill ï'ïé'ÏT and7"l99—OS—5 Incubated at 37^ C on Day Four
Fifty trypomastigotes from each of I#IMT and 199—CS-5 were measured for PK, KN, NA, and P. PN is the sum of PK and KN, while the total body length is the sum of PK, KN, NA, and P, The body width was measured from the breadth through the center of the nucleus. The nuclear index (Nl) was calculated from NI = PN/NA, and the kinetoplastic index (Kl) from KX = PN/KN,
The above measurements obtained were studied statistically.
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
CHAPTER III
RESULTS
GROWTH STUDIES OP T. IŒLOPHAGIUM IN CULTURE MEDIA -- ,---^ __■■■■■ P ■ -- ■■ ■ —
All counts reported throughout this section will he in number of organisms/ml.
Inoculum SizeIn order to establish a base line to investigate growth charac
teristics of T. melophagium in MI'IMT culture media, the effects of different concentrations of the organisms at varied temperatures were studied.
Figure 5 shows growth characteristics of cultures inoculated with8.1 X 10^ and 4*5 X 10^ in Mt/ILîT at 27° and 30° C. No lag phases were observed in each instance. At 27° 0, cultures inoculated with 8.1 X 10^ increased their numbers rapidly to attain a maximum population of 48 X 10-" on day six, six times their initial inoculum size. After this time, the number of organisms decreased rapidly. Cultures initiated with an inoculum of 4 .5 X 10^ and incubated at 27° C increased their numbers slowly and peaked at 17 X 10^ on day twelve, 2.6 fold their initial inoculum.These cultures peaked six days later than those inoculated with the larger inoculum size. The number of organisms then decreased gradually during the following days.
Similar results were obtained for cultures incubated at 30° 0. However, maximum populations were much more higher than those incubated
28
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
29
Figure 5. Effect of inoculum size on the growth of T.O 3 inO ”*melophagium in MI-fflT at 2? and 30 C
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
10
3 0 %
E
§E>o
*oo
Z
10*
6 8 16 18 2042 12 1 4O 10
3 0
Days
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
31
at 27° C. At 30° Cl cultures inoculated with 8.1 X 10^ increased their numbers rapidly and peaked at 66.6 X 10^, about eight fold their inoculum size I on day eight* This was followed by a rapid decrease during the subsequent two days, then more gradually on the following days. Cultures initiated with 4«5 X 10^ increased their numbers rapidly and peaked at
R42 X t about nine times the initial inoculum size on day ten, then decreased gradually during the following days. It also took these cultures two days longer to peak than those established with larger inoculum.
Effect of Temperature on MMMT Cultures of T. melophagium
Figure 6 shows that the optimum growth temperature for T.melophagium in I#3MT is 30° C.
OAt 37 Ci the organisms attained a population of 16X10 on day two from an initial inoculum of 8.1 X 10^. This was followed by a very
sslow increase to a peak at day six when 18 X 10- were counted. The number of organisms decreased during the following six days. Prom day twelve to sixteen the population of the organisms remained at about the same level.On day twenty the number of organisms decreased below the inoculum size.
Cultures growing at 27° and 30° C were described under the previous heading.
Effect of pH on I'II#IT Cultures of T. pielophafrium Incubated at 30° C ' ' ' '
RIn this study the size of the initial inoculum was 7*9 X 10 .In M1-3.it held at pH 5*93 the organisms did not multiply, and few sluggish organisms were found in medium taken from the bottom of the culture tube during days two to eight. At pH 8 .2 5 and 8 .5 5 only sediment in about
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
32
Figure 6# Effect of temperature on the growth of T, melophagium in ""
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
33
10
E> 3 0 C
Io'oôz
2 7
3 7
18 201 410 1 66 84 1220Days
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
34
one—third of the medium was found in each culture tube. Occasionally few disintegrated organisms were found on day two.
Figure 7 depicts MMIT cultures held at pH 6.3, 6.331 7.25» and 7*7. At pH 6.3 the organisms multiplied rapidly in the first two days then increased slowly in numbers to 26 X 10^ on day twenty. At pH 6 .6 6
the organisms increased in numbers rapidly in the first four days to 56 X 10^1 then showed a tendency toward a long stationary phase until day twenty. The maximum population at pH 6 .6 6 was seen on day twelve at 64 X 10 , eight fold its inoculum size. At pH 7«7 the cultures increased in numbers, rapidly in the first four days then increased slowly to attain a maximum population of 52 X 10^ two days later, 6 .5 times their initial inoculum size then decreased more rapidly than at any other pH.
The growth of the organisms in I®ÎT at pH 7.25 was shown previously in this chapter.
Growth of T. melophagium in Medium 199—CS Plus Different Concentrations of Hemolyzed Defibrinated Rabbit Blood at 27 C
Figure 3 shows that 199—CS—15 induced the fastest growth rate ofthe organisms in the three different media tested. The protozoans increased
15rapidly in numbers to attain a maximum population of 53 X 10 on day six,6 ,5 fold increase over its initial inoculum. Then the count decreased
5gradually to 11.5 X 10 on day twenty. On day ten a second peak in the number of organisms (39 X 10^) was observed. The second fastest growth was observed in 199-CS—10, in which the number of cells increased rapidly and peaked at 44 X 10^ on day six, 5.5 fold its initial inoculum, but decreased rapidly on the following two days to 32 X 10^ before increasing
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
35
Figure 7» Effect of pH on the growth of T. melophagium in I-miT at 30° c. ”
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
36
6.66
7 .2 56 .3E
Co2»ood
Z
7 .7
201 81 66 10 12 14842ODays
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
37
Figuré 8, Effect of concentrations of hemolyzed defibrinated rabbit blood on the growth of T. melophagium in 199—CS at 27° C.
