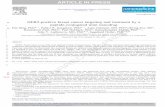
StrandAdvantage Tissue-Specific Cancer Genomic Tests ......Breast carcinoma (ER+ve/PR+ve/HER2-ve)...
Transcript of StrandAdvantage Tissue-Specific Cancer Genomic Tests ......Breast carcinoma (ER+ve/PR+ve/HER2-ve)...

StrandAdvantage Tissue-Specific Cancer Genomic Tests
Empowering Crucial First-Line Therapy Decisions for Your Patient

Precision medicine in cancer refers to the delivery of personalized treatment based on the characteristics of the individual’s tumor genetics, using targeted therapies that efficiently kill tumor cells.
As advances in cancer research and drug development provide multiple treatment options, oncologists are faced with the challenging task of deciding which therapy works best for their patients. Delivering highly targeted, actionable treatment options based on the patients genetic profile, StrandAdvantage enables optimised decision making.
StrandAdvantage matches actionable cancer genes in solid tumors to all relevant, FDA-approved and NCCN recommended cancer therapies, enabling timely decisions about effective personalized therapies for patients. Strand’s NGS-based genomic profiling combined with validated molecular markers eliminates the need for multiple tests plus, provides a comprehensive view of therapy-relevant genomic and proteomic changes in an easy-to-read report.
Harness the power of precision medicine with StrandAdvantage

StrandAdvantage: Lung Carcinoma Report
StrandAdvantage provides targeted, actionable, standard-of-care treatment options in days instead of weeks
Easy-to-read report highlights the genomic alterations, and summarizes available treatment options for your patient by recommending appropriate therapies, approved by FDA or recommended by NCCN for the cancer tissue type.
Report shown for representative purposes only.
Strand Center for Genomics and Personalized Medicine
(A unit of Strand Life Sciences Pvt. Ltd.)
UAS Alumni Association Building, Veterinary College Campus, Bellary Road, Bangalore - 560024
+91-80-23095252, [email protected], www.strandcenters.com
Report shown for representative purposes only.
PatientXXXXXXXXX
TRF IDSOMATIC-XX
TestStrandAdvantage Test
GenderFemale
Block IDXXXXXX
TypeSOC Report
06/10/15
10/10/15
21/10/15
Age75 years
Specimen Type FFPE Block
Date Collected
MRNNA
Specimen SiteLung
Date Received
ClinicianXXXXXXXXX
XXXXXXXXX
Date Generated
Hospital
Clinical Indications Lung adenocarcinoma
Summary for Standard Drugs
The patient does not meet the inclusion criteria for Ceritinib, Crizotinib, Vemurafenib, Dabrafenib and Trastuzumab
based on the results of this test.
Therapy
Relevant Marker
Tested Markers
Predicted Response**
GefitinibEGFR +
EGFR, KRAS, ERBB2, MET
ErlotinibEGFR +
EGFR, KRAS, ERBB2, MET
AfatinibEGFR +
EGFR, KRAS, ERBB2, MET
Bevacizumab--
KRAS, VHL
Markers for Vinorelbine, Carboplatin, Paclitaxel, Topotecan, Docetaxel, Mitomycin, Cisplatin, Doxorubicin, Etoposide, Cabozantinib,
Ifosfamide, Pemetrexed, Irinotecan, Mechlorethamine, Methotrexate, Gemcitabine, Nivolumab, Ramucirumab, Vinblastine are not part of
the StrandAdvantage SOC Report.
Enhanced Response - Better than standard population response due to the presence or absence of specific markers.
Enhanced but Limited Response - Better than standard population response mitigated by the presence of conflicting markers.
Standard Response - Expected response similar to the typical population response for the specific therapy.
Poor response - Likely poorer response or increased toxicity than the standard population response due to the presence of specific markers.
** Clinical Correlation Recommended

