Strong elevational trends in soil bacterial community composition on Mt. Halla, South Korea
-
Upload
jonathan-miles -
Category
Documents
-
view
213 -
download
0
Transcript of Strong elevational trends in soil bacterial community composition on Mt. Halla, South Korea

lable at ScienceDirect
Soil Biology & Biochemistry 68 (2014) 140e149
Contents lists avai
Soil Biology & Biochemistry
journal homepage: www.elsevier .com/locate/soi lbio
Strong elevational trends in soil bacterial community composition onMt. Halla, South Korea
Dharmesh Singh a, Larisa Lee-Cruz a, Woo-Sung Kim a, Dorsaf Kerfahi a, Jung-Hwa Chun b,Jonathan Miles Adams a,*
aDepartment of Biological Sciences, College of Natural Sciences, Seoul National University, Seoul 151-742, South KoreabDepartment of Forest Conservation, Forest Ecology Division, Korea Forest Research Institute, Seoul 130-712, South Korea
a r t i c l e i n f o
Article history:Received 26 June 2013Received in revised form23 September 2013Accepted 26 September 2013Available online 8 October 2013
Keywords:JejuMt. HallaElevationPyrosequencingBacteriaVolcanic
* Corresponding author. Tel.: þ82 2 880 4404.E-mail addresses: foundinkualalumpur@yahoo
(J.M. Adams).
0038-0717/$ e see front matter � 2013 Elsevier Ltd.http://dx.doi.org/10.1016/j.soilbio.2013.09.027
a b s t r a c t
Elevational trends in the ecology of macroorganisms have been studied extensively; by contrast verylittle is known of such trends in microbial diversity. Previous studies on soil bacteria have found either adiversity decline, a ‘peak’ in mid altitudes, or no trend with increasing elevation. Here we studied bac-terial diversity and community composition in relation to elevation on Mt. Halla, Jeju Island, South Korea,a massive shield volcano. Samples were taken along two transects, from 100 m.a.s.l. to the summit at1950 m.a.s.l., at elevational intervals of 200 m. PCR-amplified soil DNA for the bacterial 16S rRNA genetargeting V1 to V3 region was 454-pyrosequenced, and taxonomically classified against EzTaxon-edatabase.
Elevation was the best predictor of variation in bacterial community composition along the twotransects, even when considering other soil parameters. Elevation was itself highly correlated with meanannual temperature (MAT) and mean annual precipitation (MAP), suggesting that soil bacterial com-munity composition on Mt. Halla is more strongly affected by climate than by geochemical or soiltextural factors. The two transects showed certain consistent differences in bacterial phyla composition,with one transect having significantly higher abundance of Planctomycetes and Gemmatimonadetesthan the other. Certain other phyla (e.g. Acidobacteria) also showed striking trends in abundance withelevation, but the trends differed between the two transects.
Bacterial diversity and richness were also most strongly correlated with elevation, MAT and MAP,although soil pH explained a part of the variation. Moreover, vegetation cover type, irrespective ofelevation, had an effect on soil bacterial diversity and richness. We found a ‘dip’ in diversity at lower midelevations (700e1300 m) in both transects; a trend which has not been found before. Our results, whencompared with other studies, emphasize that no simple rule can be applied to mountain systems ingeneral, but that climate itself is a major influence on community composition.
� 2013 Elsevier Ltd. All rights reserved.
1. Introduction
Understanding howbacterial communities are distributed at thelandscape scale is still rudimentary (Dequiedt et al., 2009).Encouraging progress has been made recently on the horizontaldistribution of microbial communities (Chu et al., 2010; Griffithset al., 2011; Lauber et al., 2009; Lozupone and Knight, 2007;Nemergut et al., 2011; Rousk et al., 2010), but few studies haveinvestigated elevational trends in microbial distribution (Bryant
.com, [email protected]
All rights reserved.
et al., 2008; Fierer et al., 2011; Singh et al., 2012a, 2012b; Wanget al., 2012; Zhang et al., 2009), showing a broad range of rich-ness/diversity patterns in relation to elevation. For instance, a studyof soil samples from varying elevations on a broad geographicalscale across the Americas (Lauber et al., 2009) suggested no trendwith elevation; whereas a more localized systematic study inmountains of the SW USA suggested a decline in bacterial richness/diversity towards higher elevations (Bryant et al., 2008). Althoughboth studies provide insight on the relationship between elevationand microbial richness/diversity, other factors that could influencemicrobial distribution were not accounted for. Factors controllingsoil bacterial community composition have recently been studiedacross a variety of spatial scales (Baker et al., 2009; Chu et al., 2010;Fierer and Jackson, 2006; Griffiths et al., 2011; Jenkins et al., 2009;

D. Singh et al. / Soil Biology & Biochemistry 68 (2014) 140e149 141
Lauber et al., 2009; Philippot et al., 2009), but mainly on horizontalscales. The study by Lauber et al. (2009) mixes different latitudes,climate zones and geologies, whereas the study by Bryant et al.(2008) mixes different rock types. A more comprehensive studyby Fierer et al. (2011) in the eastern Andes which analyzed bacteriain the phyllosphere, soil organic layer and soil mineral layer, did notfind any diversity/richness trendwith elevation. However, they alsosampled across a range of geologies, complicating the picture.
Two different studies on geologically uniform sites offer con-tradictory results. Shen et al. (2012) in a study on Changbai Mts.,China, did not find any elevational gradient in soil bacterial rich-ness/diversity. Instead, pH was the primary factor controlling bac-terial diversity and community composition. By contrast, Singhet al. (2012a, 2012b) found a mid-elevation richness/diversity‘peak’ for both bacteria (Singh et al., 2012b) and archaea (Singhet al., 2012a) on Mt. Fuji, a high mountain of uniform basalticcomposition.
Some of these studies, including that by Shen et al. (2012) havefound an important role of vegetation in shaping the soil bacterialcommunity. Vegetation distribution is itself partly a product oftopographic variation within the montane environment, withelevation, aspect, and slope being the three main topographicvariables determining vegetation patterns and distribution, com-bined with other influences of land use and disturbance history(Titshall et al., 2000). Thus, it is possible that an effect of elevationon the soil bacterial community is mainly due to an effect ofvegetation. It is now well established that plant species composi-tion affects the community composition of soil microbial pop-ulations (Garbeva et al., 2006; Saetre and Baath, 2000), and thecommunity composition of these soil microbes may in turn havestrong influence on plant community structure (Ettema andWardle, 2002).
Given the few existing studies of bacterial communities inmontane systems and their contradictory results, it is important toadd new work on mountain systems to approach a better under-standing of underlying mechanisms behind microbial patternsalong the elevational gradients. Especially desirable for study arethose mountain systems which offer a fairly uniform geology andsimpler climate gradients suitable for studies of biodiversity andbiogeography (Lomolino, 2001; Rahbek, 2005; Reche et al., 2005). Itis only whenmore observations of actual patterns have been made,that a theoretical framework for bacterial richness/diversity onmountains can be formulated and discussed in broader ecologicalterms.
In this study we set out to answer the following questions:
What trends in richness/diversity and community compositionare seen in soil bacteria with elevation on Mt. Halla, and whatidentifiable environmental parameters e including vegetationcover - seem to control them? Do two separate transects showthe same trends? Are there distinct communities of soil bacteriaassociated with each elevational zone? How do trends seen onMt. Halla compare to those seen on other mountain systems?