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
38
10 I
15
10
4«AEcOeno'o
1 10
15
10
8 1 62 10 18 206 12 1 44ODays
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
39
Rslowly again to a population of 39 X 10'' on day fourteen. However, the numhers decreased slowly on the following four days then rapidly to 18 X 10^ on day twenty.
C u ltu re s o f organisms in 199—CS—5 pro v id ed th e low est numbers o f
organisms o f th e th re e d i f f e r e n t media p rep ared . Numbers o f organisms in —Rcreased rapidly during the first four days to 30 X 10 . Then the cultures
continued the same level to day twelve. On day fourteen the same cultures attained a maximum population of 37 X 10^, 4 *5 times their initial inoculum,
Rthen gradually decreased to 20 X lO* on day twenty.
Growth of fC. Ipphafrium in 199—CS—5 at 37° CAs shown in Figure 9» the organisms attained the maximum population
of 26 X 10^ on day four, three times their initial inoculum, then rapidly decreased during the following eight days. From day twelve to sixteen thepopulation of the organisms remained stationary. By day twenty, thenumber of organisms had slowly decreased to 9*7 X 10^.
STAGS CŒPCSITION STUDIES
I,miT at 27° CFigure 10 shows that the percentage of epimastigotes increased
slightly during the first four days from 92 to 9 7 * 5• The percentage of organisms decreased gradually to 45*5 on day sixteen, then increased slowly to 5 0 .5 on day twenty. On the other hand, the percentage of the degenerating forms decreased slightly during the first four days from 8.1 to 2 .9 . Subsequently, degenerating forms increased gradually to 93.5 per cent on day sixteen, then decreased slowly to 49 per cent on day twenty.
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
40
Figure 9« Growth curve of T. melophagium in 199-CS-5 at 37 C.
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
41
o'odZ
2016124 8ODays
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
42
Figure 10. Changes in proportions of stages of T« melophagium in culture in w m? at 2?° G. Di degenerating forms. E, epimastigotes* T| trypomastigotes.
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
43
CO
— M
Cls u i i o j 3 jn 4 |n 3 40 a B o i u a o j s j
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
44
A very low percentage of trypomastigotes were observed, on days six, eight, sixteen, eighteen, and twenty, varying from 0*5 per cent to 1 per cent.
at 30° CFigure 11 shows that the percentage of the epimastigotes increased
slightly during the first two days from 92 to 9 4 •5» The percentage then decreased gradually to 19 on day twenty. On the other hand, the percentage of degenerating forms decreased slightly during the first four days from8.1 to 2.5, increasing gradually to 77*5 on day twenty. The percentage of the trypomastigotes found was very low, varying from 1.5 on day two to8.5 on days fourteen and sixteen.
I3.31T at 37° CFigure 12 shows that the percentage of epimastigotes decreased
gradually from 92 on day 0 to 0 on day twenty. On the other hand, degenerating forms gradually increased in the percentage from 8.1 on day 0 to 100 on day twenty. Trypomastigotes attained their peak on day four with a percentage of 23.5. The following four days represented a stationary phase followed hy a gradual decrease to 0 on day twenty.
199-C3-5 at 37° CFigure 13 shows the percentage of epimastigotes decreased rapidly
from 92 on day 0 to 8.3 on day eight with a gradual decrease to 0 on day twenty. On the other hand, the percentage of degenerating forms increased slowly during the first four days from 8.1 on day 0 to 10.4 on day four. This was followed by a rapid percentage increase to 7^.5 on day eight then a slowed rate of increase to 98*5 on day twenty. Trypomastigotes peaked
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
45
Figure 11. Changes in proportions of stages of T. melophagium in culture in MIiMT at 30° 0. D| degenerating forms, E| epimastigotes. T, trypomastigotes.
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
46
_o
eo
— CO
— Ci
00«uuoj ajn|{n3 p
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
47
Figure 12. Changes in proportions of stages of T. melophagium in culture in MJMT at 37° C. D, degenerating forms, E» epimastigotes, Tf trypomastigotes.
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
4 8
tu
— eo
osiujo^ djn(|no @6o(uaoj@j
>.o
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
49
Figure 13» Changes in proportions o f stages o f T . melophagium in cu ltu re in 199-GS-5 a t 37° C« Di degenerating form s. E , ep im astigotes, T» trypom astigo tes .
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
5 0
CO
COsuijo^ e jn ijro aBo^uaajaj
I
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
51
at 32*5 per cent on day four, then decreased gradually to 15«2 per cent on day eight. From day eight to twelve trypomastigotes decreased slowly to1.5 per cent on day twenty.
Culture Forms of T. mel.Q.Ph.a/tLum Observed in the Cultures
Only the culture forms of T. melophagium in MI-MT incubated at 2?°, 30®, and 37° c, and in 199—GS—5 at 37° C were studied.
Epimastigotes, trypomastigotes, amastigotes, sphaeromastigotes, promastigotes, and degenerating forms have been observed (Figure 14) in both media used. No difference was observed between the kinds of the stages found in both culture media. Epimastigotes were seen abundantly during the exponential phase of cultures at 27°» 30°, and 37° G. Trypomastigotes were found in higher percentage at 37° C, lower at 30° C, and very low at 27° C. Amastigotes, promastigotes, and sphaeromastigotes were rarely seen in the cultures. Degenerating forms increased in the percentage in the later incubation period of the cultures at any temperature studied. heta— trypanosomes were rarely found in cultures and most of those found were immature.
