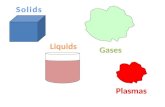
States of Matter! Molecules are vibrating. Molecules are moving faster. Definite volume No definite...
-
Upload
brooke-mcdonald -
Category
Documents
-
view
254 -
download
1
Transcript of States of Matter! Molecules are vibrating. Molecules are moving faster. Definite volume No definite...
States of Matter!
Molecules are vibrating.
Molecules are moving faster.
Definite volume
No definite shape
Definite volume
Definite shape
From CPO focus on Physical Science
Molecules are moving quickly.
No definite shape
No definite volume
Melting point: Temp. at which a solid changes to a liquid.
H2O 0o C
Freezing point: Temp. at which a liquid changes to a solid.
H2O 0o C
Boiling point: Temp. at which liquid changes to a gas.
H2O 100o C
Condensation: gas to liquid
Evaporation: liquid to gas at the surface
Vaporization: liquid to gas
Sublimation: solid to gas
Latent Heat hidden heat
•During melting, the energy is used to break up the arrangement of molecules in the ice crystals.
•The temperature doesn’t rise until the ice is melted.
Latent Heat Lab!
1. Wear goggles!
2. Use Celsius oC
3. Fill the beaker 2/3 full with ice. Add H20 just until the bulb of the thermometer is covered.
4. Let it sit for a minimum of 1 min. to stabilize, then
♠record temp. @ time=0
Latent Heat Lab Continued
5. Light the bunsen burner and record the temperature every 30 seconds
6. Also, record the time and temperature when all ice is melted and again when it is boiling!
7. Let it boil for 1 minute (continue taking data)
TAKE DATA THE WHOLE TIME !!!!!!!!!!!!!