2. Methodology
2.1. Geological background
Jeju Island is a volcanic island (33.3667� N, 126.5333� E) domi-nated by a large volcanic cone, Mount Halla (1950 m). Mt. Halla is ashield volcano, consisting of alkaline lavas: basalts and trachytes(Park et al., 2000a, 2000b, 1998). The island is pocked by some 360pyroclastic cones with most of them being scoria cones and about20 being tuff cones and rings (Sohn, 1996). The main cone of Mt.Halla is of Quaternary age (last 2 million years), but the present day
covering of volcanic rocks is much younger. The extensive trachy-basalt and the other eruptive products that make up the surfacelayers of Mt. Halla were deposited around 25,000 years ago (lateQuaternary age) (Sun et al., 2005) from separate feeder pipes. Foursmall flank eruptions have taken place in the last 1000 years, butthese have not had a significant effect on the surface covering/li-thology of the island (Cheong et al., 2007).
2.2. Climate and vegetation zones
The whole island has a moist climate, with the smallest annualtemperature range in South Korea. The annual mean rainfallaveraged from stations across Jeju Island is 1975 mm; however,due to orographic and local effects, the annual mean rainfallrecorded on the southern part of Jeju is almost twice the observedin the western part. Similarly, more rainfall occurs at higher ele-vations, and thus rainfall received in the central mountainous partof the island is almost twice the observed in coastal areas (Leeet al., 1999; Won et al., 2006). According to the climate classifi-cation system of Koeppen, the climate of the lowlands of Jeju Is-land is Cfa, a subtropical moist climate. The mean annualtemperature is 14.7 �C with lowest and highest temperatures inJanuary (5.1 �C) and August (25.4 �C), respectively. By contrast theannual mean air temperature of the summit of Mt. Halla is only3.7 �C, with much colder winters (Lee et al., 1999; Won et al.,2006). The peak normally regains its winter snow cover duringmid to late October.
Mt. Halla is almost completely covered by vegetation, whichvaries in composition with altitude due to differences in climate.Vegetation can also differ locally depending on local geologicalfeatures such as cinder cones e although the areas we sampled didnot contain any of these. The vegetation of Jeju Island can bedivided into six categories: coastal vegetation, pasture/croplands,evergreen broadleaf forest, deciduous forest, coniferous forest andshrub. Coastal vegetation at the foot of Halla includes many rockycliffs with chasmophytes. Evergreen broadleaf forest, dominated byCastanopsis cuspidata var. sieboldii and Quercus salicina e Quercusglauca community, is themain natural vegetation in the lowlands ofJeju, and remains widespread where not cleared for agriculture.The lower parts of Mt. Halla have a long history of human land usefor wood cutting and more locally for livestock grazing, but itsslopes above 800 m are in a semi-natural state and are now pro-tected as a national park. Deciduous forest is distributed both belowand within the park, around 600 me1400 m in an altitudinal zonebetween evergreen broadleaf forest and coniferous forest. The de-ciduous Quercus serrata forest also contains Acer palmatum, Prunussargentii, Quercus mongolica, and Carpinus laxiflora. Coniferoussubalpine forest dominated by Abies koreana is particularlyconcentrated around 1400 masl, on the north face of Mt. Halla. Ashrub zone is concentrated around 1600 masl on the north side,and around 1500 masl on the south side, with species such asJuniperus chinensis var. sargentii, Empetrum nigrum var. Japonicum,Ilex crenata, and Ligustrum obtusifolium (Lee et al., 2010). HallasanNational Park has been designated a World Heritage Site (2007;http://www.hallasan.go.kr/english/content.php?page¼0102) andan UNESCO Biosphere Reserve (2002; http://www.hallasan.go.kr/english/content.php?page¼0102) for its high level of endemismand unique distribution of vegetation.
Large sections of Jeju are of fairly uniform volcanic composition,with similar lava types (all essentially of basaltic composition)extending from the lowest to the highest elevations of Mt. Halla,and indeed all across the island (Fig.1). Thus, it is possible to study across section of elevations with different climates, with most of thecomplicating factor of substrate geological variation removed.However, we did allow for the possible effect of variations in lava

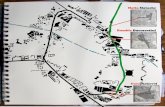
Fig. 1. Sampling scheme followed on Mt. Halla. Sampling locations are located at an approximate interval of 200 m intervals along transects. Sampling points on Gwaneumsatransect are shown as red dots whereas the sampling points on Yeongsil transect are shown as blue dots. Contour interval is 50 m. (For interpretation of the references to color inthis figure legend, the reader is referred to the web version of this article.)
D. Singh et al. / Soil Biology & Biochemistry 68 (2014) 140e149142
type (between basalt and trachybasalt) by sampling on two sides ofthe volcano, each dominated by different lava flows.
2.3. Sampling
In early September 2010, we took an elevational transect whichroughly followed the Yeongsil Hiking Trail (Lee, 2012) along thesouth-west slope of Mt. Halla, and down into the more gentlysloping surrounding lowland areas, leading down to sea level to-wards the south-east coast of Mt. Halla. This transect was located inareas mainly covered by the more Na- and K-rich trachybasalt lavadeposits (Fig. 1) (Park et al., 2000a, 2000b, 1998).
A second altitudinal transect, which followed the GwaneumsaHiking trail (Lee, 2012), was also taken on the north-eastern side ofMt. Halla and down into the more gently sloping lowlands. Thisarea is by contrast covered mostly by basaltic lavas (Fig. 1). For bothtransects, Yeongsil e ranging from 150 to 1700 masl- and Gwa-neumsa e ranging from 500 to 1700 masl, we avoided sampling inthe immediate vicinity (within 50 m) of intensively used trails toavoid the various possible human influences. We sampled at nineand seven elevation points, at 200m intervals (determined by GPS),on the Yeongsil and Gwaneumsa transects respectively, within theprevalent vegetation types for those elevational bands. Finally, fouradditional samples were taken at the summit encircling the edgesof the Crater Lake located at 1950 masl. Because of the complexmosaic of eruptives, many reworked and mixed by different phasesof volcanic activity at the rim of the crater, these four samples acted
as the summit samples for both transects. The use of two transectson Mt. Halla allows one to observe if there is a consistent patternemerging with elevation, and at the same time allows us toexamine if different lava types have any apparent effect on thebacterial community. Amap of the spatial sample distribution, witha geological map of Mt. Halla superimposed, along with samplingtrails and elevational contours is shown in Fig. 1.
‘Sample areas’ were all 0.01 ha (a 10 m by 10 m square), locatedon the same elevational contour at least 100 m apart from oneanother, except where access difficulties or the tapering width ofthe summit prevented this (inwhich case theywere located at least50 m apart). Each individual sample consisted of 5 equal sub-samples (50 g) of soil from the top 5 cm underneath the litter layer.The five subsamples, one taken at each corner and one at the center,were gathered from each 0.01 ha area, and mixed into a singlesample bag (Fulthorpe et al., 2008). Four replicates were taken ateach elevational point resulting in a total of 68 samples from bothtransects and the summit (Supplementary Table. S1).
GPS coordinates taken during the field survey were used toconstruct a spatial point layer from which Mean Annual Tempera-ture (MAT) and Mean Annual Precipitation (MAP) values wereextracted for each altitudinal band using digital climate mapsproduced by the Korea Meteorological Administration (KMA) andthe National Center for Agrometeorology (NCA). MAT data is basedon the data observed from 1971 to 2008, and precipitation amountis based on data from 1981 to 2008. The spatial resolution of theraster data is 30 m for temperature and 270 m for precipitation.

D. Singh et al. / Soil Biology & Biochemistry 68 (2014) 140e149 143
2.4. DNA extraction and pyrosequencing
The samples were crushed and sieved while still frozen througha 3 mm sieve. DNAwas extracted using the MOBIO Power Soil DNAextraction kit (MOBIO Laboratories, Carlsbad, CA, USA) as directedby the manufacturer, and was subsequently stored at �80 �C forfurther analysis. DNAwas amplified using primers targeting the V1to V3 regions of the bacterial 16S rRNA gene and PCR reactions werecarried out as described previously (Chun et al., 2010). The DNAsequencing was performed by Macrogen Incorporation (Seoul,Korea) using 454 GS FLX Titanium Sequencing System (Roche),according to the manufacturer’s instructions.
