Spatio-temporal pattern of cells expressing the clock genes period and timeless and the lineages of...
-
Upload
santiago-ruiz -
Category
Documents
-
view
214 -
download
2
Transcript of Spatio-temporal pattern of cells expressing the clock genes period and timeless and the lineages of...

Gene Expression Patterns 10 (2010) 274–282
Contents lists available at ScienceDirect
Gene Expression Patterns
journal homepage: www.elsevier .com/locate /gep
Spatio-temporal pattern of cells expressing the clock genes period and timelessand the lineages of period expressing neurons in the embryonic CNSof Drosophila melanogaster
Santiago Ruiz a,1, Christof Rickert b,1, Christian Berger b,1, Gerhard M. Technau b, Rafael Cantera a,*
a Department of Developmental Neurobiology, Instituto de Investigaciones Biológicas Clemente Estable, Av. Italia 3318, Montevideo, Uruguayb Institute of Genetics, University of Mainz, D-55099 Mainz, Germany
a r t i c l e i n f o
Article history:Received 30 April 2010Received in revised form 31 May 2010Accepted 4 June 2010Available online 15 June 2010
Keywords:DrosophilaNeural developmentNeuroblastCell lineagePeriodTimeless
1567-133X/$ - see front matter � 2010 Elsevier B.V.doi:10.1016/j.gep.2010.06.001
* Corresponding author.E-mail address: [email protected] (R. Cantera
1 These authors contributed equally to this paper.
a b s t r a c t
The initial steps towards the generation of cell diversity in the central nervous system of the fruitfly Dro-sophila melanogaster take place during early phases of embryonic development when a stereotypic pop-ulation of neural progenitor cells (neuroblasts and midline precursors) is formed in a precise spatial andtemporal pattern, and subsequently expresses a particular sequence of genes. The clarification of thepositional, temporal and molecular features of the individual progenitor cells in the nerve cord and brainas well as of their specific types of neuronal and/or glial progeny cells forms an essential basis to under-stand the mechanisms controlling their development. The present study contributes to this effort by trac-ing the expression of period and timeless, two genes that encode transcription factors with a key role inthe molecular mechanism of the biological clock. Using a combination of genetic markers and immuno-cytochemistry with antibodies specific for period and timeless we define the number, location, origin andlineage of period cells in the nerve cord throughout embryogenesis. We also provide the first descriptionof the expression of timeless in the embryonic central nervous system. We found a major transformationin the number and types of cells that express period and timeless takes place between embryonic and lar-val life.
� 2010 Elsevier B.V. All rights reserved.
Cell diversity within the developing central nervous system(CNS) of the fruitfly Drosophila melanogaster correlates with diver-sity in the genes expressed by each cell type. During early phases ofCNS development, each stem cell (also called neuroblast, NB) del-aminates from the neuroectoderm with specific position and tim-ing (Hartenstein and Campos-Ortega, 1985) and subsequentlyexpresses a particular sequence of genes, several of which encodetranscription factors. The confluence of morphological, temporal,and molecular features defining the formation of each NB madepossible to generate graphical representations called ‘‘NB maps”.This was first achieved for the NBs of the nerve cord (Doe, 1992)and more recently for those of the brain, which is considerablymore complex (reviewed in Urbach and Technau, 2004). In theory,it might be possible to generate ‘‘maps” representing anatomical,molecular and functional information about all neurons and glialcells produced by each NB. Important advances have been donein this direction. The labeling of a single NB with a non-toxic fluo-rescent marker (DiI) makes possible to trace the entire embryonic
All rights reserved.
).
lineage and accurately map its particular set of neurons and/or glia(Bossing et al., 1996; Schmidt et al., 1997).
Combining DiI-based lineage analysis with other methods al-lowed for the assignment of most motorneurons (Landgraf et al.,1997), some interneurons (as for example serotonin and corazoninneurons, Lundell et al., 1996; Novotny et al., 2002) and all glial cells(Beckervordersandforth et al., 2008; Schmidt et al., 1997) to spe-cific NB lineages in the nerve cord. Complementary methods, suchas time-course analysis of global gene expression, made possible toidentify large numbers of genes that are expressed by particularpopulations of cells as for example glial cells (Altenhein et al.,2006; Beckervordersandforth et al., 2008; von Hilchen et al.,2008) and midline cells (Kearney et al., 2004; Wheleer et al.,2006). However, much remain to be learned before a map of allCNS cells including lineage, gene expression, morphological pheno-type and function can be drawn.
The present study contributes to this effort. Using antibodiesspecific for the proteins encoded by the clock genes period (per)and timeless (tim), in combination with antibodies specific for thesegmental marker Engrailed (En) and the glial marker Repo, thedistribution, number and glial or neuronal identity of cells express-ing those two clock genes was mapped throughout the CNS. Theproteins Per and Tim are transcription factors that play a key role

S. Ruiz et al. / Gene Expression Patterns 10 (2010) 274–282 275
within the molecular mechanism that sustains the function of thebiological clock inside ‘‘clock neurons” of the brain, but also haveother functions in other cells and tissues (Hardin, 2005; Williamsand Sehgal, 2001).