The Test
The StrandAdvantage Non-Small Cell Lung Carcinoma (NSCLC) test comprehensively maps actionable mutations in the tumor and matches them to appropriate FDA approved/NCCN recommended therapy regimens. The test detects low-frequency sequence variations with high sensitivity and specificity from a single biopsy sample.
Overview of StrandAdvantage Non-Small Cell Lung Carcinoma (NSCLC) Test
Erlotinib, Gefitinib, Afatinib: Most EGFR alterations indicate response to erlotinib, gefitinib and afatinib, while some mutations indicate resistance to them. Alterations in MET or KRAS may indicate poor response to erlotinib, gefitinib and afatinib. Alterations in ERBB2 indicates poor response to erlotinib and gefitinib but possible response to afatinib.
Osimertinib: Tumors with EGFR T790M mutation may respond to osimertinib.
Trastuzumab: Alterations in ERBB2 are associated with response to trastuzumab while mutations in EGFR, MET or PIK3CA are associated with poor response.
Bevacizumab: Mutations in KRAS may indicate limited response to bevacizumab while alterations in VHL indicates possible response.
Crizotinib, Ceritinib, Alectinib: ALK rearrangement indicates response to crizotinib, ceritinib, and alectinib. Alterations in MET and ROS1 rearrangement may indicate response to crizotinib.
Vemurafenib, Dabrafenib: Mutations in BRAF indicate possible response to vemurafenib and dabrafenib.
FDA Approved and NCCN Recommended Therapies Impacted by Genetic Markers
Erlotinib, Gefitinib
Afatinib
Osimertinib
Crizotinib
Ceritinib
Alectinib
EGFR, ERBB2, KRAS, MET
EGFR, ERBB2, KRAS, MET
EGFR
ALK*
ALK*
ALK*
Bevacizumab
Vemurafenib
Dabrafenib
Trastuzumab
KRAS, VHL
BRAF
BRAF
EGFR, ERBB2,MET, PIK3CA
* Measured by FISH

StrandAdvantage: Breast Carcinoma Report
StrandAdvantage provides targeted, actionable, standard-of-care treatment options in days instead of weeks
Easy-to-read report highlights the genomic alterations, and summarizes available treatment options for your patient by recommending appropriate therapies, approved by FDA or recommended by NCCN for the cancer tissue type.
standard-of-care treatment options in days instead of weeks
Easy-to-read report highlights the genomic alterations, and summarizes available treatment options for your patient by recommending appropriate therapies, approved by FDA or recommended by NCCN for the cancer tissue type.
Report shown for representative purposes only.
Strand Center for Genomics and Personalized Medicine
(A unit of Strand Life Sciences Pvt. Ltd.)
UAS Alumni Association Building, Veterinary College Campus, Bellary Road, Bangalore - 560024
+91-80-23095252, [email protected], www.strandcenters.com
PatientSTRAN-xxx
TRF IDSOMATIC-xx
TestStrandAdvantage Test
GenderFemale
Block IDXXXXXX
TypeSOC Report
AgeNot Available
NA
Specimen Type FFPE DNA
Sample Collected 08/10/15
11/10/15
22/10/15
MRN
Specimen SiteBreast
Sample Received
ClinicianXXXXXXXXXX
Report Generated
HospitalXXXXXXXXXXX
Clinical Indications Breast carcinoma (ER+ve/PR+ve/HER2-ve)
Summary for Standard Drugs
The patient does not meet the inclusion criteria for Lapatinib, Pertuzumab and Trastuzumab based on the results of
this test.
Therapy
Relevant Marker
Tested Markers
Predicted Response**
EpirubicinTOP2A IHC +
PgP, TOP2A
DoxorubicinTOP2A IHC +
PgP, TOP2A
EverolimusPTEN +
HER2, ER, PIK3CA, PTEN
TamoxifenER IHC +
EGFR, HER2, ER, ERBB2
Bevacizumab--
VHL
Palbociclib--
HER2, ER
DocetaxelTUBB3 IHC +
PgP, TUBB3
PaclitaxelTUBB3 IHC +
PgP, TUBB3
FluorouracilTS IHC +
SMAD4, TS
Markers for Exemestane, Toremifene, Carboplatin, Goserelin, Fulvestrant, Letrozole, Anastrozole, Capecitabine, Ixabepilone, Cisplatin,
Cyclophosphamide, Megestrol, Methotrexate, Gemcitabine, Thiotepa, Vinblastine, Eribulin are not part of the StrandAdvantage SOC Report.
Enhanced Response - Better than standard population response due to the presence or absence of specific markers.
Enhanced but Limited Response - Better than standard population response mitigated by the presence of conflicting markers.
Standard Response - Expected response similar to the typical population response for the specific therapy.
Poor response - Likely poorer response or increased toxicity than the standard population response due to the presence of specific markers.