MCHFHOLOGICAL STUDIES Œ THE TRYPOMASTIGOTES IN PM-iT AND I99-CS- 5 INCUBATED AT 37° C
Fifty trypomastigotes were measured in each medium used on day four when both cultures peaked. Some of the trypomastigotes studied are illustrated in Figures I3 and 16. The results of measurements are shown in Table 1.
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
52
Figure 14* Various stages of T. melophagium from cultures in * T at 27° I 30°, and 37° C, and 199-CS-5 at 37° C. 1-4#Various sizes of epimastigotes. 5 a.nd 6, Aberrant epimastigotes with undulating membranes partly detached or absent. 7#Aberrant epimastigote with two nuclei. 8—10, Dividing epimastigotes. 11—131 Various sizes of trypomastigotes, 14, Dividing trypomastigote, 15, Aberrant trypomastigote with twonuclei, 16—27, Different stages of metacycl©genesis. 28 and 29, Sphaeromastigotes, 30, Amastigote. 31, Promastigote.32-41, Different stages of degeneration of culture forms.
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
^— 2 3 ^
lo wm
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
54
Figure 15* Trypomastigotes from four-day M # T cultures at 37° C. (Camera lucida drawingsj at 2000 X from air-dried Giemsa preparations.)
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
5 5
10 wm
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
56
Figure 16« Trypomastigotes from four*-day 199“*CS—5 culture at 37^ C. (Camera lucida drawings, at 2000 X from air—dried Giemsa preparations*)
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
5 7
JSUtiB-
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
58
Tala le 1Mensural Ranges and Means, and Nuclear and Kinetoplastic
Indices of the Trypomastigotes Cultured in lÆMI'IT and I99—CS-5 on Day Four of Incubation at 37° C
Medium Total Length PI age Hum WidthMean 3 4.33+ 0.87 9 .82± 0 .55 1.88+0 .0 5
M'ÏÏ.IT S. D. 64I6 3 .8 8 0 .3 8
Range 20.50-4 7 .7 5 3 .50- 17.50 1.25-3 .0 0
Mean 35.38- 0.60 9 .46* 0.41 2 .01*0 .06
I99-CS- 5 S. D. 4 .2 8 2.93 0.41Range 2 5.66-4 4 .5 0 3 .33- 14.75 1.00-3.33
Medium - PK KN PN NAMean 9 .75- 0.33 3.87*0.22 13.63i 0 .4 0 10.88* 0 .4 8
M.3.IT S, D. 2.37 1.58 2 .8 4 3 .4 0
Range 4 .50-17 .00 1.50-8 .5 0 8 .00-21 .00 4 .25- 17.25
Mean 11.66i 0 .3 3 3 .97*0 .2 7 15.63* 0 .4 6 10.29* 0.41
I99-CS- 5 S. D. 2 .3 5 1.93 3.26 2.87Range 7.75- 17 .00 1.00-9 .0 0 9 .75-25 .00 4 .50-17.00
Medium Nucleus NuclearIndex
KinetoplasticIndex
Mean 2.43*0 .0 7 X 1.30*0 .0 3 1.40*0.09 3 .99* 0.29i-n.n-iT S. D. 0 .5 2 0 .2 2 0 .6 0 2 .05
Range 1.33-3 .5 0 X 0 .75- 1.75 0 .61-3.41 1.94- 8 .23
Mean 2.32*0.08 X 1.25*0 .0 4 1.68*0.09 4.70* 0 .28
199-CS- 5 S. D. 0 .5 3 0 .2 9 0.66 2.00Range 1.50-3 .3 3 X 1.00-2.00 0 .63-3 .9 2 1.97- 11.22
Note: All measurements except indices are in micrometers.
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
59
As shown in Table 1 and Figure 171 there was no significant difference between trypomastigotes measured from MI#T and those from 199~CS—5» Parameters measured included total body length, body width, length of free flagellum, NA, and size of nucleus. In M-IMT, total body length ranged from 20,5um to 47.75am (mean = 34.33\uii), body width ranged from 1,25um to 3um (mean = 1.88um) , length of free flagellum ranged from 3 .5 0um to 17.5 0am (mean = 9.82um), NA ranged from 4.25am to 17.25am (mean = 10.88um) , and size of nucleus ranged from 1.33 to 3.50um X 0.75 to 1.75am (mean = 2.43um X 1.30um). Measurements for trypomastigotes cultured in 199—OS—5 were total body length from 2 5.6 6um to 4 4.5 0um (mean = 35.38um), body width from 1.00um to 3.33am (mean = 2.01 um) , length of flagellum from 3«33um to 14.75 am (mean = 9 «48 um), NA from 4.50am to 17.00um (mean = 10.29 um) , and size of nucleus from I.5O to 3.33am X 1.00 to 2.00um (mean = 2.32um X 1.25 tun) •
A statistically significant difference between trypomastigotes from MTffîT and those from 199—CS-5 was demonstrated (Figure 17) with respect to PKj KN, PN, and nuclear and kinetoplastic indices. Measurements and calculations on trypomastigotes cultured in MI.iMT were as follows:PK from 4 .5 0am to 17.00um (mean = 9«75am) , KN from 1.50um to 8.50um (mean = 3.87am), PI'I from 8.00um to 21 .OOum (mean = 13.63am), nuclear index from 0 ,61 to 3 .4 1 (mean = 1.4 0), and kinetoplastic index from 1.94 to 8 .2 3
(mean = 3.99). Trypomastigotes cultured in 199—OS—5 yielded the following values: PK from 7.75am to 17.OOum (mean = 11.6 6um) , KN from 1.OOum to9 .OOum (mean = 3.97am), PN from 9.75am to 25.OOum (mean = 15.63am), nuclear index from O .63 to 3.92 (mean = 1.6 8), and kinetoplastic index from 1 .9 7 to 11 .22 (mean = 4 .70).