The expression of per in the embryonic CNS has been describedwith several methods (Houl et al., 2008; James et al., 1986; Liuet al., 1988), albeit a good resolution at the single cell level is miss-ing, with the only exception of per expression in midline neurons ofthe nerve cord (Wheleer et al., 2006, 2008). Here, by applying acombination of genetic markers (transgenic expression of GFP asreporter of cells expressing the gene of interest), DiI labeling andimmunocytochemistry with antibodies specific for Per, we wereable to define the number, location and lineage of per cells in thenerve cord from the first moment of expression until the end ofembryogenesis. The expression of tim in the CNS of the embryohas not been described so far. We provide such a description,and report that a major transformation in the number and typesof cells that express tim and per takes place between embryonicand larval life.
1. Results
1.1. Expression of period and timeless in the brain
Transgenic expression of GFP driven by the per or tim promoters(per-Gal4 or tim-Gal4 flies crossed with UAS-gfp, see ExperimentalProcedure) was largely coincident with the corresponding immu-nostaining, but differences were observed in the number of cellsdetected by each method (see below). The specificity of the anti-bodies reported in the original publications (Stanewsky et al.,1997) was confirmed here by the absence of staining in the corre-sponding null mutants and the specific staining of clock neurons inthe larval brain (not shown).
In the larval brain, around 20 cells co-express per and tim and alower number expresses either per or tim (Hamasaka and Nässel,2006; Kaneko and Hall, 2000; Kaneko et al., 1997). To clarify theexpression pattern in the embryonic brain we analyzed embryosdoubly stained for Per and Tim. The expression of Per in the brainstarted around stage 12 (Fig. 1A) and extended to more cells bystage 13 (Fig. 1B). At stage 16 around 130 cells were positive foranti-Per (Fig. 1C), of which about 100 (97.8 ± 3.1, n = 5) were alsopositive for anti-GFP (when the immunostaining was done in em-bryos with per-Gal4 expression), and about 30 detected with anti-Per only. None of the 130 Per-positive cells expressed the glialmarker Repo, indicating that they are all neurons (data not shown).The expression of Tim started around stage 12 (Fig. 1D) and ex-tended to more cells progressively through stage 13 (Fig. 1E) and16 (Fig. 1F). In tim-Gal4/UAS-gfp embryos double-stained withanti-Tim and anti-GFP, approximately 160 cells (160.3 ± 4.6,n = 8) were detected with anti-Tim of which about 130 also ex-pressed GFP (Fig. 1G–I). The majority of the Tim-expressing cellswere also positive for the glial marker anti-Repo. We observedtwo populations of Tim-expressing cells with regards to the inten-sity of their anti-Tim staining and the presence of anti-Repo stain-ing. One of them showed strong levels of Tim and were negative forRepo (arrows in Fig. 1J). The other showed low to very low levels ofTim and often co-expressed Repo (Fig. 1K–L).
Both Per and Tim-expressing cells were widely distributedthroughout the embryonic brain but with partial segregation, withmany Tim cells located more rostrally and many Per cells locatedmore caudally (Fig. 1M–O, see arrowheads). A subset of up to 20cells co-expressed Tim and Per in regions of the protocerebrumclose to where the clock neurons are located in the larval brain(20 neurons that co-express per and tim in the larval brain, re-viewed by Helfrich-Förster, 2003; Kaneko and Hall, 2000; Kaneko
et al., 1997) and similar also in location and number to the cellsthat have been found to co-express Per and Clock at this embryonicstage (Houl et al., 2008). The two most anterior and dorsal pairswere located in a position close to that occupied by the two pairsof dorsomedian clock neurons in the larva (Helfrich-Förster,2003). The rest (Fig. 1M–O) were distributed in clusters along thelateral protocerebrum, in an area roughly coincident with thatwhere the lateral clock neurons, which also co-express tim andper, are located in the larval brain (Helfrich-Förster, 2003).
1.2. Expression of timeless and period in the nerve cord
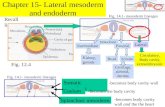
The anti-Tim antibodies gave no immunostaining in wild-typeembryos younger than stage 11 (Fig. 2A). The earliest expressionwas observed by stage 12 in a pair of bilateral cell clusters in eachsegment of the nerve cord (Fig. 2B). Some of the first cells to ex-press Tim were neurons because we observed immunoreactiveaxons along the commissures and axons leaving the CNS alongthe nerve roots (arrow in Fig. 2B and see Supplementary Fig. 1).The commissural axons were found in the anterior commissure,forming two fascicles of at least two axons each. Later on, addi-tional cells were stained in a more median position although withmuch weaker intensity (Fig. 2C, stage 13). By stage 16, the lateralclusters maintained strong immunofluorescence while the cellscloser to the midline were barely detectable, and the number ofcells in the three thoracic segments had increased compared withabdominal segments (Fig. 2D). The co-staining for Tim and En-grailed (En) showed that almost all the tim cells were locatedanterior to the Engrailed domain. However, we found two cellsthat co-expressed Tim and En (Fig. 2E–G). None of the cells withstrong immunostaining (lateral clusters) were positive for theglial marker anti-Repo (Fig. 2H–J). However, Repo staining wasdetected in the Tim-cells close to the midline, which had verylow Tim immunofluorescence.