2.5. Processing of pyrosequencing data and taxonomic analysis
Data obtained after pyrosequencing were processed usingMothur (Schloss et al., 2009) except for the step of removingchimeric sequences. Briefly, sequences shorter than 150 nt, withhomo-polymers longer than 8 nt, or reads containing ambiguousbase calls or incorrect primer sequences were removed. Next, thesequences were aligned against the EzTaxon-extended database(http://eztaxon-e.ezbiocloud.net/; Kim et al., 2012) and then trim-med, so that subsequent analyses were constrained to the sameportion of the 16S rRNA gene (V1eV3 region). Putative chimericsequences were detected and screened using a similarity-basedapproach, which splits each query sequence into two even lengthfragments and then assigns each fragment to a taxon using BLASTsearch against EzTaxon-e database, followed by removal of thesequences when two fragments differ at the order level or percentidentities are greater than 95% for both fragments despite beingassigned to different taxonomies. The remaining reads were pre-clustered using the pre-cluster command (http://www.mothur.org/wiki/Pre.cluster) to remove erroneous sequences derivedfrom sequencing errors and then clustered using Mothur’s averagealgorithm. Taxonomic classification of each OTU (clustered at �97%sequence similarity) was obtained by classifying alignments againstEzTaxon-e reference bacterial taxonomy and non-redundantnucleotide bacterial databases files using the classify command at50% Bayesian bootstrap cutoff with 1000 iterations.
2.6. Soil analysis
Soil pH, texture, total nitrogen, available phosphorus, and totalcarbonwere measured based on the standard protocol of SSSA (SoilScience Society of America) at National Instrumentation Center forEnvironmental Management (NICEM, South Korea)(Supplementary Table. S1).
2.7. Statistical processing and analysis of results
To assess the relationship between soil bacterial species rich-ness/diversity and elevation, as well as with edaphic factors such aspH, soil nutrients and environmental parameters, the number ofOTUs and other diversity indices were calculated using the Mothurplatform (Schloss et al., 2009) for samples standardized to 566 reads(size decided by default, set to the size of smallest sample size), asdiversity is directly correlated with the number of sequencescollected (Supplementary Table. S2). One sample from the Yeongsiltransect at 1700masl was removed due to lownumber of reads. Soilbacterial diversity was estimated using non-parametric Shannonindex and Faith’s PD. We used Shannon index as it is influenced byboth richness and evenness and is more sensitive to changes inabundance of the rare groups (Hill et al., 2003); and Faith’s PD as itdescribes the evolutionary history of each bacterial community.Faith’s PD reports the shared length between species in an
assemblage as a proportion of the total branch lengths in the speciespool phylogeny (Faith, 1992). To assess the relationship of elevationwith OTU richness, non-parametric Shannon and PD indices of thetotal bacterial community, we fitted a linear, cubic or quadraticregression (Sigma plot ver. 10). Similarly, relative abundances of thefivemost abundant phyla (i.e. Acidobacteria, Alpha, Beta- &Gamma-proteobacteria and Bacteroidetes) were fitted against elevation inthe samemanner as above. Goodness of fit was evaluated bymeansof adjusted R2 and root mean square error (RMSE). Before applyingmultiple regression to the dataset, we looked for redundant edaphicfactors using the Varclus procedure in the Hmisc package (Sarle,1990) in the R platform. The highest correlation was betweenelevation and MAT/MAP (Spearman’s r2 � 0.82; on both the trails;Supplementary Fig. S1). Total organic carbonand total nitrogenwerethe next most correlated (Spearman r2 � 0.54; on both the trails;Supplementary Fig. S1). Therefore, we removedMAT, MAP and totalnitrogen from the analysis, and used the remaining seven environ-mental variables, {i.e. pH, available phosphorus, C. N ratio, elevation,salinity, total organic carbon and soil texture (% silt and clay)} formultiple regression analyses for both trails. To evaluate in moredetail the effect of these seven remaining environmental variableson OTUs richness, Shannon Index and Faith’s PD, we performedmultiple regression analyses. Non-significant predictor variableswere removed sequentially until only significant variables were leftin the model. Goodness of fit of the linear models was assessed bynormality of residuals. To assess if the relative abundance of the ninemost abundant phyla differed between the two transects, we per-formed linear models for normal data, or generalized linear modelsfor not normal data, with site, elevation and their interaction asfactors. Non-significant interactions and terms were removedsequentially from themodel. To assess if Planctomycetes abundancein soil was correlated with the soil nitrate (NO3N) levels, we tooksingle soil samples (one randomly chosen per elevational samplingband) from a range of elevations between 500 and 1700 masl onboth transects, and measured their NO3N content. Taking percentrelative abundanceof Planctomycetes as a response variable and soilNO3Nas the predictor,while incorporating data fromboth transects,was analyzed usingMicrosoft excel to generate graphs and statistics.Correlations between the mean annual/monthly precipitation andrelative abundance of Gemmatimonadetes were tested for signifi-cance using Spearmann rank correlations tests in R. Analyses wereperformed with R version 2.15.1.
We performed a Non-metric Multi Dimensional Scaling (NMDS)using Primer v6 (Clarke and Gorley, 2006) to explore patterns inspecies composition using a Bray Curtis similarity matrix. As totalnumber of reads was used for this analysis, abundance data weresquare root transformed and then standardized by sample total.
To determine the amount of dissimilarity between any pair ofbacterial communities, we employed two metrics: Bray and Curtis(1957) and unweighted UniFrac (Lozupone and Knight, 2005).Bray Curtis quantifies the compositional dissimilarity between eachpair of sites based on counts at each site. UniFrac dissimilarity onthe other hand is based on the fraction of branch length sharedbetween any pair of communities within a phylogenetic tree con-structed from the 16S rRNA gene sequences from all communitiesbeing compared. A relatively small UniFrac distance implies thattwo communities are compositionally similar, harboring lineagessharing a common evolutionary history. Mantel-type tests(Legendre and Legendre, 1998) were performed to look at the effectof all the environmental factors on Bray Curtis and UniFracdissimilarity matrices (obtained using Mothur e (Schloss et al.,2009)). Euclidean distance matrices of each environmental vari-able were calculated on averaged normalized data using PRIMER-6(RELATE Function) (Clarke and Gorley, 2006). Significance of theMantel-type tests was assessed by 999 permutations Finally, to

D. Singh et al. / Soil Biology & Biochemistry 68 (2014) 140e149144
tease apart the relative importance of the environmental variableson the bacterial community similarity and phylogenetic structure,we used a Multiple Regression on Matrices (MRM) approach(Legendre et al., 1994). Non-significant factors were removedsequentially and the MRM analysis was repeated until only signif-icant factors were left in the model. Significance was tested bypermutations (9999) and P-values of the two tailed tests are re-ported for this analysis.
To investigate whether there is an effect of vegetation typeregardless of elevation, we performed an ANOVA on phylogeneticdiversity, chao Index and number of OTUs taking into account onlythe five main types of vegetation (i.e. coniferous forest, deciduousforest, evergreen forest, pine forest and shrub land). Post-hocTukey’s HSD test was used for pairwise comparisons when the ef-fect of vegetation was significant. All variables were checked fornormality or transformed to meet a normal distribution beforeanalysis. To partition the variance in diversity and communitycomposition due to vegetation, we performed a permutationalmatrix-based multivariate analysis of variance using the adonisfunction in the vegan package in R (Anderson, 2001) with thebacterial abundance file a as response variable and vegetation andelevation as factors. MAT and MAP were highly correlated withelevation, thus they were excluded from this analysis. Statisticalanalyses were performed in R version 2.15.1.