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
60
Figure 17» Dice squares for trypomastigotes incubated at 37° C in IM-IT and 199-CS-5* Extreme limits of variation shown by length of horizontal line; means marked by vertical lines; dark rectangles represent standard deviation; light areas represent twice the standard error of the mean. Where light areas do not overlap, the differences in mean are statistically significant. Where dark areas do not overlap, separation of population is on an 84 per cent level. (Additional abbreviations: W = body width; LN = nuclear length;WW = nuclear width.)
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
61
as ao 15 10T T“i rn I— pi— I— I— r-|— r i " i j— pi— i— r r n— r~t— r-r
I I I I I I I I I I I I I I— I— I— I— I— I— I— i - J — I— I— I— L
PN
NA
KN
PK
50
as
45
ao
40
15
35
1030
5
25
O um
ao[ I I r- 1— I— I I 1 1— I -I- — I I I " T - ' I .....I— I ' I I I r T" I - r
B
I I I I - I I I I I I I I I i I L-J I I— l_J— I— I— I > 1 J— I— L__L50 45 40 35 30 25 20 wm
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
12T"
12
4T 3T 2T
mm i 1 ^ » — JL— ^ — ^ a j L
T I I I I f 1--------1--------1--------r
J I I 1 L I » I I L
62
W
LN
W N
NI
0 wm
Kl
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
CHAPTER IV
DISCUSSim AND CONCLUSIONS
Culture media contained hemolyzed defibrinated blood from three different rabbits. Several workers have shown that unknown factors in blood from various rabbits may affect the growth of Trypanosoma species in blood-based culture media. For example, Dusanic (I96 8) found that variations in growth of Trypanosoma lewisi were observed in culture media prepared with blood collected from different rabbits. For this reason, he heated defibrinated rabbit blood for thirty minutes at 5^° C for use in preparing the modified diphasic medium described by Tobie et al. (1950). Variations resulting from the different sources of rabbit blood were not determined in this study. Calf sera, used for preparing the media in this study, were obtained from the same pool and were also inactivated at 56° C for thirty minutes. Thus, any factors of complement that might have affected the in vitro growth of T. melophagium could not be detected by comparison of the different cultures prepared with different sera.
The concentrations of penicillin G and streptomycin sulfate in the media may have had an effect on the growth of T. melophagium. This possibility was not ascertained in the present problem. In this regard, studies on the effects of blood and/or calf serum from various sources as well as varying the concentration of antibiotics, in culture media will be investigated in the future.
63
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
64
The size of the inoculum affected the subsequent accretion of T. melophagium in IvUttlT both at 27° and 30° C. Maximum populations in cultures initiated with larger inocula were much greater in numbers than were those initiated with smaller inocula. The former cultures developed maximum numbers two to six days earlier than the latter cultures at 30° C and at 27° C| respectively. Because aging of the culture caused dénaturation of the medium, cultures initiated with small inocula were not able to attain peak populations as high as those cultures initiated with larger inocula. However, Dusanic (1968), working with diphasic blood-agar cultures of T. lewisi, found that the growth limits were similar regardless of inoculum size. ‘He also stated that limitation of growth may be a result of the diffusion of toxic products and/or availability of substrates and growth factors. Further investigations may indicate a great variation in the limitations of different culture media.
Though no lag period in the accretion curves for T. melophagium were found, other workers (Chang, 1947; Dusanic, I968) have stated them to be present in other trypanosome species in other culture media. Cultures described in this study were not examined before the two—day post- inoculation period, and a minor decrease in counts corresponding to a lag phase may have occurred within this two—day period.
According to Holler (1919); the optimum temperature for culturing T. melophagium in Holler’s medium was 30° C. Holler (1919) stated further that T. melophagium would not grow at lower temperatures. In this study,T. melophagium was isolated from the midgut of the sheep ked in N. N. H. medium incubated at 27° C. Herbert (I9 6I) also isolated and maintained sheep trypanosomes in an enriched W. N. N. medium at 28° C. Results
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
65
obtained in this study support Holler's findings since the optimum temperature for incubation of T. melophagium cultures in I#1MT of the three temperatures used in this study (27°, 30°, and 37° C) was 30° C.
Preparing with a specific hydrogen ion concentration was difficult since the pH was adjusted in the trypticase soy broth which had a high buffering property. The addition of inactivated calf serum and hemolyzed defibrinated rabbit blood to the autoclaved I»H<nvlT with trypticase soy broth altered the pH, as did the inoculum added later. The pH values reported in this study were obtained as soon as possible after inoculation. Without adjustment, the pH of I#34T prepared according to the usual formula was 7»25»
The growth of T. melophagium in I«H«tHT of various pH values showed marked differences. Growth in media of pH 6,66 and 7-25 was more profuse than in media of pH 6.3 and 7*7. The culture forms in I®MT having a pH of 6.3 continued increasing in numbers even by day twenty, while those in I®niT at pH 6.66 showed a tendency toward a long stationary phase at least to day twenty. Cultures in IffilMT with a pH of 6.3 or 6,66 should be studied further for periods of more than twenty days.