The expression of per in the embryonic nerve cord has been de-tected with in situ hybridization (James et al., 1986), reporter-geneexpression in transgenic flies (Kaneko et al. 1997; Kaneko and Hall,2000) and specific antibodies (Liu et al., 1988; Siwicki et al., 1988)but, with the exception of per neurons at the midline (Wheleeret al., 2006) very little is known about the identity, number andlocation of these cells. Identical to Tim expression, we did not de-tect Per immunoreactivity at stage 11 or earlier (Fig. 3A). The ear-liest expression was detected at stage 12 close to the midline asreported recently (Houl et al., 2008) and that we determined hereto be a single cluster of about 2–3 cells per segment showing weakimmunofluorescence (Fig. 3B). Both cell number and fluorescenceintensity increased at stage 13 (Fig. 3C), when we found more cellsin the midline cluster, and several pairs of bilateral cells. At leasttwo cells with larger cell bodies and stronger fluorescence thanthe others were consistently found at the most anterior positionof Per cells close to the midline (Fig. 3C). By stage 16 the numberof cells had further increased and comprised 8 cells at the midlineand eleven pairs of lateral cells per segment (Fig. 3D, see Supple-mentary Fig. 2 and also our lineage analysis below for details).We found no segmental differences between thoracic and abdom-inal segments (Fig. 3E). None of the Per cells in the nerve cord ex-pressed the glial marker Repo (Fig. 3E–G). Co-staining withantibodies against Per and En confirmed that four midline neuronsper neuromere (only one of the UMI and three VUMs) co-expressthese genes (Wheleer et al., 2006). We also found co-expressionof En and Per in 4 other cells at the midline (see below) and intwo more lateral cells per hemisegment that often form directpairs (Fig. 3H–J). Of the six VUM neurons in each neuromere,the three that express En are interneurons (Bossing and Brand,2006; Bossing and Technau, 1994) and we confirmed here,with anti-Per, that these cells express Per as reported with

Fig. 1. Period and timeless expression in the embryonic brain. (A) In wild-type embryos Per expression was found first in a few cells at stage 12, (B) more cells by stage 13, (C)around 130 cells at late stage 16, (D) the expression of Tim started around stage 12, (E) extended to more cells at stage 13, (F) even more at stage16, (G–I) in tim-Gal4/UAS-gfpembryos double-stained with anti-Tim and anti-GFP, approximately 160 (160.3 ± 4.6, n = 8) were Tim positive of which about 130 also expressed GFP. Tim-expressing cells
276 S. Ruiz et al. / Gene Expression Patterns 10 (2010) 274–282

Fig. 2. Timeless expression in the nerve cord throughout embryonic development, its relation to glia and its segmental relationship with the Engrailed domain. (A) Cellspositive for anti-Tim were not found at stage 11 or earlier. (B) The earliest expression was observed by stage 12 in a pair of bilateral cell clusters per segment. Arrowheads in Bindicate immunoreactive axons along commisures. (C) Later on additional cells were stained in a more median position although with much weaker intensity (stage 13). (D)By late stage 16, the lateral clusters maintained strong immunofluorescence while the cells closer to the midline were barely detectable, and the cells number in the threethoracic segments increased compared with abdominal segments. (E–G) Co-localization of Tim and En showed that almost all Tim-expressing cells are located anterior to theEngrailed domain. However, there are two cells that co-express Tim and En (see arrows). (H–J) None of the cells with strong Tim expression (lateral clusters) were positive forthe glial marker anti-Repo but Repo staining was detected in the Tim-cells close to the midline, which have very low Tim immunofluorescence (arrowheads in H–J showexamples of co-localization of Tim an Repo with the corresponding antibodies). Anterior is to the top, T2 and T3 indicate the second and third thoracic segments and A1 thefirst abdominal segment. In all cases, the proteins (TIM, EN, REPO) were detected in wild-type embryos using the corresponding antibodies.
S. Ruiz et al. / Gene Expression Patterns 10 (2010) 274–282 277
per-GAL4/UAS-gfp (Wheleer et al., 2006). In accordance with areport that the three per-positive VUM cells are interneurons(Wheleer et al., 2006) we found no labeled axons in the peripheralnerves either with anti-Per or per-Gal4 (not shown).
can be subdivided into two types with regards to the intensity of anti-Tim staining and thebetween brain and nerve cord, (J) one consists of Repo-negative cells with strong anti-Timco-expresses Repo (see frame), (K–L) larger magnification of the frame in (J) showing (Kcorrespond to Repo-positive cells in K showing low levels of Tim expression, (M–O) per anlocated rostrally and many Per cells located more caudally. Arrowheads indicate cells that c(G–O) All brains correspond to late stage 16 and anterior is to the top (horizontal views). (A–(PER, TIM, GFP, REPO) were detected using the corresponding antibodies.