Everolimus: In ER positive, HER2 negative breast cancer, mutations in PIK3CA or loss of PTEN expression or function may indicate enhanced response to everolimus.
Tamoxifen: In ER positive breast cancer, alterations in EGFR and ERBB2, and high levels of HER2 may indicate poor response to tamoxifen.
Bevacizumab: Loss of VHL may indicate response to bevacizumab.
5-fluorouracil: Loss of SMAD4 and high levels of TS indicate poor response to 5-fluorouracil-based therapy, where as low levels of TS indicate response.
Paclitaxel, Docetaxel: High levels of Pgp and TUBB3 indicate poor response to paclitaxel and docetaxel.
Doxorubicin, Epirubicin: High levels of TOP2A indicate response to doxorubicin and epirubicin indicates response to doxorubicin and epirubicin. High levels of Pgp indicate poor response to doxorubicin and epirubicin.
Trastuzumab, Pertuzumab, Lapatinib: Alterations in ERBB2 indicate response to anti-HER2 therapy such as trastuzumab, pertuzumab and lapatinib. In HER2 positive breast cancers, mutation in PIK3CA, alterations in EGFR or low levels of PTEN indicate poor response to trastuzumab and pertuzumab.
The Test
The StrandAdvantage Breast Carcinoma test comprehensively maps actionable mutations in the tumor and matches them to appropriate FDA approved/NCCN recommended therapy regimens. The test detects low-frequency sequence variations with high sensitivity and specificity from a single biopsy sample. The test also includes IHC (immunohistochemistry) to assess the expression level and cellular localization of specific markers. These markers have been selected based on their therapeutic significance.
Overview of StrandAdvantage Breast Carcinoma Test
FDA Approved and NCCN Recommended Therapies Impacted by Genetic Markers
Everolimus
Doxorubicin,Epirubicin
5-fluorouracil
Bevacizumab
ER*, HER2*, PIK3CA, PTEN*
Pgp*, TOP2A*
SMAD4, TS*
VHL
Lapatinib
Trastuzumab
Pertuzumab
Paclitaxel, Docetaxel
Tamoxifen
ERBB2/HER2*, PIK3CA
EGFR, ERBB2/HER2*, PIK3CA, PTEN
EGFR, ERBB2/HER2*, PIK3CA, PTEN
Pgp*, TUBB3*
ER*, ERBB2/HER2*, EGFR
* Indicates IHC markers

StrandAdvantage: Colorectal Carcinoma Report
StrandAdvantage provides targeted, actionable, standard-of-care treatment options in days instead of weeks
Report shown for representative purposes only.
Easy-to-read report highlights the genomic alterations, and summarizes available treatment options for your patient by recommending appropriate therapies, approved by FDA or recommended by NCCN for the cancer tissue type.
Strand Center for Genomics and Personalized Medicine
(A unit of Strand Life Sciences Pvt. Ltd.)
UAS Alumni Association Building, Veterinary College Campus, Bellary Road, Bangalore - 560024
+91-80-23095252, [email protected], www.strandcenters.com
Report shown for representative purposes only.
PatientXXX
TRF IDSOMATIC-
XXXXXX
XXXXXX
XXXXXX
XX
TestStrandAdvantage Test
GenderMale
Block ID
TypeSOC Report
02/12/15
06/12/15
16/12/15
Age40 years
Specimen Type FFPE Block
Sample Collected
MRNNA
Specimen SiteColon
Sample Received
Clinician
Report Generated
Hospital
Clinical Indications Colorectal cancer
Summary for Standard Drugs
Therapy
Relevant Marker
Tested Markers
Predicted Response**
OxaliplatinKRAS +
KRAS
Fluorouracil--
SMAD4, APC
BevacizumabKRAS +
KRAS
PanitumumabKRAS +
EGFR, NRAS, KRAS, BRAF, MET, PIK3CA,
PTEN
CetuximabKRAS +
EGFR, NRAS, KRAS, BRAF, MET, PIK3CA,
PTEN
Markers for Capecitabine, Ziv-Aflibercept, Irinotecan, Leucovorin, Regorafenib are not part of the StrandAdvantage SOC Report.
Enhanced Response - Better than standard population response due to the presence or absence of specific markers.
Enhanced but Limited Response - Better than standard population response mitigated by the presence of conflicting markers.
Standard Response - Expected response similar to the typical population response for the specific therapy.
Poor response - Likely poorer response or increased toxicity than the standard population response due to the presence of specific markers.
** Clinical Correlation Recommended