The growth of T. melophagium in 199~CS preparations having different concentrations of hemolyzed defibrinated rabbit blood and incubated at 27° C indicated that blood concentrations were very important in their effects. The most rapid grovrth was observed in 199—OS cultures containing 15 per cent hemolyzed defibrinated rabbit blood. Further studies are needed on 199—CS containing concentrations of hemolyzed defibrinated blood greater than I5 per cent to determine the optimum concentration.
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
66
The developmental stages of T. melophagium observed in I##IT cultures incubated at 27°» 30®, and 37° C» as well as those observed from 199“CS—5 culture incubated at 37° C (Figure 14)» were identical to those reported by Hoare (1923) in Roller's and Wenyon-Noguchi's media incubated at 30® C.
When T. melophagium was cultured in I®MT medium at 30® or 37° C» the relative proportion of epimastigotes decreased and that of degenerating forms increased with greater incubation time. In I#!# cultures incubated at 27° C» however, the relative proportion of epimastigotes increased while that of the degenerating forms decreased during the final four days of incubation. This fact was probably attributable to my inability to detect numerous degenerating forms in the stained smears at days eighteen and twenty. Further evidence for an error in detection was an apparent failure of the cultures incubated at 27° C to increase in numbers as did cultures held at 30° and 37° C. Organisms in #BIT cultures incubated at 27® C did not increase during the last four days. This was apparently caused by the increasing of the number of uncountable degenerating forms in the stained smears on days eighteen and twenty.
The stage composition of T. melophagium cultured in IGIWT at pH of 6.3, 6.66, and 7*7 were not studied in this research. Castellani, Ribeiro, and Fernandes (19^7) have reported that the optimal pH in HIL medium for induction of metacyclogenesis in Trypanosoma cruzi was 6.7 at 28® C. The optimal pH required for metacyclogenesis of T. melophagium in IHGIT and 199—CS has not been determined in this study and remains to be studied.
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
67
In this study only 199~CS-5 was used to study the stage composition of T. melophai°:ium at 37^ C. The other two media, 199—CS-10 aiid 199— CS— 19, were not studied. The relative proportions of different stages of T. melophagium in 199—CS—5 at 37° C were similar to those cultured in M.n.lT at 37° C. Possibly, the stage composition of those cultured in 199—CS—10 and 199—CS—15 at 27° C are similar to those found in I€#IT cultures at27° C.
The differentiation of epimastigotes to trypomastigotes of T. melophagium in cultures in I€®T was found to occur at 27°, 30°, and 37° C, and in 199—CS—5 at 37° C. The highest percentage of trypomastigotes was found in KIvMT and 199-CS-5 at 37° C, both on day four. The trypomastigotes were found in a low percentage in M Œ T at 30° C while rarely found in the cultures at 27° C. Nc511er (I92O) was the first to demonstrate the true nature of the ked—flagellates through cultivation in Noller’s medium at 37° C when the epimastigote forms were transformed into blood forms of T. melophagium. Hoare (1923) also found trypomastigotes in cultures in Holler's and Wenyon—Noguchi's medium at 30° C. In his hands, the proportion of trypomastigotes rose from 1 per cent to 11.5 per cent by Bubculturing these forms through fourteen transfers. In this study, the proportion of trypomastigotes found in M.MT was 8 .5 per cent on day fourteen and sixteen on after the first subculture of transfer from 27° C to 30° 0. Decreased proportion of trypomastigotes may occur in the subsequent transfers at the same temperature.
The metatrypanosomes have been rarely found in the cultures in I'HlI'iT at three temperatures studied as well as in 199-CS-5 at 37° C. Almost all of these metatrypanosomes were immature. The reason for the
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
68
observed, immaturity of the metatrypanosomes may be due to the fact that a twenty—day incubation period is not sufficient time for metacyclogenesis.
Significant differences were observed in PK, KN, PN, NI, and KI, but none on total body length, body width, length of free flagellum, and size of nucleus of T. melophagium kept in and 199—CS—5 cultures onday four at 37° C.
Nuclear indices of the trypomastigotes of T, melophagium in LM'ÎT (ratio: 1,4 0) and 199“CS—5 (ratio: 1.68) show that the nucleus in formscultured in was much further apart from the anterior end of the bodythan in those cultured in 199—CS—9. Kinetoplastic indices show the kinetoplast in I-M'IT forms (ratio: 3,99) to be much further apart fromthe nucleus than in 199—CS—5 (ratio: 4*70),
If the data observed in this study (Table 2) is compared with the most reliable data of Hoare (1972) that was based on eight specimens from stained blood smears, the trypomastigotes cultured both in fflffiT and 199— CS—5 were much shorter and thinner than the blood forms. The flagellar lengths, however, were much longer in cultured than in blood forms. The PK of the culture forms were shorter than those of the blood forms. Averages of KI'I and KI indices of trypomastigotes in I.ff.lIT and 199—CS—5 were within the range of those reported for blood forms. Thus, it is clear that the trypomastigotes cultured in M M T and 199-CS-5 were much smaller in size than blood forms reported by Hoare (1972), The differences in size and morphs are probably due to the different nutritional elements and/or the growth conditions in artificial media compared to sheep blood. In view of the complexities of the undefined blood system, the problem to obtain size and morphs indicated in all the different media used is difficult to accomplish.
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
CD■DOQ.CgQ .
■DCD
o'3O
Table 2Comparison of Blood Form Trypomastigotes of T. melophagium with Those Cultured in #BIT and 199-CS-5 at 37° C on Hay Four
8
( O '3"i3CD
"nc3.3"CD
CD■DOQ.Cao3■oo
CDQ.