3
1.3. Clonal origin of per-expressing neurons in the nerve cord
To identify which NBs generate each of the neurons that ex-press per in the nerve cord, we chose two approaches. First we
presence of the glial marker Repo. The dashed line in (I) shows the approximate limitfluorescence (see arrows) and the other shows low to very low levels of Tim and often) both channels or (L) only anti-Tim in single focal planes. The white outlines in (L)d Tim-expressing cells were distributed with partial segregation, with many Tim cellso-express Per and Tim. (A–F) Anterior is to the left and dorsal to the top (lateral views).J) and (M–O) maximum projections of brains are represented. In all cases, the proteins

Fig. 3. Period expression in neurons of the nerve cord throughout embryonic development and their segmental relationship with the Engrailed domain. (A) Perimmunoreactivity was not detected in the nerve cord of wild-type embryos at stage 11 or younger. (B) The earliest expression was detected at stage 12 close to the midline asreported recently (Houl et al., 2008) in a single cluster of about 2–3 cells per segment showing weak immunofluorescence. (C) By stage 13, the staining was more intense andextended to more cells in the midline cluster and several pairs of bilateral cells. At least two cells with larger cell bodies and stronger fluorescence were consistently found atthe most anterior position of Per cells close to the midline. (D) At late stage 16 expression had further extended to eight cells at the midline and eleven pairs of lateral cells persegment (see also lineage analysis and Supplementary Fig. 2). (E–G) None of the Per cells in the nerve cord co-expressed the glial marker Repo (the yellow signal observed in(G) corresponds to superposition of cells that are apart in the dorsoventral axis). The co-staining with antibodies against Per and En confirmed that four midline neurons perneuromere co-express these genes (Wheleer et al., 2006). (H–J) Per and En were also co-expressed by four other cells at the midline and two more lateral cells perhemisegment (see arrowheads in (J)). (A–J) Maximum projections of the nerve cord are represented. In all cases, the proteins (PER, REPO, EN) were detected in wild-typeembryos using the corresponding antibodies.
278 S. Ruiz et al. / Gene Expression Patterns 10 (2010) 274–282
made use of the Eve pattern that allows to individually identifycells based on their position (Fig 4). We found that the motorneu-rons aCC and pCC (that stem from NB1-1), RP2 (from NB4-2), U1and U2 (from NB7-1) often coexpress Eve and per-Gal4. U3 (alsofrom NB7-1) and one to two cells of the Eve-Lateral Cluster (EL-cells, NB3-3) sometimes show this co-expression. Secondly, welabeled lateral and midline stem cells with the lipophilic dyeDiI in embryos expressing GFP as a reporter of per expression(per-Gal4/UAS-gfp). To check that per-Gal4 expression is an accu-rate reporter of per expression in the embryonic nerve cord we
studied embryos from this cross that were immunostained withanti-Per. We found a good correlation between both markers,with only a few cells detected with anti-Per but not with GFPand/or with GFP but without anti-Per (see SupplementaryFig. 3). The DiI labeling was done according to Bossing et al.(1996) at stage 6 and the embryos were fixed at stage late 16to early stage 17 to analyze the resulting clones. The lineageswere identified with the help of cell number and location, axonalmorphology and other morphological criteria firmly establishedin previous studies (Bossing and Technau, 1994; Bossing et al.,

Fig. 4. Some Per neurons can be identified because they also express Eve. (A–E) Anti-Eve (magenta) and per-Gal4/UAS-gfp (green). (A) All the neurons that can be individuallyidentified by position and Eve expression are shown here in two abdominal segments. Co-expression identifies (B) RP2 and aCC neurons, (C) aCC and pCC, (D) U1 and (E) U2,U3 and one cell of the EL cluster. (F) Schematic summary of all the cells that we found to express per-Gal4 in one segment (see also Figs. 5 and 6) indicating also the originwith a color code. Note that although each VUM clone has only one per-Gal4 positive interneuron, due to the existence of three VUM clones a total segment displays three per-Gal4 positive VUM neurons.
Fig. 5. VUM and MNB DiI labeled clones contain per-Gal4 positive cells. Views are from dorsal except otherwise marked. DiI is in magenta and per-Gal4/UAS-cd8-gfp in green.(A) Schematic VUM clone taken from Bossing and Technau (1994). (B) DiI labeled clone consisting of two cells, a motorneuron (VUMm) and an interneuron (VUMi). While themotorneuron does not express per-Gal4 (see B0 for projections and C, C0 for cell bodies), the interneuronal axon (arrows in b0–b00 0), axonal branches (arrowheads in b0–b00 0) andcell body, clearly are per-Gal4 positive. (D) A schematic MNB clone taken from Bossing and Technau (1994), (E) DiI labeled clone has 5–8 neurons with at least onemotorprojection. As with VUM clones, the MNB motorneuron (see F) is per-Gal4 negative while ending from interneurons (arrowheads in F0 and F00) show per-Gal4. Thedifferent layers in G and G0 (lateral view) compile the cell bodies, demonstrating that four cells (highlighted 1–4) have per-Gal4.