3. Results
3.1. Broad taxonomic features of bacterial community on Mt. Halla
A total of 189,409 quality sequences were classified into 5, 256OTUs at �97% similarity level, distributed across all 67 samples. On
Fig. 2. Phyla breakdown for the Gwaneumsa
average, 355 OTUs (�6.65 SE) were found in each sample stan-dardized at 566 reads. Proteobacteria was the most abundantphylum on Mt. Halla, accounting for approximately 34% of the totalsequences obtained, followed closely by Acidobacteria with around32.5% of the total sequences (Fig. 2, Supplementary Table S3). Themost abundant single phylotype across all samples was classifiedunder genus Acidobacteria Gp2 with a total of 4563 sequences(around 2.4%). The second most abundant phylotype was from thefamily Sinobacteriaceae of Proteobacteria with a total of 2640 se-quences (1.4%).
3.2. Comparison of two transects
The two transects differed in terms of community compositionof the dominant phyla. On the Gwaneumsa transect, Acidobacteriawas the most dominant phylum (31819 seq, 35.14%) followed byProteobacteria (28960 seq, 31.98%; Fig. 2a), whereas the oppositewas found at the Yeongsil transect (Proteobacteria, 37460 seq,35.47%; Acidobacteria, 30719 seq, 28.95%; Fig. 2b), although nomajor differences were found at lower taxonomic levels withinthese two phyla between transects. Notably, the most abundantsingle phylotype (belonging to Acidobacteria Gp_2) on Mt. Hallawas also the most abundant phylotype on both transects, with ahigher abundance on Gwaneumsa (2716 seq, 3.0%) than on Yeongsiltransect (1847 seq,1.74%). Comparison of the relative abundance forthe nine most abundant phyla between the two transects revealscertain striking and consistent differences (SupplementaryTable S4; Supplementary Fig. S2). On the Yeongsil transect, Planc-tomycetes (Yeongsil 2.11%; Gwaneumsa 0.90%) were more abun-dant than on the Gwaneumsa transect (F1, 48 ¼ 49.88, P < 0.0001).Gemmatimonadetes (Yeongsil 2.09%; Gwaneumsa 1.30%) possibly
(top) and Yeongsil transects (bottom).

Table 1Multiple regression between richness (OTUs) and diversity (PD and Shannon) withenvironmental and edaphic factors. Coefficients are shown for significant predictorvariables.
Variables Whole community
Gwaneumsa Trail OTUs(R2 ¼ 0.11*b)
Faith’s PD(R2 ¼ 0.16*b)
Shannon(R2 ¼ 0.28**b)
Intercept 149.19 7.00 3.67eþ00***pH 46.27* 2.78* 3.38e�01**Elevation e 0.002* 1.55e�04*Salinity e e 1.76e�04*
Yeongsil Trail OTUs(R2 ¼ 0.114*b)
Faith’s PD(R2 ¼ 0.094*b)
Shannon(R2 ¼ 0.164**b)
Intercept 403.54*** 30.55*** 5.80eþ00***pH e e e
Elevation �0.041* �0.003* �2.29e�04**Salinity e e e
P � 0 ‘***’ 0.001 ‘**’ 0.01 ‘*’ 0.05. The analysis was performed with a total of sevenvariables (based on Varclus results; see material and methods); non-significantvariables were sequentially removed from the model, and only the significantones are reported here.
D. Singh et al. / Soil Biology & Biochemistry 68 (2014) 140e149 145
followed the same trend, but this was marginally non-significant(F6, 42 ¼ 5.55, P ¼ 0.08). The interaction between transect andelevation was significant for Acidobacteria (F6, 42 ¼ 7.87,P < 0.0001), Proteobacteria (F 6, 42 ¼ 4.63, P < 0.001) and TM7 (F6,42 ¼ 2.36, P ¼ 0.05).
The results also show a significant relationship of bacterial di-versity/richness with elevation, although richness/diversity tend tovary differently along the elevational gradient on the two transects(Fig. 3). On the Gwaneumsa transect, there was a “peak” in rich-ness/diversity in higher elevations at around 1700 masl. Maximumrichness with approximately 56% of total OTUs was observed at500 masl whereas minimum richness was observed at 1300 masl(only around 19% of total OTUs) (see Fig. 3). On the other hand,Yeongsil transect showed a “hollow” pattern in richness/diversitytowards higher altitudes with a maximum richness observed at700 masl (57% of total OTUs observed) while minimum richnesswas observed at 1700 masl (20% of total OTUs observed). Theseresults show that the bacterial communities were strikinglydifferent among the different elevations on Mt. Halla. To confirmthat there is no role of sampling scheme in the presence of differentpatterns on the two transects, we removed the 100 and 300maslsampling points from the Yeongsil transect to make it more com-parable to the Gwaneumsa transect and reanalyzed the data. Thisresulted in no significant relationship between richness/diversityand elevation on the Yeongsil transect (for both Shannon andFaith’s PD; results not shown).
When we further examined the percent relative abundance ofthe five most abundant phyla individually, most of them weresignificantly correlated with elevation on at least one of the twotransects. Specifically, the relative abundance of Acidobacteria(Gwaneumsa: R2 ¼ 0.45, P < 0.001; Yeongsil: R2 ¼ 0.52, P < 0.001)and Alpha-proteobacteria (Gwaneumsa: R2 ¼ 0.43, P < 0.001;Yeongsil: R2 ¼ 0.53, P < 0.001) was significantly correlated withelevation on both transects (Supplementary Fig. S3). Soil pH wasalso significantly correlated with the relative abundance of Acid-obacteria and Beta-proteobacteria on both transects (results notshown). In addition, soil total phosphorus was also significantlycorrelated with the relative abundance of different dominant phyla(Acidobacteria on both transects, Beta-proteobacteria only onGwaneumsa. and Bacteroidetes only on Yeongsil; results notshown).
Fig. 3. Relationship between elevation and phylotype richness (left), phylogenetic diversity (row) and Yeongsil trail (second row). We tested three models (linear, quadratic, and cubic) tand RMSE (root mean square error; value not shown). Significance level is shown at ***P <
The multiple regression analyses showed that out of the sevenenvironmental variables tested (see Varclus; Materials andMethods, Table 1), elevation was the most important correlate forFaith’s PD, Shannon index and OTUs on the Yeongsil transect;whereas on the Gwaneumsa transect it was only significantlycorrelated with the diversity indices and not with OTU richness.Soil pH was significantly correlated with all the richness/diversityindices, but only on the Gwaneumsa transect.
An NMDS performed on the Bray Curtis similarity matrix,calculated from the total community, assesses changes in bacterialcommunity composition, incorporating both taxon abundance andidentity. The NMDS showed that composition of the bacterialcommunities was highly variable across the soils represented bydifferent elevations (Fig. 4). Both transects showed significantvariability across the elevational gradient, however the clusteringpattern observed on the two transects was different. Gwaneumsasamples formed three separate clusters according to the sampleelevation range, showing that the samples belonging to thedifferent elevational zones harbored distinct communities. Yeong-sil, on the other hand, had samples arranged in a pattern on the
middle), phylotype diversity (right) for the whole community on Gwaneumsa trail (firsto describe the relationships, and model selection was carried out based on adjusted R2
0.001; **P < 0.01; and P < 0.05.

Fig. 4. NMDS of Bray Curtis similarity of overall community composition in relation to elevation for the Gwaneumsa (left) and Yeongsil (right) transects.