The Test
The StrandAdvantage Colorectal Carcinoma test comprehensively maps actionable mutations in the tumor and matches them to appropriate FDA approved/NCCN recommended therapy regimens. The test detects low-frequency sequence variations with high sensitivity and specificity from a single biopsy sample.
Overview of Colorectal Carcinoma Test
Cetuximab, Panitumumab: Mutations in KRAS or NRAS indicate poor response to cetuximab and panitumumab while wild type status of these genes indicates favorable response. Additionally, alterations in BRAF, MET, PTEN or PIK3CA may indicate poor response. Alterations in EGFR may indicate favourable response to cetuximab and panitumumab in the absence of alterations in the above genes.
Bevacizumab: Mutations in KRAS may indicate limited response to bevacizumab.
5-fluorouracil: Loss of APC and SMAD4 may indicate poor response to 5-fluorouracil.
Oxaliplatin: Mutations in KRAS indicate possible response to oxaliplatin.
FDA Approved and NCCN Recommended TherapiesImpacted by Genetic Markers
5-fluorouracil
Cetuximab
Panitumumab
Oxaliplatin
Bevacizumab
APC, SMAD4
BRAF, EGFR, KRAS, MET,NRAS, PIK3CA, PTEN
BRAF, EGFR, KRAS, MET,NRAS, PIK3CA, PTEN
KRAS
KRAS

Why Strand
StrandAdvantage offers oncologists and pathologists a powerful new tool to determine optimal therapeutic strategies for cancer patients and start treatments quickly.
StrandAdvantage is designed for oncologists to use as a resource when making first-line decisions
Standard-of-care gene profile results are delivered in ten working days.
Best-in-class interpretation and reporting
Expert Interpretation and reporting using proprietary technology with deep curation and interpretation conducted by a very large team of expert scientists.
Easy-to-read reports.
Comprehensive view of treatment options
Strand’s comprehensive database of genomic variants links to FDA-approved and NCCN recommended targeted cancer therapies.
Clinical Utility of StrandAdvantageStrandAdvantage Test Performance Characteristics
Validation Summary
Graph denotes percentage of cases where clinically actionable variation(s) were reported
100
75
50
25
0LUNG BREAST COLON
*Slightly lower specificity and concordance can be explained by the limitation of Sanger to detect SNPs at <30% supporting reads. Validation performed by Sanger sequencing.
*Clinical utility and assay validation parameters are only applicable to the gene loci sequenced by NGS
66% 67%
78%
Assay Validation Parameter Results
Assay Accuracy / concordance 95.8%
Analytical sensitivity 100%
Analytical specificity 95%
Reproducibility 99.7%
Affordability
StrandAdvantage provides an affordable opportunity to obtain faster and more actionable results compared to other tests.
Chemotherapeutic Response StrandAdvantage provides information related to
chemotherapeutic response for use in standard-of-care setting. StrandAdvantage provides extensively curated information from the literature on the genetic basis of chemotherapy response and identifies markers that are linked specifically to chemotherapy sensitivity, response or toxicity.

Optimized to fit the needs of clinical practice, StrandAdvantage uses next-generation sequencing to analyze cancer-related genes taken from solid tumor samples. The test results are annotated using a comprehensive knowledge base curated by proprietary technology and an experienced team of scientists at Strand.
The StrandAdvantage Testing Process
From Prescription to Report
Test Prescription
Provide prescription to your patient for StrandAdvantage testing.
Sample collection
The hospital or our product specialist collects sample from patient in kits provided by us. Sample is sent to our lab for processing.
Sample processing & analysis
Samples are processed through state-of-the art analysis, interpretation, and reporting platforms by our team of expert scientists.
Report
Receive your clinical report securely through email. The final results are delivered in an easy-to-read report containing actionable genomic variant information. Our clinical experts are available to help you review the results and answer any questions you may have.
StrandAdvantage Lung, Breast, Colon Cancer Tests – 10 working days Complete 48 gene report – 4 weeks
Turnaround Time (TAT) for StrandAdvantage Test
Sample Requirement to Perform StrandAdvantage Test
Formalin-fixed, paraffin embedded tissue (FFPE)