S. Ruiz et al. / Gene Expression Patterns 10 (2010) 274–282 279
1996; Schmidt et al., 1997). A total of 11 clones from three differ-ent midline precursors (UMI, VUM, MNB) and two NBs (NB2-1,NB4-1) were found to include per-positive cells.
The three types of midline neurons that we report here to ex-press per based on anti-Per antibodies, per-gal4/UAS-gfp and DiI
labeling had been previously reported to express per with a combi-nation of per-gal4/UAS-gfp and other markers. These are the VUMinterneurons, an unspecified number of cells from the MNB prog-eny and one of the UMIs (also called H-cell sibling) (Wheleeret al., 2006). In the three VUM clones successfully labeled with

Fig. 6. per-Gal4 cells in UMI, NB2-1 and NB4-1 clones. Views are from dorsal except otherwise marked. DiI is in magenta and per-Gal4/UAS-cd8-gfp in green. (A) Schematicand (B) DiI label of an UMI lineage. It comprises two interneurons with different shapes: the H-cell sib projects more anteriorly before bifurcating and the H-cell with shorteranterior projection and more lateral projection reaching the lateral region of the connective. We found always only the H-cell sib to be per-Gal4 positive (arrow heads in (C)and (D)). (E) Schematic view of the NB2–1 clone, (F) a DiI labeled NB2–1 clone consists of around eight interneurons most of which stay ipsilateral (2-1Ii) and at least oneprojects contralaterally (2-1Ic). (G) Only the anterior ipsilateral interneurons are per-Gal4 positive (arrowheads) while posterior ipsilateral and contralateral cells are negative(hollow arrowheads). (H) Shows that four cells (numbered 1–4) are per-Gal4 positive. Views in H0 and H00 are from lateral. (I) and (J) show a schematic and a DiI preparation ofNB4-1. It has 12–18 interneurons that send projections through both commissures and into both directions within the ispilateral connective. (K) Only an ipsilateral fasciclethat turns anteriorly expresses per-Gal4 (arrowheads) and stems from two cells in the ventrolateral cortex (1 and 2 in (L) an (L0)). The view in (L0) is from lateral.
280 S. Ruiz et al. / Gene Expression Patterns 10 (2010) 274–282
DiI, only one of the two siblings expressed per-Gal4 and noneshowed DiI labeling in efferent axons (Fig. 5A–C), in agreementwith the lack of anti-Per staining of motor axons (see previous sec-tion) and thus their interneuronal identity. The MNB lineage gener-ates 5–8 interneurons and at least one motorneuron (Bossing andTechnau, 1994). In the MNB clones four cells were labeled withper-Gal4 all of which were interneurons because they did not formaxons leaving the CNS (Fig. 5D–G).
The third type of midline neurons expressing per belong to theUMI lineage, which consists of two interneurons with two differ-ent axonal branching types (Bossing and Technau, 1994) and ineach of the three UMI clones studied here, only one of the twoneurons was clearly per-Gal4 positive (Fig. 6A–D) and corre-sponded to the more medially ending cell. A previous report indi-cated that some of these cells express per-Gal4, withoutspecifying cell type or numbers (Wheleer et al., 2006). Since wefound eight cells at the midline co-expressing Per (detected withanti-Per) and En, and four of them were subsequently demon-strated to belong to the VUM and UMI lineages (four cells in to-tal), four cells of this type remain to be assigned to midlinelineages, which based on the known expression pattern of En atthe midline (see for example Bossing and Brand, 2006 for a recentstudy) should belong to the MNB lineage.
The rest of the per cells belong to two neuroectodermal NB2-1(Fig. 6E–H00) and NB4-1 (Fig. 6I–L). The NB 2–1 lineage comprisesabout eight interneurons (Bossing et al., 1996). Four of them thatstay ipsilateral and don’t turn posterior were per-Gal4 positive(Fig. 6H–H00). The NB 4–1 lineage consists of 12–18 interneurons(Bossing et al., 1996). Within this lineage we found two interneu-
rons to be per-Gal4 positive with ipsilateral axons that stay verylateral in the anterior connective (Fig. 6L–L). Since these cells arethe lateral most per-Gal4 positive cells they can be identified asthe cells that also express En. To our knowledge this is the first re-port of En positive cells that stem from an En negative NB in theembryo.
2. Discussion
We provide here a comprehensive analysis of the expression ofthe clock genes per and tim in the Drosophila embryonic CNS. Byusing a combination of cell lineage tracing and molecular markerswe also uncover the neuroblast or midline precursor origin of theneurons that express per in the nerve cord.