D. Singh et al. / Soil Biology & Biochemistry 68 (2014) 140e149146
NMDS plot from left to right in order of their decreasing elevation.Bray Curtis similarity index on Yeongsil shows minimal overlapbetween communities that differ by more than 400masl.
A Mantel-type test was used to determine which environmentalfactors significantly correlated with the community composition(Bray Curtis and UniFrac). Elevation and MAT were significantlycorrelated with community composition on both transects, butMAP and pH were correlated with community composition only onthe Yeongsil transect (Table 2).
To further assess the importance on determining communitycomposition of the seven environmental variables that did notshow covariation (see Varclus results; Supplementary Fig. S1), weused a MRM. MRM was able to explain a significant portion of thevariability in bacterial community composition on both the Gwa-neumsa (Bray Curtis: R2 ¼ 32%, P < 0.0004; UniFrac: R2 ¼ 35%,P < 0.003) and the Yeongsil (Bray Curtis: R2 ¼ 74%, P < 0.0002;UniFrac: R2 ¼ 55%, P < 0.002) transects. Elevation was the onlyvariable which was able to explain a significant proportion ofvariability on both transects for both Bray Curtis and UniFrac dis-similarities (Table 3). pH had a significant effect on communitysimilarity only on the Yeongsil transect; and notably, soil texture (%silt and clay) also explained a small portion of the variation on thistransect (Table 3).
Table 3Results of the multiple regression on matrices (MRM) analysis for the whole bac-terial community.
3.3. Effect of vegetation type
An ANOVA showed that, irrespective of elevation, vegetationhad a significant effect on phylogenetic diversity (F4, 65 ¼ 10.25,P < 0.0001; Fig. 5), Shannon index (F4, 65 ¼ 6.26, P ¼ 0.0002; Fig. 5)and number of OTUs (F4, 65 ¼ 6.23, P < 0.0003; Fig. 5). This resultwas supported by the permutational multivariate analysis whichshowed that variance in community similarity was significantlyrelated to vegetation (R2 ¼ 0.33, P < 0.001), when controlling byelevation.
Table 2Correlation between BrayeCurtis and unweighted UniFrac dissimilarity with envi-ronmental and edaphic factors (Spearman rank correlations estimated using Manteltests).
Variables BrayeCurtis UniFrac
Gwaneumsa Trail Elevation 0.562** 0.595**MAT 0.601** 0.558*MAP e e
pH e e
Yeongsil Trail Elevation 0.688** 0.648**MAT 0.729** 0.702**MAP 0.765** 0.733**pH 0.773** 0.670**
Significance level is shown at ***P � 0.001, **P � 0.01, *P � 0.05 (Bonferroni cor-rected P values). Only values for significant relationships are shown.
4. Discussion
We found clear elevational patterns in taxonomic richness,phylotype diversity, phylogenetic diversity and communitycomposition in the soil bacterial communities of Mt. Halla. Soilbacterial richness/diversity exhibited different patterns withelevation on different transects of Mt. Halla; however none of thesepatterns was unimodal or monotonous as observed in other studies(Bryant et al., 2008; Fierer et al., 2011; Singh et al., 2012b). Thissuggests that different factors (biotic and abiotic) could beresponsible for the patterns observed between bacterial richness/diversity and elevation on different sampled landscapes.
4.1. Consistent differences in phyla breakdown between thetrachybasalt and basalt transect
The consistently high abundance of Planctomycetes and Gem-matimonadetes on the Yeongsil transect is a particularly intriguingresult (Supplementary Table. S4; Supplementary Fig. S2).
One other study reported that Planctomycetes abundance in soilwas correlated with the soil nitrate (NO3N) levels, suggesting thatthe diversity of the Planctomycetes community may be a functionof either heterogeneity in the NO3N levels themselves, heteroge-neity in soil processes, or on characteristics correlated with soilNO3N levels (Buckley et al., 2006). Correlations between Plancto-mycetes abundance with the soil nitrate (NO3N) on Mt. Halla,showed that the phylum Planctomycetes abundance was indeedcorrelated to the soil NO3N content (Supplementary Fig. S4). Thus,NO3N content might explain the difference in Planctomycetes be-tween the two transects. However, this correlation in itself does not
Variables Whole community
Gwaneumsa Trail BrayeCurtis(R2 ¼ 0.316a)
UniFrac(R2 ¼ 0.354a)
pH e e
Elevation 0.045*** 0.018**Soil texture e e
Yeongsil Trail BrayeCurtis(R2 ¼ 0.735a)
UniFrac(R2 ¼ 0.551a)
pH 0.023* 0.008*Elevation 0.026** 0.009*Soil texture �0.013* e
The variation in community composition (both R2 values are significant at P� 0.001)explained by only significant variables. Partial regression coefficients (a) of the finalmodels are reported for only significant values***P � 0.0001, **P � 0.001, *P � 0.01,evaluated by 9999 permutations. Other non-significant variables included in themodel, were salinity, total organic carbon, available phosphorus and C: N ratio. Soiltexture here equals to total of % silt and clay content.

Fig. 5. Bacterial Richness (OTUs), Diversity (Shannon Index) and Phylogenetic Diversity (Faith’s PD) for the five main vegetation types sampled at Mt. Halla. Mean � SD. Abbre-viations: A e Coniferous with dwarf bamboo, B e Deciduous with dwarf bamboo, C e Evergreen Deciduous with dwarf bamboo, D e Pine with evergreen deciduous and E e Shrubland.
D. Singh et al. / Soil Biology & Biochemistry 68 (2014) 140e149 147
clearly explainwhat the underlying cause of this difference in soil Nmight be and how it relates to geology and soil ecosystem pro-cesses. As such, the pattern remains unexplained.
Gemmatimonadetes abundance, on the other hand, has beensuggested as inversely correlated to soil moisture (DeBruyn et al.,2011) with larger numbers of phylotypes being reported fromsemiarid and arid environments (Acosta-Martinez et al., 2008; Caryet al., 2010; Chanal et al., 2006; Costello et al., 2009; Kim et al.,2008; Mendez et al., 2008). Using Mean Annual and MeanMonthly (for September) precipitation (see SupplementaryTable S1) values as a proxy for soil moisture, while comparingthese against abundance of Gemmatimonadetes, we found thatindeed low precipitation areas of Mt. Halla had increased abun-dance of this phylum (Spearman correlation: MAP, r ¼ �0.38,P ¼ 0.002; mean monthly temperature for September, r ¼ �0.44;P ¼ 0.0002).
4.2. Diversity/richness patterns with altitude
A trend in richness/diversity with elevation is found for bothtransects, with a ‘dip’ in richness/diversity that reaches its lowpoint at around 1100 m elevation. This dip was then followed up-slope by a subtle “peak” on Gwaneumsa, whereas on Yeongsil thepatterns of both bacterial species richness and diversity were hol-low towards higher elevations (Fig. 3). This hollow elevationalrichness/diversity pattern has rarely been observed in nature(Rahbek, 2005), although Wang et al. (2012) reported it for speciesrichness and phylogenetic diversity on biofilm bacterial commu-nities along an elevational gradient from 1820 to 4050 m in China,for the whole community as well as for the phyla Proteobacteria. Itis not clear however, what exactly has caused this elevation-diversity trend. One possibility is that it represents some unde-tected gradient in soil chemistry produced by either the physico-chemical weathering conditions of the underlying lava rock.Another possibility is that the observed trend is product of agradient in disturbance rates in the soil. Disturbance gradients mayhave a role in the elevational gradient in soil bacterial diversity onFuji (Singh et al., 2012a). However, there is no obvious quantifiabledisturbance factor available for Jeju to test this hypothesis. UnlikeFuji, with its unstable volcanic ash fields from mid-elevations up-wards, Mt Halla is mainly vegetated at all elevations. Thus it isunlikely that soil movement or frost heave of surface stones is asignificant factor on Mt. Halla, as it may be on Fuji. Therefore, thegenerality of these observations for microbes still needs to beaddressed by more extensive studies for specific habitats, as well asacross habitats.