MSI
ATGCATGC
Cytogenetics
FISH
Single gene
ATGCATGCATGATGCATGCATGCATGCATGCATGACATGCATGCATGAATGCATGCATGATGCATGCATGCATGCATGCATGACATGCATGCATGAATGCATGCATGATGCATGCATGCATGCATGCATGACATGCATGCATGA
Biopsy ResectionSpecimen
NGStargeted panels
IHC
“The StrandAdvantagetest indicates that your
tumor contains actionable mutations which uniquely
qualifies you for an alternate targeted drug
therapy.”
Over the years, rapid advances in cancer diagnostic technology has enabled oncologists to obtain deeper
insights about the patients tumor and genetics, thereby helping make informed and actionable
therapy decisions for them.
“We tested your tumor sample and looked at it under the microscope. The morphology of the
tumor suggests the tumor may be of subtype X.”
tumor suggests the tumor
Oncologists change in approach to cancer diagnosis
and
ther
apy
sele
ctio
n
Evolution of Cancer DiagnosticsCancer DiagnosticsCancer Diagnostics
Evolution of Evolution of Evolution of Evolution of Evolution of Evolution of Evolution of Cancer DiagnosticsCancer DiagnosticsCancer DiagnosticsCancer DiagnosticsCancer DiagnosticsCancer DiagnosticsCancer DiagnosticsCancer DiagnosticsCancer DiagnosticsCancer DiagnosticsCancer DiagnosticsCancer DiagnosticsCancer DiagnosticsCancer DiagnosticsCancer DiagnosticsCancer DiagnosticsCancer DiagnosticsCancer DiagnosticsCancer DiagnosticsCancer DiagnosticsCancer DiagnosticsCancer DiagnosticsCancer DiagnosticsCancer DiagnosticsCancer DiagnosticsCancer DiagnosticsCancer DiagnosticsCancer Diagnostics
Short TAT to start therapy, including first-line options.
Testing of many genes concurrently (simultaneous identification of a driver mutation, as well as a secondary mutation in the gene).
Identification of resistance to targeted therapy for the driver gene, thus helping predict the contraindications of a targeted drug.
Targeting tumors of different origins with the same driver mutations, using a single drug.
Testing a tumor for mutations and genes other than the ones typically associated with that tumor for more information.
Determining with very high sensitivity extremely subtle mutations that are unique to the tumor.
Capturing tumor heterogeneity with high sensitivity, often missed with other molecular testing approaches.
Compared to single gene testing approaches, Strand’s NGS-based multi-gene testing allows :
Benefits of Using Strand’s NGS-Based Multi-Gene Tests vs. Single Gene Tests
Histopathology
Patients with solid tumors, especially those with breast, lung, and colon cancers for whom individual, tissue specific panels are available.
Prior to starting therapy, as part of your molecular work-up.
Patients who have failed first-line therapy, or have a metastatic presentation.
During the neoadjuvant period and soon after surgery.
For Which of My Patients Should I Order the StrandAdvantage Test?

About StrandA History of Innovative Genomic Research
Founded in 2000, Strand Life Sciences is a personalized medicine company offering next-generation sequencing (NGS)-based, targeted multi-gene panels for cancer and other diseases. Strand works with oncologists, pathologists, and community hospitals to enable faster clinical decision support for accurate molecular diagnosis, prognosis, therapy recommendations, and clinical trials. Strand’s central reference laboratories are located in Bangalore, India and Colorado, USA. For larger hospital systems, Strand offers an innovative customizable turnkey solution called Strand SmartLab (www.strandcenters.com). Our genomics products and solutions are used at over 2,000 laboratories and 100 hospitals worldwide.
www.strandls.com
A Trusted Partner to Companies WorldwideFor 15 years, our products have facilitated the work of leading researchers and medical geneticists around the world.
Contact UsGet started with targeted cancer treatments. Order a test kit today or talk to one of our product specialists to learn more.
Strand Center for Genomics & Personalized Medicine (A unit of Strand Life Sciences Pvt. Ltd.) UAS Alumni Association Building, Veterinary College Campus, Bellary Road, Hebbal, Bangalore – 560 024 Phone: +91-80-2309-5252, [email protected], www.strandcenters.com
SCG
PM-M
KTG
-000
30-V
2