Shapes of Simple Organic Molecules
-
Upload
antonique-headman -
Category
Documents
-
view
221 -
download
0
Transcript of Shapes of Simple Organic Molecules
-
8/16/2019 Shapes of Simple Organic Molecules
1/21
SHAPES OF SIMPLEORGANIC MOLECULES
HYBRIDIZATION AND RESONANCE
-
8/16/2019 Shapes of Simple Organic Molecules
2/21
Types of Atomic Orbitalpreset i carbo t!at
cotai electros1s orbital- spherical
2s orbital- spherical
2p orbitals- lobes
-
8/16/2019 Shapes of Simple Organic Molecules
3/21
P orbital
There are three porbitals- 2px 2p! a"#
2p$
-
8/16/2019 Shapes of Simple Organic Molecules
4/21
P orbital
-
8/16/2019 Shapes of Simple Organic Molecules
5/21
Bonding
Energy
1s
2s
2px
2py
2pz
Ground State of Carbon
The %ro&"# state co"'%&ratio" o(carbo" is &"s&itable (or bo"#i"%)
I" the %ro&"# state carbo" ca"o"l! (or* three bo"#s +&"e,&alle"%th a"# stre"%th) .or /correct0 bo"#i"% to occ&rso*e cha"%es hae to be *a#e i"the orbitals)
-
8/16/2019 Shapes of Simple Organic Molecules
6/21
Orbital Changes- Excitation
Energy
1s
2s
2px 2py 2pz
Ground State of Carbon
Energy
1s
2s
2px 2py 2pz
Excited State of Carbon
-
8/16/2019 Shapes of Simple Organic Molecules
7/21
Orbital Changes- Hybridizationsp3
Energy
1s
2s
2px 2py 2pz
Excited State of Carbon
Energy
1s
sp3 Hybridized State of Carbon
sp3
-
8/16/2019 Shapes of Simple Organic Molecules
8/21
sp3 hybridized orbital
-
8/16/2019 Shapes of Simple Organic Molecules
9/21
sp3 hybridized orbital
S a"# p orbitalsSp orbitals
-
8/16/2019 Shapes of Simple Organic Molecules
10/21
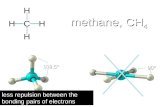
"o#i$
i
Met!ae
%CH&'0
Oerlap o( s a"# sp orbitals (or* si%*a +3 bo"#
0 Carbo" ato* is sp3 hybridized
0
Bo"#s are (or*e# b!
oerlappi"%
o(
the
1s
orbitals o( (o&r h!#ro%e" ato*s 4ith the sp3 orbitals o( the carbo" ato*
Met!ae !as & (
bo#s
-
8/16/2019 Shapes of Simple Organic Molecules
11/21
N ote that each bond contains 2
electrons: 1 from s orbital of hydrogen
1 from sp3 orbital of carbon
-
8/16/2019 Shapes of Simple Organic Molecules
12/21
"o#i$
i
Et!ee
%C)H&'
0 Ethe"e co"tai"s a #o&ble bo"#
0
I"
carbo"
o(
#o&ble
bo"#
there
are
three sp2 h!bri# orbitals a"# one &"&se# p orbital
0 Each carbo" i" the #o&ble bo"# is sp2
hybridised 0
5"&se# p orbital oerlaps aboe a"# belo4 the
pla"e
to
(or*
a
pi
+6
bo"#
-
8/16/2019 Shapes of Simple Organic Molecules
13/21
!ttp*++,,,-citycolle$iate-com+!ybri#i.atio)-!tm
http://www.citycollegiate.com/hybridization2.htmhttp://www.citycollegiate.com/hybridization2.htmhttp://www.citycollegiate.com/hybridization2.htmhttp://www.citycollegiate.com/hybridization2.htmhttp://www.citycollegiate.com/hybridization2.htmhttp://www.citycollegiate.com/hybridization2.htmhttp://www.citycollegiate.com/hybridization2.htmhttp://www.citycollegiate.com/hybridization2.htmhttp://www.citycollegiate.com/hybridization2.htmhttp://www.citycollegiate.com/hybridization2.htmhttp://www.citycollegiate.com/hybridization2.htmhttp://www.citycollegiate.com/hybridization2.htmhttp://www.citycollegiate.com/hybridization2.htmhttp://www.citycollegiate.com/hybridization2.htmhttp://www.citycollegiate.com/hybridization2.htmhttp://www.citycollegiate.com/hybridization2.htmhttp://www.citycollegiate.com/hybridization2.htmhttp://www.citycollegiate.com/hybridization2.htmhttp://www.citycollegiate.com/hybridization2.htmhttp://www.citycollegiate.com/hybridization2.htmhttp://www.citycollegiate.com/hybridization2.htmhttp://www.citycollegiate.com/hybridization2.htmhttp://www.citycollegiate.com/hybridization2.htmhttp://www.citycollegiate.com/hybridization2.htmhttp://www.citycollegiate.com/hybridization2.htmhttp://www.citycollegiate.com/hybridization2.htmhttp://www.citycollegiate.com/hybridization2.htmhttp://www.citycollegiate