In the embryonic brain we found three populations of cellsregarding expression of tim and per. One of them comprises 130cells expressing per, other comprises 160 cells expressing tim andthe third is a group of about 20 cells that co-express both genes.Cells expressing either tim, or per, or both exist also in the larvalbrain, although in much smaller numbers (Hamasaka and Nässel,2006; Kaneko et al., 1997) and part of them initiate expression ofper or tim first after some hours of larval life. Considering their lackof expression and their number, the 20 cells that co-express perand tim in the brain at embryonic stage 16 could be the same 20cells that co-express these genes in the larval brain and functionas clock neurons. This is reinforced by their approximate distribu-tion within the protocerebrum and the detection of oscillator fea-tures in a similar, probably identical array of per and clockexpressing brain neurons at the same stage (Houl et al., 2008).

S. Ruiz et al. / Gene Expression Patterns 10 (2010) 274–282 281
Since synaptic activity in central neurons develops in Drosophiladuring the same phase of development (from late stage 16–17,Baines and Bate, 1998) it is possible that a network of clock neu-rons becomes functional at this time or shortly thereafter as pro-posed (Houl et al., 2008; Sehgal et al., 1992).
The number of per and tim-expressing cells in the brain in-creases again during metamorphosis and reaches values in theadult (Helfrich-Förster, 2003) similar to those reported here forthe embryo, suggesting that such a large population of cellsexpressing these clock genes is required twice during life. This isin accordance with the identification, in a careful study of the glo-bal profile of gene expression during the life cycle of Drosophila, ofa group of genes with expression peaks late in embryogenesis andpupal life (Arbeitman et al., 2002).
Another considerable difference between embryonic and otherlife stages regards the expression of per and tim in glial cells. Hun-dreds of glia of different types express per and/or tim in the adultbut none or few in the larval brain (Helfrich-Förster, 2003; Kanekoand Hall, 2000). Here we found that per is not expressed by glialcells in the embryonic CNS, indicating that either per expressionis turned on later in some of these cells, or in glial cells born duringpostembryonic stages. On the contrary, we found that embryonicglial cells do express tim, although at apparently lower levels thanin neurons. The approximate number of cells that we found toco-express repo and tim in the embryo is smaller than the totalnumber of repo-positive cells at this stage, defining two new sub-populations of glial cells. The correlation found here between weakanti-Tim fluorescence in Repo-positive cells and strong anti-Timfluorescence in Repo-negative cells in both the brain and the nervecord suggests a common regulation of the expression of both geneswithin CNS cells. In summary, our results indicate that during thefew hours from late stage 16 to shortly after larval eclosion there isa significant change in the transcriptional profile of CNS cells, turn-ing off the expression of per and tim in neurons and the expressionof tim in glia or eliminating these cells through programmed celldeath.
In the nerve cord the per and tim neurons defined here are lo-cated in close vicinity but are generated by many different NBs.In each NB lineage, only one, or very few cells turn on the expres-sion of either tim or per. Thus, expression of these genes is notinherited by lineage, but needs to be induced in individual postmi-totic progeny cells. As suggested by the occurrence of only one po-sitive cell in some of the lineages this is also true for sibling cellsderived from individual ganglion mother cells. Differential cell fateamong such sibling cells may result form asymmetric division in-duced by Notch signaling as previously demonstrated (e.g. Udolphet al., 2001).
We found a clear, although not complete segregation of per andtim cells along the CNS. Within the nerve cord some of the mostposterior per cells were located in the engrailed domain (the pos-terior compartment of the segment) and almost all tim cells wereanterior to the engrailed domain. The same relationship was foundin the brain, although without the clear segmental pattern of thenerve cord (i.e. lacking correlation with the small engrailed do-mains of the brain). Instead, the distribution of per and tim cellsin the brain can roughly be described as two large fields with abun-dant overlap, with the majority of the tim cells located in the moreanterior field i.e. the same relative position with regards to per cellsas observed in the nerve cord.
The best known function of Per and Tim is as transcriptionalregulators acting within molecular feedback loops responsible forthe timekeeping mechanism of the biological clock (Hall, 2005).Within this conceptual frame, the ‘‘traditional” function of Perand Tim in the CNS is restricted to ‘‘oscillator cells” or clock neu-rons, in which the cycling of gene expression, mRNA and protein,as well as the shuttling of Per and Tim between cytoplasm and nu-
cleus has been documented with several methods (see Houl et al.,2008 for a recent study on Per and Clock; Helfrich-Förster, 2003;Yuand Hardin, 2006). Thus, the embryonic expression of per and timin cells other than the clock neurons, as the glia, interneuronsand motorneurons identified here in the nerve cord, suggests thatthese proteins might have other functions, perhaps in neuronaldevelopment.
3. Experimental procedure
3.1. Fly strains
Flies were raised on standard Drosophila medium and kept at25 �C. The following fly strains were used: Oregon R as wild type,period-Gal4 (per-Gal4; strain 3), timeless-Gal4 (tim-Gal4; strain6212) kindly provided by R. Stanewsky, M. Kaneko and J. Hall,UAS-gfp or UAS-cd8-gfp and the clock loss-of-function mutants per-iod01 (per01) and timeless01 (tim01) kindly provided by F. Ceriani.