■DCD
observers Total Length Width Plagellum PK KN KI
Hoare (1972): In Blood 41 -60.5 2.3 -4 2.5 - 7.5 13 -21 2.4 -9 .0 3.3 - 6.0
This Paper: In imiT 20.50-47.75
(34.33)1,25-3.00(1.88)
3.50-17.50(9.82)
4 .50-17.00(9 .75)
1.50-8.50(3.87)
1.94- 8.23 (3 .99)
In 199-CS-5 25.66-44.50(35.38)
1.00-3.33(2.01) 3.33-14.75
(9 .46)7.75-17.00 (11.66)
1.00-9.00(3 .97)
1,97-11.22(4.70)
Petes: 1, All measurements except ICI are in micrometers.2, Pumbers shown in the parentheses are means.
if)C/)
CHAPTER V
SUT®1ARY
1 • The size of inoculum affected the growth of T. melophagium in M#IT both at 27° and 30° C,
2. The optimum temperature for culturing T. melophagium in M-ffllT was 30° 0.
3. Culture forms of T. melophagium survived but did not multiply after eight days in MMMT at pH 5*93 at 30° C. These forms died and disintegrated on day two in IIMI>IT at pH 8.25 and 8.55 at 30° C.
4* Culture forms of T. melophagium in M#IT at pH 6.66 and 7*25increased more profusely than those in MI-IMT at pH 6.3 and 7*7* Medium WI.II'ÎT at pH 7*25 provided the most suitable condition but the number of organisms decreased more rapidly than that at pH 6.66 after the culture had pealced.
5* Medium 199-CS-15 induced the fastest growth of the three different media (199“CS-5t 199-CS-10, and I99-CS-I5) tested.
6. In JMIT held at 30° and 37° 0» the proportion of epimastigotes decreased while that of degenerating forms increased in direct proportion to incubation time. The results were similar at 27° C to cultures held at 30° C except on days eighteen and twenty when the proportion of epimastigotes increased and that of degenerating forms decreased. Similar results were observed in 199-CS-5 held at 37° C as in I#n,IT at 37° C.
70
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
71
7» The differentiation of epimastigotes to trypomastigotes in I-HIMT cultures held at 27°; 30°, and 37° C, as well as in 199~CS-5 at 37° C* The highest percentage of trypomast igotes was found in the cultures on day four of incubation in both media incubated at 37° C. Thus, temperature seems to be a critical factor.
8. The stages of epimastigotes, trypomastigotes, amastigotes, sphaeroraastigotes, promastigotes, and degenerating forms of T. melophagium have been observed and described for H>a.IT at 27°; 30°; and 37° C as well as in 199-CS- 5 at 37° C.
9 . The morphology between the trypomast igotes in MBIT and 199—CS—5 at 37° G on day four of incubation was statistically studied and also compared with blood forms.
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
CHAPTER VI
BIBLIOGRAPHY
Beiini P* 1911. Trypanosomen beim Schafe. Berlin Tierarztl. V/ochenschr, ^:?68.
________ • 1912. Weitere Trypanosomenbefunde beim Schafe. Ibid.^:934.
Bequaertj J. 1942. A monograph of the Melophaginae, or ked-flies, of sheep, goats, deer and antelopes (Diptera, Hippoboscidae). Entomol. Amer. 22;1-210,
Bogdanov, B. N., and Baldicina, K. S. I94O. [A case of intrauterine infection of a lamb with Trypanosoma melophagium.] Trudy Kazakh Sci, Res, Vet. Inst. ^:90-91 • [.In Russian.J
Bozhenko, V. P., and Zeiss, A. L. 1928. [Trypanosomiasis of sheep.]Rev. Microbiol. (Saratov) _7:417—420. (in Russian.)
Bruce, S. D., Hamerton, A. E., and Bateman, H. R. I9 0 9. A trypanosoma from Zanzibar. Proc. Roy. Soc. 81:14-3O.
Buchner, P. E. C. 1922. Sind die Crithidien der Schaflaus fur Rause pathogen? Ztschr. Hyg. u. Infektionskr, 95 :115-118.
Castellan!, 0., Kibeiro, L. V., and Fernandes, J, p. 196?. Differentiation of Trypanosoma cruzi in culture. J. Protozool, 14;447—451.
Cauchemez, L. 1912. Recherche sur la transmission héréditaire de Crithidia melophagia Plu. C. R. Soc. Biol. J2:1062—IO6 4.
CelisSev, A, A. 194&. [Results of a study of parasitic protozoa in livestock of Kazakh SSR.] Izvestia Acad. Sci. Kazakh SSR. (Ser. Parasitol.) ^:34—4O. (in Russian.]
Chang, S. L. 1947* Studies on hemoflagellates. II. A study of thegrowth rates of Leishmania donovani, L. braziliensis, L. tropica, and Trypanosoma cruzi in culture. J. Inf. Dis, 80;172-18 4.
Chatton, E., and Delanoë, P. I9 1 2. Observations sur 1'evolution et la propagation de Crithidia melophagia Plu. C. R. Soc. Biol. 72;942-944<
Colas—Belcour, J. 1931. Notes sur la fauna parasitologique des oasis de Tozeur et Kebili. Arch, Inst. Pasteur Tunis, 20:66.
72
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
73
Davis, B. S. 1952. Studies on the trypanosomes of some California mammals. Univ. Calif. Publ. Zool. 145-250.
Dice, L. R., and Leraas, H. J. 1936. A graphie method for comparing several sets of measurements. Contr. Lab. Vert, Gen, Univ, Mich,3:1-3.
Dios, R, L. 1928, Presence du Trypanosoma melophagium dans le sang des ovides. C. R, Soc. Biol, 99:1502-1903.