.com/hybridization2.htmhttp://www.citycollegiate.com/hybridization2.htmhttp://www.citycollegiate.com/hybridization2.htmhttp://www.citycollegiate.com/hybridization2.htmhttp://www.citycollegiate.com/hybridization2.htmhttp://www.citycollegiate.com/hybridization2.htmhttp://www.citycollegiate.com/hybridization2.htmhttp://www.citycollegiate.com/hybridization2.htmhttp://www.citycollegiate.com/hybridization2.htmhttp://www.citycollegiate.com/hybridization2.htmhttp://www.citycollegiate.com/hybridization2.htmhttp://www.citycollegiate.com/hybridization2.htmhttp://www.citycollegiate.com/hybridization2.htmhttp://www.citycollegiate.com/hybridization2.htmhttp://www.citycollegiate.com/hybridization2.htmhttp://www.citycollegiate.com/hybridization2.htmhttp://www.citycollegiate.com/hybridization2.htm
-
8/16/2019 Shapes of Simple Organic Molecules
14/21
"o#i$
i
Et!ye
%C)H)'
0
Eth!"e co"tai"s a triple bo"#0
I" carbo" o( triple bo"# there are two sp h!bri#
orbitals
a"#
two
&"&se#
p
orbital0
Each carbo" i" the triple bo"# is sp hybridised 0
The t4o &"&se# p orbitals oerlap aboe a"# belo4 the pla"e to (or* t4o pi +6 bo"#s
-
8/16/2019 Shapes of Simple Organic Molecules
15/21
✓ The
triple
bo"#
co"sists
o(
o"e
si%*a bo"#
a"#
t4o
pi
bo"#s✓ The %eo*etr! aro&"# each carbo" is
li"ear 4ith a bo"# a"%le o( 178o
http9::pa%es)to4so")e#&:la#o":carbo")ht*l
http://pages.towson.edu/ladon/carbon.htmlhttp://pages.towson.edu/ladon/carbon.htmlhttp://pages.towson.edu/ladon/carbon.htmlhttp://pages.towson.edu/ladon/carbon.htmlhttp://pages.towson.edu/ladon/carbon.htmlhttp://pages.towson.edu/ladon/carbon.htmlhttp://pages.towson.edu/ladon/carbon.htmlhttp://pages.towson.edu/ladon/carbon.htmlhttp://pages.towson.edu/ladon/carbon.htmlhttp://pages.towson.edu/ladon/carbon.htmlhttp://pages.towson.edu/ladon/carbon.htmlhttp://pages.towson.edu/ladon/carbon.htmlhttp://pages.towson.edu/ladon/carbon.htmlhttp://pages.towson.edu/ladon/carbon.htmlhttp://pages.towson.edu/ladon/carbon.htmlhttp://pages.towson.edu/ladon/carbon.htmlhttp://pages.towson.edu/ladon/carbon.htmlhttp://pages.towson.edu/ladon/carbon.htmlhttp://pages.towson.edu/ladon/carbon.htmlhttp://pages.towson.edu/ladon/carbon.htmlhttp://pages.towson.edu/ladon/carbon.htmlhttp://pages.towson.edu/ladon/carbon.htmlhttp://pages.towson.edu/ladon/carbon.htmlhttp://pages.towson.edu/ladon/carbon.htmlhttp://pages.towson.edu/ladon/carbon.htmlhttp://pages.towson.edu/ladon/carbon.htmlhttp://pages.towson.edu/ladon/carbon.htmlhttp://pages.towson.edu/ladon/carbon.htmlhttp://pages.towson.edu/ladon/carbon.htmlhttp://pages.towson.edu/ladon/carbon.htmlhttp://pages.towson.edu/ladon/carbon.htmlhttp://pages.towson.edu/ladon/carbon.htmlhttp://pages.towson.edu/ladon/carbon.htmlhttp://pages.towson.edu/ladon/carbon.html
-
8/16/2019 Shapes of Simple Organic Molecules
16/21
"e.eeSix carbo" ato*s i" a ri"%
Sho4s reso"a"ce h!bri#isatio"
Hexa%o"al i" shape - at each apex there is acarbo" bo"#e# to a h!#ro%e"
Each carbo" is bo"#e# to three other ato*s; a
h!#ro%e" a"# t4o other carbo" ato*s
Each carbo" &ses sp2 h!bri# orbitals
Each carbo" co"tai"s a p&re p orbital
perpe"#ic&lar to the pla"e o( the ri"%
-
8/16/2019 Shapes of Simple Organic Molecules
17/21
"e.ee
Each &"h!bri#i$e# p orbitaloerlaps 4ith t4o other porbitals o"e o" each o( the t4o
"ei%hbo&ri"% carbo" ato*s
A lar%e circ&lar pi-t!pe bo"# is(or*e# aboe a"# belo4 thepla"e
Electro"s are #elocali$e# i" the
be"$e"e ri"%
-
8/16/2019 Shapes of Simple Organic Molecules
18/21
"e.ee
Oerlappi"% o( p orbitals
-
8/16/2019 Shapes of Simple Organic Molecules
19/21
"e.ee
-
8/16/2019 Shapes of Simple Organic Molecules
20/21
"e.ee
Ca"o"ical (or*s
-
8/16/2019 Shapes of Simple Organic Molecules
21/21
"e.ee
H!bri#i$e# str&ct&re