3.2. Immunohistochemistry and laser confocal microscopy
Embryos were raised in LD cycle 12:12 h and collections wereperformed at similar times (in the first 2–3 h of the light phase).Embryos were dechorionated, fixed and immunostained accordingto standard protocols (Patel, 1994). The following primary antibod-ies were used: rabbit anti-Period (1:700; kindly provided by R. Sta-newsky), rat anti-Timeless (1:100; kindly provided by A. Sehgal),mouse anti-GFP (1:800; from Molecular Probes, Invitrogen), mouseanti-Repo (8D12, 1:10; Developmental Studies Hybridoma Bank)and mouse anti-Invected (4D9, 1:4; Developmental StudiesHybridoma Bank) which recognizes the protein Engrailed (Gustav-son et al., 1996). The secondary antibodies were goat anti-rabbitCy3 and goat anti-rat Cy3 (Jackson ImmunoResearch) and goatanti-mouse Alexa 488 (Molecular Probes). Stained embryos wereflattened, mounted in PBS-buffered glycerin and studied with laserconfocal microscopy on an Olympus Fluoview FV300 or a Leica TCSSPII.
3.3. Lineage analysis
DiI labeling of single neuroectodermal progenitor cells followedthe protocol of Bossing et al., 1996. Here, we used embryos hemi-zygous for per-Gal4 and UAS-cd8-gfp. Late stage 16 to early stage17 embryos were subjected to flat preparation, fixed for 10 minin 3.7% formaldehyde in PBS and scanned with a Leica TCS SPII con-focal microscope using 488 and 514 nm excitation wavelength forGFP and DiI detection, respectively.
Acknowledgements
The authors wish to express their gratitude to R. Stanewsky, M.Kaneko, J. Hall and F. Ceriani for the fly strains, and R. Stanewsky, A.Sehgal and the Developmental Studies Hybridoma Bank for anti-bodies. The study was supported by grants from the Swedish Re-search Council and the Agencia Nacional de Investigación eInnovación (ANII, Uruguay) to R.C., PEDECIBA, ANII and BoehringerIngelheim fellowships to S.R., and from the Deutsche Forschungs-gemeinschaft (DFG) to G.M.T.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, inthe online version, at doi:10.1016/j.gep.2010.06.001.

282 S. Ruiz et al. / Gene Expression Patterns 10 (2010) 274–282
References
Altenhein, B., Becker, A., Busold, C., Beckman, B., Hoheisel, J.D., Technau, G.M., 2006.Expression profiling of glial genes during Drosophila embryogenesis. Dev. Biol.296, 545–560.
Arbeitman, M.N., Furlong, E.E.M., Imam, F., Johnson, E., Null, B.H., Baker, B.S.,Krasnow, M.A., Scott, M.P., Davos, R.W., White, K.P., 2002. Gene expressionduring the life cycle of Drosophila melanogaster. Science 2970, 2275.
Baines, R.A., Bate, M., 1998. Electrophysiological development of central neurons inthe Drosophila embryo. J. Neurosci. 18, 4673–4683.
Beckervordersandforth, R.M., Rickert, C., Altenhein, B., Technau, G.M., 2008.Subtypes of glial cells in the Drosophila embryonic ventral nerve cord asrelated to linage and gene expression. Mech. Dev. 125, 542–557.
Bossing, T., Brand, A.H., 2006. Determination of cell fate along the anteroposterioraxis of the Drosophila ventral midline. Development 133, 1001–1012.
Bossing, T., Technau, G.M., 1994. The fate of the CNS midline progenitors inDrosophila as revealed by a new method for single cell labeling. Development120, 1895–1906.
Bossing, T., Udolph, G., Doe, C.Q., Technau, G.M., 1996. The embryonic centralnervous system lineages of Drosophila melanogaster. I. Neuroblast lineagesderived from the ventral half of the neuroectoderm. Dev. Biol. 179, 41–64.
Doe, C.Q., 1992. Molecular markers for identified neuroblasts and ganglion mothercells in the Drosophila central nervous system. Development 116, 855–863.
Gustavson, E., Goldsborough, A.S., Komberg, T.B., 1996. The Drosophila engrailed andinvected genes: partners in regulation, expression and function. Genetics 142,893–906.
Hall, J.C., 2005. Systems approaches to biological rhythms in Drosophila. MethodsEnzymol. 393, 61–185.
Hamasaka, Y., Nässel, D.R., 2006. Mapping of serotonin, dopamine, and histamine inrelation to different clock neurons in the brain of Drosophila. J. Comp. Neurol.494, 314–330.
Hardin, P.E., 2005. The circadian timekeeping system of Drosophila. Curr. Biol. 15,714–722.
Hartenstein, V., Campos-Ortega, J.A., 1985. Fate mapping in wildtype Drosophilamelanogaster: the spatio-temporal pattern of embryonic cell divisions. WilhelmRoux’s Arch. Dev. Biol. 194, 181–195.