4.3. Explaining variance in bacterial community diversity andcommunity composition
We used NMDS ordination to provide a more complete pictureof important compositional features of biodiversity relating to theabundance of different taxa encompassing the total community.The NMDS provided different clustering patterns for microbialcommunity on both transects (Fig. 4). These patterns could beexplained in terms of environmental filtering mainly due to MATandMAP, as these two climatic variables co-vary very strongly withelevation (Varclus results; Supplementary Fig. S1). In fact, an NMDSplot on the Euclidean distances from MAT and MAP values lookedvery similar to the patterns observed on both transects (graph notshown). Climatic factors have been shown to be the strongestpositive factor shaping elevational richness/diversity patterns inseveral studies (Currie et al., 2004; Forister et al., 2010; Griffithset al., 2011; Hawkins et al., 2003; von Storch et al., 2004). The re-sults of the Mantel-type tests in our study give additional supportto the relation between community composition and MAT/MAP.MAT along with elevation was the only other variable which wassignificantly correlated with the community composition (BrayCurtis) and phylogenetic structure of the community (UniFrac) onboth transects (Table 2). Temperature has previously been shown tocontrol bacterial communities in natural environments (Adamset al., 2010; Hall et al., 2008; Miller et al., 2009). Moreover, watertemperature was the strongest environmental filter for phyloge-netic structure in one study on elevational gradient in a biofilmbacterial community (Wang et al., 2012). MRM results furtheremphasize this point where elevation (MAT and MAP removed dueto high co-variance with elevation) was the only variable whichconstantly explained a significant portion of variability observed forcommunity composition and phylogenetic structure on both tran-sects (Table 3). Overall, these results indicate that ecological pro-cesses related to temperature and rainfall, may play a dominantrole in structuring bacterial biodiversity along the elevationalgradient, analogous to what was recently found in a largely hori-zontal study across a range of ecosystems in Britain (Griffiths et al.,2011).
Our study has confirmed the generality of certain patterns in soilbacterial richness/diversity seen in other parts of theworld. Soil pH,generally found to be the most important identifiable factor con-trolling soil bacterial diversity, has recently been found to drive thespatial distribution of bacterial communities along an elevationaltransect on Mt. Changbai (Shen et al., 2012). However, in this study,although a pH effect is detectable, it is not consistently present: soilpH explained part of the variation on bacterial community

D. Singh et al. / Soil Biology & Biochemistry 68 (2014) 140e149148
composition and phylogenetic structure only on the Yeongsiltransect. The reason for the absence of anymajor role of soil pH as amajor factor on Gwaneumsa could be the presence of a very narrowrange of pH (3.67e4.95) on this transect compared to the Yeongsiltransect (4.01e5.76). In general, the narrow range of soil pH on Mt.Halla may be the key to revealing the importance of climate incommunity variation.
4.4. Diversity and vegetation cover
Vegetation cover was significantly correlated with bacterialphylotype diversity (Chao), phylogenetic diversity (Faith’s PD) andrichness (OTUs) on Mt Halla. Comparison of data points at the samealtitude on Jeju but with different vegetation cover does revealstatistically significant differences in diversity, but this pattern isnot consistent across different elevational zones. Furthermore,controlling for elevation, vegetation type significantly explained33% of the variance on community similarity on Mt. Halla, whichindicates that the effect of elevation on bacterial richness/diversityis related to vegetation change, which on Mt. Halla, follows a uni-form pattern with elevational gradient, accompanied closely byclimatic factors such as MAT and MAP. It has been suggested earlierthat local scale variation in dominant microbial communities canbe explained by plant identity and substrate hotspots (Bezemeret al., 2010; Chu et al., 2011; Orwin et al., 2010; Thomson et al.,2010). In a recent study on forest refugia at Mt. Fuji, elevationwas one of the main drivers of the vegetation patterns in the forests(Dolezal et al., 2012). Thus, wemight infer that vegetation typemayindirectly affect bacterial distribution along elevation gradientsthrough altering the soil composition, mainly the C and N status.
5. Conclusion
Our results suggest that a range of factors, including abioticfactors such as mean annual temperature, mean annual precipita-tion, pH, and also biotic factors such as vegetation, operate togetherto explain variation in soil microbial community distribution alongelevational gradients. Dominant amongst these seems to be eleva-tion, a proxy for climate, suggesting that soil bacteria are to someextent adjusted in their niches to the prevailing climate. On Mt.Halla, variation in soil chemistry (e.g. pH) is not broad, and it is likelythat holding soil chemistry relatively constant allows the impor-tance of climate to be revealed. Nevertheless, there were consistentdifferences inbacterial communities between the two transects. Justas for the whole community, certain bacterial phyla (e.g. Acid-obacteria) also showed striking trends in diversity with elevation,which differed in form between the two transects. Also, the twotrails showed somewhat different clustering trends, and differencesin the prevalence of certain bacterial phyla such as Planctomycetes.
Comparing our results with those of other studies, given therange of different patterns seen, it is clear that no unifying patterncan be expected in terms of soil bacterial richness/diversity trendsamongst the world’s mountain systems. Even different transects onthe same mountain may show different diversity and communitycompositional trends. However, it is clear that in the local context ofrelatively constant background ecology, there are strong commu-nity composition trends with elevational gradients that followclimate rather than soil chemistry, suggesting the importance ofclimate adaptation in bacterial niches.
Acknowledgements
This paper is based onwork supported by the National ResearchFoundation under Grant NRF-2010-0021413, Ministry of Education,Science and Technology, South Korea.
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.soilbio.2013.09.027.
References
Acosta-Martinez, V., Dowd, S., Sun, Y., Allen, V., 2008. Tag-encoded pyrosequencinganalysis of bacterial diversity in a single soil type as affected by managementand land use. Soil Biol. Biochem. 40, 2762e2770.
Adams, H.E., Crump, B.C., Kling, G.W., 2010. Temperature controls on aquatic bac-terial production and community dynamics in arctic lakes and streams. Envi-ron. Microbiol. 12, 1319e1333.
Anderson, M.J., 2001. A new method for non-parametric multivariate analysis ofvariance. Austral Ecol. 26, 32e46.
Baker, K.L., Langenheder, S., Nicol, G.W., Ricketts, D., Killham, K., Campbell, C.D.,Prosser, J.I., 2009. Environmental and spatial characterisation of bacterialcommunity composition in soil to inform sampling strategies. Soil Biol. Bio-chem. 41, 2292e2298.
Bezemer, T.M., Fountain, M.T., Barea, J.M., Christensen, S., Dekker, S.C., Duyts, H.,van Hal, R., Harvey, J.A., Hedlund, K., Maraun, M., Mikola, J., Mladenov, A.G.,Robin, C., de Ruiter, P.C., Scheu, S., Setala, H., Smilauer, P., van derPutten, W.H., 2010. Divergent composition but similar function of soil foodwebs of individual plants: plant species and community effects. Ecology 91,3027e3036.
Bray, J.R., Curtis, J.T., 1957. An ordination of the Upland forest communities ofsouthern Wisconsin. Ecol. Monogr. 27, 326e349.
Bryant, J.A., Lamanna, C., Morlon, H., Kerkhoff, A.J., Enquist, B.J., Green, J.L., 2008.Microbes on mountainsides: contrasting elevational patterns of bacterial andplant diversity. Proc. Natl. Acad. Sci. U. S. A. 105, 11505e11511.