Douwes, J. B, 1920. Trypanosomen bij het schaap (Trypanosoma melophagium Plu). Tijdschr. Diergeneesk, 47:408-409,
Dunkerley, J, S. 1913. Plagellata and Ciliata. Proc, Roy, Irish Acad, 21:61-62,
Dusanic, D. G. I9 6 8, Growth and immunologic studies on the culture forms of Trypanosoma lewisi, J, Protozool. 15 :328-333.
Evans, G. P. 1949* Studies on the bionomics of the sheep ked,Melophagus ovinus L,, in West Wales, Bull, Entomol. Res, 40:459.
Flu, P, C. 1908, Ueber die Plagellaten im Darm von Melophagus ovinus.Arch, Protistenk. 12:147-153.
Gut t man, H, R. , and Wallace, P, G. I9 6 4. Nutrition and physiology of the Trypanosomatidae in ’'Biochemistry and physiology of protozoa," (ed.Hutner, S, H.), Academic Press, New York,
Herbert, I. V, I9 6I, Bovine trypanosomiasis due to Trypanosoma theileri Laveran, I902 and its occurrence in Eire, Irish Vet, J. I5 :23Q-236.
________. 1965a. Cytoplasmic inclusion and organelles of in vitro cultured Trypanosoma theileri arid T, melophagium and some speculations on their functions. Exp. Parasitol, 1?:24—4Q» , 1965b, Some observations on the isolation and in vitro cultureof two mammalian trypanosomes, Trypanosoma theileri Laveran, 1902 and T. melophagium Plu, I9O8 , with special reference to T, theileri.Ann, Trop, Med, and Parasitol, 59:277-293.
Hoare, C. A. 1921a, Some observations and experiments on insect flagellates, with special reference to artificial infection of vertebrates, Parasitology. 13 :67-85.
1921b. Trypanosomiasis in British sheep. Trans. Roy, Soc.Trop. Med. and Hyg, I5 :6-7«
1922, Trypanosomiasis in British sheep. (Preliminary communication.) Ibid. 16 :l88-194i
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
74
Hoare, C. A. 1923a. On a trypanosoma occurring in British sheep and its transmission "by the sheep-ked. Vet. J. 79 ;271-^74»
. 1923b. An experimental study of the sheep—trypanosorae(Trypanosoma melophagium Plu, I9O8 ) and its transmission by the sheep-ked '(Melophagus ovinus L.). Parasitology 13 :363—424.
________ . 1970» The mammalian trypanosomes of Africa, Systematic description of the mammalian trypanosomes of Africa: In the African Trypanosomiases (ed. Mulligan, H, W.), London: 3. . 1972. The trypanosomes of mammals (a zoological monograph).Blackwell Sci, Pub,
, and Wallace, P. G, Developmental stages of Trypanosomatidflagellates: a new terminology. Nature (London) 212:1383-1306.
Kleine, P. K. 1919» Beitrag zur Kenntnis des Trypanosoma melophagium Flu. Deutch Tierarztl. Wochenschr, 27:408—4 IQ.
Léger, L. 1902, Sur un flagellé parasite de 1*Anopheles maculipennis. C. R, Soc. Biol. ^:334-356.
1904. Sur les affinités de l'Herpetomonas subulata et laphylogenie des trypanosomes. Ibid. 6 7 ;6l3-6l7.
Lehmann, D. L., and Sorsoli, W. A. 1962. The culture forms of Trypanosoma ranarum (Lankester, I87 1). I. Relation between cyclic development of culture forms, oxygen consumption in the presence of glucose, and malonate inhibition. J. Protozool. 2:90—60.
Mackerras, M. J. 1939* The Haematozoa of Australian mammals. Austral, J. Zool. 2:103—133*
Morgan, J. P., Morton, H. J., and Parker, R. C. 1930. Nutrition of animal cells in tissue culture I. Initial studies on a synthetic medium. Proc. Soc. Exp. Biol. Med. 73 :1-8.
Nakamura, M. 1967* An autoclaved medium for routine cultivation ofTrypanosoma cruzi. Trans. Roy. Soc, Trop. Ked. and Hyg. 6l :792-794*
Nelson, W. A. 1956* Mortality in the sheep ked, Melophagus ovinus (L,), caused by Trypanosoma melophagium Plu. Nature 178:750*
1958. Transfer of sheep keds, Melophagus ovinus (L.), fromewes to their lambs. Nature I8 I:56—57* , 1961. Experimental elimination of Trypanosoma melophagiumplu from its hosts, the sheep and the ked, Melophagus ovinus (L.), Nature 190:739.
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
75
Noble, E. R. 1955* The morphology and life cycles of trypanosomes. Quart. Hev. Biol. 30:1-28.
Nôller, W« 1917» Blut— und Insektenflagellatenzüchtung auf Flatten. Arch. Schiffs- u. Tropen.- Hyg. 21:53-94.
. 1919a* Beitrag zur Kenntnis des Schaftrypanosomas. Ibid.23:99-100.
1919%* Zur Verbeitung des Schaftrypanosomas bei heimischenSchafen. Deutch Tierarztl. Wochenschr. 32:327-328.
1920, Neuer Porschungen auf dem Gebiete der Trypanos orne n-züchtung. Arch. Achiffs- u. Tropen.- Hyg. 24:168-172.
Novy, F. G., and MacKeal, W. J. 1904* On the cultivation of Trypanosoma bruce i. J. Inf. Dis. J[:1-30.