Helfrich-Förster, C., 2003. The neuroarchitecture of the circadian clock in the brainof Drosophila melanogaster. Microsc. Res. Tech. 62, 94–102.
Houl, J.H., Ng, F., Taylor, P., Hardin, P.E., 2008. CLOCK expression identifiesdeveloping circadian oscillator neurons in the brains of Drosophila embryos.BMC Neurosci. 119.
James, A.A., Ewer, J., Reddy, P., Hall, J.C., Rosbash, M., 1986. Embryonic expression ofthe period clock gene in the central nervous system of Drosophila melanogaster.Embo. J. 5, 2313–2320.
Kaneko, M., Helfrich-Förster, C., Hall, J.C., 1997. Spatial and temporal expression ofthe period and timeless genes in the developing nervous system of Drosophila:newly identified pacemaker candidates and novel features of clock-geneproduct cycling. J. Neurosci. 17, 6745–6760.
Kaneko, M., Hall, J.C., 2000. Neuroanatomy of cells expressing clock genes inDrosophila: transgenic manipulation of the period and timeless genes to markthe perikarya of circadian pacemaker neurons and their projections. J. Comp.Neurol. 422, 66–94.
Kearney, J.B., Wheeler, S.R., Estes, P., Parente, B., Crew, S.T., 2004. Gene expressionprofiling of the developing Drosophila CNS midline cells. Dev. Biol. 275, 473–492.
Landgraf, M., Bossing, T., Technau, G.M., Bate, M., 1997. The origin, location andprojections of the embryonic abdominal motorneurons of Drosophila. J.Neurosci. 17, 9642–9655.
Liu, X., Lorenz, L., Yu, Q.N., Hall, J.C., Rosbash, M., 1988. Spatial and temporalexpression of the period gene in Drosophila melanogaster. Genes Dev. 2, 228–238.
Lundell, M.J., Chu-LaGraff, Q., Doe, C.Q., Hirsh, J., 1996. The engrailed and huckebeingenes are essential for the development of serotonin neurons in the DrosophilaCNS. Mol. Cell Neurosci. 7, 46–61.
Novotny, T., Eiselt, R., Urban, J., 2002. Hunchback is required for the specification ofthe early sublineage of neuroblast 7–3 in the Drosophila central nervoussystem. Development 129, 1027–1036.
Patel, N.H., 1994. Imaging neuronal subsets and other cell types in whole-mountDrosophila embryos and larvae using antibody probes. Methods Cell Biol. 44,445–487.
Schmidt, H., Rickert, C., Bossing, T., Vef, O., Urban, J., Technau, G.M., 1997. Theembryonic central nervous system lineages of Drosophila melanogaster. II.Neuroblast lineages derived from the dorsal part of the neuroectoderm. Dev.Biol. 189, 186–204.
Sehgal, A., Price, J., Young, M.W., 1992. Ontogeny of a biological clock in Drosophilamelanogaster. Proc. Natl. Acad. Sci. USA 89, 1423–1427.
Siwicki, K.K., Eastman, C., Petersen, G., Rosbash, M., Hall, J.C., 1988.Antibodies to the period gene product of Drosophila reveal diversetissue distribution and rhythmic changes in the visual system. Neuron1, 141–150.
Stanewsky, R., Frisch, B., Brandes, C., Hamblen-Coyle, M.J., Rosbash, M., Hall, J.C.,1997. Temporal and spatial expression patterns of transgenes containingincreasing amounts of the Drosophila clock gene period and a lacZ reporter:mapping elements of the PER protein involved in circadian cycling. J. Neurosci.17, 676–696.
Udolph, G., Rath, P., Chia, W., 2001. A requirement for Notch in the genesisof a subset of glial cells in the Drosophila embryonic central nervoussystem which arise through asymmetric divisions. Development 128,1457–1466.
Urbach, R., Technau, G.M., 2004. Neuroblast formation and patterning during earlybrain development in Drosophila. Bioessays 26, 739–751.
von Hilchen, C.M., Beckervordersandforth, R.M., Rickert, C., Technau, G.M.,Altenhein, B., 2008. Identity, origin, and migration of periferal glial cells inthe Drosophila embryo. Mech. Dev. 125, 337–352.
Wheleer, S.R., Kearney, J.B., Guardiola, A.R., Crews, S.T., 2006. Single-cell mapping ofneural and glial gene expression in the developing Drosophila CNS midline cells.Dev. Biol. 294, 509–524.
Wheeler, S.R., Stagg, S.B., Crews, S.T., 2008. Multiple notch signaling events controlDrosophila CNS midline neurogenesis, gliogenesis and neuronal identity.Development 135, 3071–3079.
Williams, J.A., Sehgal, A., 2001. Molecular components of the circadian system inDrosophila. Annu. Rev. Physiol. 63, 729–755.
Yu, W., Hardin, P.E., 2006. Circadian oscillators of Drosophila and mammals. J. CellSci. 119, 4793–4795.