Buckley, D.H., Huangyutitham, V., Nelson, T.A., Rumberger, A., Thies, J.E., 2006. Di-versity of planctomycetes in soil in relation to soil history and environmentalheterogeneity (vol. 72, pg 4522, 2006). Appl. Environ. Microbiol. 72, 6429.
Cary, S.C., McDonald, I.R., Barrett, J.E., Cowan, D.A., 2010. On the rocks: the micro-biology of Antarctic Dry Valley soils. Nat. Rev. Microbiol. 8, 129e138.
Chanal, A., Chapon, V., Benzerara, K., Barakat, M., Christen, R., Achouak, W.,Barras, F., Heulin, T., 2006. The desert of Tataouine: an extreme environmentthat hosts a wide diversity of microorganisms and radiotolerant bacteria. En-viron. Microbiol. 8, 514e525.
Cheong, C.S., Choi, J.H., Sohn, Y.K., Kim, J.C., Jeong, G.Y., 2007. Optical dating ofhydromagmatic volcanoes on the southwestern coast of Jeju Island, Korea. Quat.Geochronol. 2, 266e271.
Chu, H., Fierer, N., Lauber, C.L., Caporaso, J.G., Knight, R., Grogan, P., 2010. Soilbacterial diversity in the Arctic is not fundamentally different from that foundin other biomes. Environ. Microbiol. 12, 2998e3006.
Chu, H.Y., Neufeld, J.D., Walker, V.K., Grogan, P., 2011. The influence of vegetationtype on the dominant soil bacteria, archaea, and fungi in a low arctic Tundralandscape. Soil Sci. Soc. Am. J. 75, 1756e1765.
Chun, J., Kim, K.Y., Lee, J.H., Choi, Y., 2010. The analysis of oral microbial commu-nities of wild-type and toll-like receptor 2-deficient mice using a 454 GS FLXTitanium pyrosequencer. BMC Microbiol. 10.
Clarke, K.R., Gorley, R.N., 2006. Primer v6: User Manual/Tutorials. Primer-E Ltd,Plymouth, UK.
Costello, E.K., Halloy, S.R.P., Reed, S.C., Sowell, P., Schmidt, S.K., 2009. Fumarole-Supported Islands of biodiversity within a hyperarid, high-elevation landscapeon Socompa Volcano, Puna de Atacama, Andes. Appl. Environ. Microbiol. 75,735e747.
Currie, D.J., Mittelbach, G.G., Cornell, H.V., Field, R., Guegan, J.F., Hawkins, B.A.,Kaufman, D.M., Kerr, J.T., Oberdorff, T., O’Brien, E., Turner, J.R.G., 2004. Pre-dictions and tests of climate-based hypotheses of broad-scale variation intaxonomic richness. Ecol. Lett. 7, 1121e1134.
DeBruyn, J.M., Nixon, L.T., Fawaz, M.N., Johnson, A.M., Radosevich, M., 2011. Globalbiogeography and quantitative seasonal dynamics of Gemmatimonadetes insoil. Appl. Environ. Microbiol. 77, 6295e6300.
Dequiedt, S., Thioulouse, J., Jolivet, C., Saby, N.P.A., Lelievre, M., Maron, P.A.,Martin, M.P., Prevost-Boure, N.C., Toutain, B., Arrouays, D., Lemanceau, P.,Ranjard, L., 2009. Biogeographical patterns of soil bacterial communities. En-viron. Microbiol. Rep. 1, 251e255.
Dolezal, J., Altman, J., Kopecky, M., Cerny, T., Janecek, S., Bartos, M., Petrik, P.,Srutek, M., Leps, J., Song, J.S., 2012. Plant diversity changes during the Post-glacial in East Asia: insights from forest refugia on Halla Volcano, Jeju Island.PLoS One 7.
Ettema, C.H., Wardle, D.A., 2002. Spatial soil ecology. Trends Ecol. Evol. 17, 177e183.Faith, D.P., 1992. Conservation evaluation and phylogenetic diversity. Biol. Conserv.
61, 1e10.Fierer, N., Jackson, R.B., 2006. The diversity and biogeography of soil bacterial
communities. Proc. Natl. Acad. Sci. U. S. A. 103, 626e631.Fierer, N., McCain, C.M., Meir, P., Zimmermann, M., Rapp, J.M., Silaman, M.R.,
Knight, R., 2011. Microbes do not follow the elevational diversity patterns ofplants and animals. Ecology 92, 797e804.
Forister, M.L., McCall, A.C., Sanders, N.J., Fordyce, J.A., Thorne, J.H., O’Brien, J.,Waetjen, D.P., Shapiro, A.M., 2010. Compounded effects of climate change and

D. Singh et al. / Soil Biology & Biochemistry 68 (2014) 140e149 149
habitat alteration shift patterns of butterfly diversity. Proc. Natl. Acad. Sci. U. S.A. 107, 2088e2092.
Fulthorpe, R.R., Roesch, L.F.W., Riva, A., Triplett, E.W., 2008. Distantly sampled soilscarry few species in common. ISME J. 2, 901e910.
Garbeva, P., Postma, J., van Veen, J.A., van Elsas, J.D., 2006. Effect of above-groundplant species on soil microbial community structure and its impact on sup-pression of Rhizoctonia solani AG3. Environ. Microbiol. 8, 233e246.
Griffiths, R.I., Thomson, B.C., James, P., Bell, T., Bailey, M., Whiteley, A.S., 2011. Thebacterial biogeography of British soils. Environ. Microbiol. 13, 1642e1654.
Hall, E.K., Neuhauser, C., Cotner, J.B., 2008. Toward a mechanistic understanding ofhow natural bacterial communities respond to changes in temperature inaquatic ecosystems. ISME J. 2, 471e481.
Hawkins, B.A., Field, R., Cornell, H.V., Currie, D.J., Guegan, J.F., Kaufman, D.M.,Kerr, J.T., Mittelbach, G.G., Oberdorff, T., O’Brien, E.M., Porter, E.E., Turner, J.R.G.,2003. Energy, water, and broad-scale geographic patterns of species richness.Ecology 84, 3105e3117.
Hill, T.C.J., Walsh, K.A., Harris, J.A., Moffett, B.F., 2003. Using ecological diversitymeasures with bacterial communities. Fems Microbiol. Ecol. 43, 1e11.
Jenkins, S.N., Waite, I.S., Blackburn, A., Husband, R., Rushton, S.P., Manning, D.C.,O’Donnell, A.G., 2009. Actinobacterial community dynamics in long termmanaged grasslands. Antonie Van Leeuwenhoek Int. J. Gen. Mol. Microb. 95,319e334.
Kim, J.S., Dungan, R.S., Crowley, D., 2008. Microarray analysis of bacterial diversityand distribution in aggregates from a desert agricultural soil. Biol. Fertil. Soils44, 1003e1011.
Kim, O.S., Cho, Y.J., Lee, K., Yoon, S.H., Kim, M., Na, H., Park, S.C., Jeon, Y.S., Lee, J.H.,Yi, H., Won, S., Chun, J., 2012. Introducing EzTaxon-e: a prokaryotic 16S rRNAGene sequence database with phylotypes that represent uncultured species. Int.J. Syst. Evol. Microbiol. 62, 716e721.
Lauber, C.L., Hamady, M., Knight, R., Fierer, N., 2009. Pyrosequencing-basedassessment of soil pH as a predictor of soil bacterial community structure at thecontinental scale. Appl. Environ. Microbiol. 75, 5111e5120.