Pan, C. T. i9 6 0. Studies on the monoxenic cultivation of Entamoeba histolytica with hemoflagellates. Ibid. 106:284—289»
1971* Cultivation and morphogenesis of Trypanosoma cruzi inimproved liquid media. J. Protozool. J8:$56—$60.
Pfeiffer, E. I9O9 . Ueber trypanosomenahnliche Plagellaten im Darm von Melophagus ovinus. Zschr, Hyg. Infektkr, 30:324—330.
Porter, A. I9 IO. The structure and life—history of Crithidia melophagia (Flu), an endoparasite of the sheep—ked, Melophagus ovinus. Quart.J. Micr. Sci. 89-224.
1911a. Some remarks on the genera Crithidia, Herpetomonasand Trypanos oma. Parasitology 4 :22—23.
1911b, Further remarks on the genera Crithidia, Herpetomonasand Trypanosoma, and Dr. Woodcock's views thereon. Ibid. 4:154-103^
Rodriguez, E., and Karinkell, C. J. 1970. Trypanosoma cruzi; Development in tissue. Exp. Parasitol. 27:78-8?*
Rodriguez Gomez, H. I9 8 4* Contribucion al estudio de la tripanosorniasis bovina, por medio de la fijacion de complernento. Rev. Pac. Med. Vet. y Zootecn., Bogota. 27:1121—1I9 1.
Roubaud, E. I9 0 9. Les trypanosomes pathogens et la Glossina palpalis. In: Rapport de la mission d'Etudes do la Maladie du sommeil au Congo Prance, I906-I9 0 8. Paris: p. 3 II.
Sikora, H. I9 1 8. Beitrage zur Kenntnis der Rickettsien. Arch. Schiffs—u. Tropen.- Hyg. 22:442.
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
76
Simpson, C. P.» and Green, J, H. 1959* Cultivation of Trypanosoma theileri in liquid medium at 37° C. Cornell Vet. 192-193*
Sluiter, C. P., Swellengrehel, K, H., and Ihle, J. E. W. 1922. DieDierlijke Parasieten van den Mensch en van onze Huisdieren, 3rd ed. Amsterdam.
Sprehn, C. 1923. Ueber die Haufigheit der Russel inf ekt ion mit Trypanosomen bei Schaflausfliegen unter naturlichen Verhaltnissen. (Dissertation: Tierarztl. Hochschule, Berlin).
Stewart, J, M., and Beck, J. S. 1967* Distribution of the DUA and the DKA-histone antigens in the nucleus of free-living and parasitic Sarcomastigophora. J. Protozool. 14:225-231.
Swingle, L. D. » A study of the life-history of a flagellate(Crithidia melophagia h. sp.) in the alimentary tract of the sheep— tick" (Melophagus ovinus ) . J. Inf. Dis. _6:98—121.
1911a. The relation of Crithidia melophagia to the sheep'sblood, with remarks upon the controversy between Dr. Porter and Dr. Woodcock. Trans. Amer. Micr. Soc. 30:275-283.
1911b. The relation of the sheep-tick flagellate (Crithidiamelophagia) to the sheep's blood. Wyoming Exp. Station Bull. 9-1:3-16.
Taliaferro, W. H. 1923. A study of size and variability, throughout the course of "pure line" infection, with Trypanosoma lewisi. J. Exp. Zool. 37:127-168.
Taylor, A. E. R., and Baker, J. R, I9 6 8. The cultivation of parasitesin vitro. Oxford Blackwell Sci. Pub.
Tobie , E. J., von Brand, T., and Me hi man, B. 1950» Cultural and physiological observations on Trypanosoma rhodesiense and T. ^^mbiense.J. Protozool. 3 6 ;48-54»
Turner, A. V/., and Murnane, D. 1930. On the presence of the non-pathogenic Trypanosoma melophagium in the blood of Victorian sheep,and its transmission by Melophagus ovinus. Austral. J. Exp. Biol. Med. Sci. _7-5-8.
Wang, L. T.» Jen, G., and Cross, J. H. 1973* Establishment of Entamoeba histolytica from liver abscess in monoxenic cultures with hemoflagellates. Am. J. Trop. Med. and Hyg. £2:30-32.
Wenyon, C. M. 1926. Protozoology. Vol. 1 and 2. London.Witzky, H. 1922. Spielt die Russel inf ekt ion der Schaflaus f liege bei der
Uebertragung des Schaftrypanosomas eine wesentliche Rolle? (Dissertation: Tierarztl. Hochschule, Berlin).
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
77
Woodcock, H, M. 1909a* The hemoflagellates and allied forms. In Lankester’s Treatise on Zoology, Pt. I, Paso.
1909b. [Remarks in Zoological record for 1909 (II. Protozoa)îp. 6 0.]
_. 1910. On certain parasites of the Chaffinch (p. ingillacoelebs) and the redpoll (Linota rufescens). Quart. J. Ilicr. Sci.H :P . 7 1 3. . 1911. A reply to Miss Porter’s note entitled "Some remarkson the genera Crithidia, Herpetomonas and Trypanos oma." Parasitology4 :150- 153.
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.































































































![Genetic and parasitological identification of Trypanosoma ...2021/01/14 · Trypanosoma evansi. Introduction Surra is a disease caused by Trypanosoma evansi [1], affecting several](https://static.fdocuments.in/doc/165x107/6149f34e12c9616cbc6918c3/genetic-and-parasitological-identification-of-trypanosoma-20210114-trypanosoma.jpg)