Lee, H.J., 2012. Frommer’s South Korea, third ed. John Wiley & Sons.Lee, K.S., Wenner, D.B., Lee, I., 1999. Using H- and O-isotopic data for estimating the
relative contributions of rainy and dry season precipitation to groundwater:example from Cheju Island, Korea. J. Hydrol. 222, 65e74.
Lee, S.C., Choi, S.H., Kang, H.M., Cho, S.C., Cho, J.W., 2010. The change and structureof altitudinal vegetation on the east side of Hallasan National Park. Korean J.Environ. Ecol. 24, 26e36 (in Korean).
Legendre, P., Lapointe, F.J., Casgrain, P., 1994. Modeling brain evolution frombehavior: a permutational regression approach. Evolution 48, 1487e1499.
Legendre, P., Legendre, L., 1998. Numerical Ecology, second ed. Elsevier, Amsterdam.Lomolino, M.V., 2001. Elevational gradients of species-density: historical and pro-
spective views. Glob. Ecol. Biogeogr. 10, 3e13.Lozupone, C., Knight, R., 2005. UniFrac: a new phylogenetic method for comparing
microbial communities. Appl. Environ. Microbiol. 71, 8228e8235.Lozupone, C.A., Knight, R., 2007. Global patterns in bacterial diversity. Proc. Natl.
Acad. Sci. U. S. A. 104, 11436e11440.Mendez, M.O., Neilson, J.W., Maier, R.M., 2008. Characterization of a bacterial
community in an abandoned semiarid lead-zinc mine tailing site. Appl. Environ.Microbiol. 74, 3899e3907.
Miller, S.R., Strong, A.L., Jones, K.L., Ungerer, M.C., 2009. Bar-coded pyrosequencingreveals shared bacterial community properties along the temperature gradientsof two alkaline Hot Springs in Yellowstone National Park. Appl. Environ.Microbiol. 75, 4565e4572.
Nemergut, D.R., Costello, E.K., Hamady, M., Lozupone, C., Jiang, L., Schmidt, S.K.,Fierer, N., Townsend, A.R., Cleveland, C.C., Stanish, L., Knight, R., 2011. Globalpatterns in the biogeography of bacterial taxa. Environ. Microbiol. 13, 135e144.
Orwin, K.H., Buckland, S.M., Johnson, D., Turner, B.L., Smart, S., Oakley, S.,Bardgett, R.D., 2010. Linkages of plant traits to soil properties and the func-tioning of temperate grassland. J. Ecol. 98, 1074e1083.
Park, K.H., Cho, D.L., Kim, J.C., 2000a. In: Korea Institute of Geology, M.a.M (Ed.),Geological Report of Moseulpo-Hanlim Sheet. Jeju Provincial Government,Daejeon, p. 56.
Park, K.H., Cho, D.L., Kim, Y.B., Kim, J.C., Cho, B.W., Jang, Y.N., Lee, B.J., Lee, S.R.,Son, B.K., Cheon, H.Y., Lee, H.Y., Kim, Y.U., 2000b. In: Korea Institute of Geology,M.a.M (Ed.), Geological Report of Segwipo-Hahyori Sheet. Jeju Provincial Gov-ernment, Daejeon, p. 163.
Park, K.H., Song, K.Y., Hwang, J.H., Lee, B.J., Cho, D.L., Kim, J.C., Cho, B.W., Kim, Y.B.,Choi, P.Y., Lee, S.R., Choi, H.I., 1998. In: Geology, K.I.o. (Ed.), Geological Report ofthe Cheju-Aewol Sheet. Jeju Provincial Government, Jeju, p. 290.
Philippot, L., Cuhel, J., Saby, N.P.A., Cheneby, D., Chronakova, A., Bru, D.,Arrouays, D., Martin-Laurent, F., Simek, M., 2009. Mapping field-scale spatialpatterns of size and activity of the denitrifier community. Environ. Microbiol.11, 1518e1526.
Rahbek, C., 2005. The role of spatial scale and the perception of large-scale species-richness patterns. Ecol. Lett. 8, 224e239.
Reche, I., Pulido-Villena, E., Morales-Baquero, R., Casamayor, E.O., 2005. Doesecosystem size determine aquatic bacterial richness? (vol. 86, pg 1715, 2005).Ecology 86, 2845.
Rousk, J., Baath, E., Brookes, P.C., Lauber, C.L., Lozupone, C., Caporaso, J.G., Knight, R.,Fierer, N., 2010. Soil bacterial and fungal communities across a pH gradient inan arable soil. ISME J. 4, 1340e1351.
Saetre, P., Baath, E., 2000. Spatial variation and patterns of soil microbial com-munity structure in a mixed spruce-birch stand. Soil Biol. Biochem. 32, 909e917.
Sarle, W.S., 1990. The VARCLUS Procedure. SAS/STAT User’s Guide, fourth ed. SASInstitute, Inc, Cary NC.
Schloss, P.D., Westcott, S.L., Ryabin, T., Hall, J.R., Hartmann, M., Hollister, E.B.,Lesniewski, R.A., Oakley, B.B., Parks, D.H., Robinson, C.J., Sahl, J.W., Stres, B.,Thallinger, G.G., Van Horn, D.J., Weber, C.F., 2009. Introducing mothur: open-source, platform-independent, community-supported software fordescribing and comparing microbial communities. Appl. Environ. Microbiol.75, 7537e7541.
Shen, C., Xiong, J., Zhang, H., Feng, Y., Lin, X., Li, X., Linag, W., Chu, H., 2012. Soil pHdrives the spatial distribution of bacterial communities along elevation onChangbai Mountain. Soil Biol. Biochem. 57, 204e211.
Singh, D., Takahashi, K., Adams, J.M., 2012a. Elevational patterns in Archaeal di-versity on Mt. Fuji. PLoS One 7.
Singh, D., Takahashi, K., Kim, M., Chun, J., Adams, J.M., 2012b. A hump-backed trendin bacterial diversity with elevation on Mount Fuji, Japan. Microb. Ecol. 63,429e437.
Sohn, Y.K., 1996. Hydrovolcanic processes forming basaltic tuff rings and cones onCheju Island, Korea. Geol. Soc. Am. Bull. 108, 1199e1211.
Sun, Y., Weon, H.H., Cha, Y.J., 2005. Geology of Hallasan (Mt. Halla), Jeju Island.J. Geol. Soc. Korea 41, 481e497 (in Korean).
Thomson, B.C., Ostle, N., McNamara, N., Bailey, M.J., Whiteley, A.S., Griffiths, R.I.,2010. Vegetation affects the relative abundances of dominant soil bacterialtaxa and soil respiration rates in an Upland grassland soil. Microb. Ecol. 59,335e343.
Titshall, L.W., O’Connor, T.G., Morris, C.D., 2000. Effect of long-term exclusion of fireand herbivory on the soils and vegetation of sour grassland. Afr. J. Range ForageSci. 17, 70e80.
von Storch, H., Zorita, E., Jones, J.M., Dimitriev, Y., Gonzalez-Rouco, F., Tett, S.F.B.,2004. Reconstructing past climate from noisy data. Science 306, 679e682.
Wang, J.J., Soininen, J., He, J.Z., Shen, J., 2012. Phylogenetic clustering increases withelevation for microbes. Environ. Microbiol. Rep. 4, 217e226.
Won, J.H., Lee, J.Y., Kim, J.W., Koh, G.W., 2006. Groundwater occurrence on Jeju Is-land, korea. Hydrogeol. J. 14, 532e547.
Zhang, L.M., Wang, M., Prosser, J.I., Zheng, Y.M., He, J.Z., 2009. Altitude ammonia-oxidizing bacteria and archaea in soils of Mount Everest. Fems Microbiol.Ecol. 70, 208e217.