Sepsis-Induced AKI Revisited_Curr Op Crit Care 2014
description
Transcript of Sepsis-Induced AKI Revisited_Curr Op Crit Care 2014
-
Seediscussions,stats,andauthorprofilesforthispublicationat:http://www.researchgate.net/publication/266975585
Sepsis-inducedacutekidneyinjuryrevisited:Pathophysiology,preventionandfuturetherapiesARTICLEinCURRENTOPINIONINCRITICALCAREOCTOBER2014ImpactFactor:2.62DOI:10.1097/MCC.0000000000000153Source:PubMed
CITATIONS3
READS658
3AUTHORS,INCLUDING:
HernandoGmezUniversityofPittsburgh33PUBLICATIONS220CITATIONS
SEEPROFILE
JohnAKellumUniversityofPittsburgh298PUBLICATIONS15,544CITATIONS
SEEPROFILE
Availablefrom:HernandoGmezRetrievedon:08October2015
-
Copyrig
CURRENTOPINION Sepsis-induced acute kidney injury revisited:n and future therapies
Gomezb,c, and John A. Kellumb,c
n critically ill patients and is associated with increasedause of AKI. Considerable evidence now suggestsI are different from those seen in other causes of AKI.a anes
s fllyflousoc
Summary
maladaptive responses to the septic insult. Preventive and therapeutic measures should be based oncounteracting these maladaptive responses of tubular epithelial cells, inflammation, and microvasculardysfunct
Keyworacute kid
INTRODUCTION
Acute kidney injury (pitalized patients anmortality [1]. The insurgery has been repothe incidence amonghigh as 70%, with anwhen AKI is part of thsyndrome [2,3]. AKI isdeath [4], and patiincreased risk to devAKI is a syndrome comditions, and outcomesdisease. The most common cause of AKI in criticallyill patients is sepsis. Despite considuring the last decades, the pathsepsis-istood.
Inwas cotion [5
Center, University of Pittsburgh, Pennsylvania, USA
Correspondence to John A. Kellum, MD, MCCM, Professor of Critical
c.edu
www.c
REVIEWthe not-so-distant past, sepsis-induced AKInsidered a disease of the renal macrocircula-] resulting from global renal ischemia, cellular
Curr Opin Crit Care 2014, 20:588595
DOI:10.1097/MCC.0000000000000153
o-criticalcare.com Volume 20 Number 6 December 2014hUSA.@upmt Lippincott Williams &pletely under- Hall, University of Pittsburgh, 3550 Terrace Street, Pittsburgh, PA 15261,Tel: +1 412 647 8110; fax: +1 412 647 2645; e-mail: kellumjanduced AKI remains incomengineering, Director, Center for Critical Care Nephrology, 604, Scaifederable researchophysiology of
Care Medicine, Medicine, Clinical and Translational Science, and Bio-ion.
dsney injury, inflammation, microvascular dysfunction, sepsis, tubular epithelial cells
AKI) occurs in 135% of hos-d is associated with highcidence of AKI after generalrted to be about 1%, whereascritically ill patients can be asin-hospital mortality of 50%e multiple organ dysfunctionan independent risk factor forents who survive have anelop chronic kidney disease.prising multiple clinical con-are influenced by underlying
damage, and acute tubular necrosis. However, anincreasing body of evidence suggests that AKI canoccur in the absence of hypoperfusion [6
&
,7]. In ahuman study, Prowle et al. [8] were able to demon-strate that decreased renal blood flow (RBF) was nota universal finding in patients with sepsis-inducedAKI. In addition,Murugan et al. [9] demonstrated, ina prospective multicenter study of more than 1800patients with community-acquired pneumonia,
aDepartment of Anesthesiology, Intensive Care and Pain Medicine,University of Munster, Munster, Germany, bCenter for Critical CareNephrology and cDepartment of Critical Care Medicine, CRISMAAn understanding of the pathologic mechanisms of sepsis-induced AKI emphasizes the important role ofpathophysiology, preventio
Alexander Zarbocka, Hernando
Purpose of reviewAcute kidney injury (AKI) is a common complication imorbidity and mortality. Sepsis is the most common cthat the pathogenic mechanisms of sepsis-induced AKThis review focuses on the recent advances in this aremight derive from these new insights into the pathoge
Recent findingsThe traditional paradigm that sepsis-induced AKI ariseevidence that total renal blood flow in is not universathe presence of normal or even increased renal bloodadaptive responses of tubular epithelial cells to injurioSimultaneously occurring renal inflammation and micrmechanisms. Wilkins. Unauthorized nd discusses possible therapeutic interventions thatis of sepsis-induced AKI.
rom ischemia has been challenged by recentimpaired during sepsis, and AKI can develop inw. Animal and human studies suggest thatsignals are responsible for renal dysfunction.irculatory dysfunction further amplify thesereproduction of this article is prohibited.
-
Copyr
that AKwithouinterleuand wopatientted toinstabiclinicalthat infrom setion anpodocypatientfunctioimmunattempchangesentingwere chwith foentirelyimpairmchangeand dea redudysfundecreasthat thperfusirate unthat apreviou[13]. Thanismsinvolve
AKI. A consistent finding in septic humans, inde-pendent of the severity of AKI, is the presence of thefollowing three pathologic findings: microcircula-
dytiviscsisape
HOT
ouceootes ishanntical
hins
onnses to injury (Fig. 1) [7]. Therefore, we hypoth-theylarmmn
ASI
is cio
KEY POINTS
The heterogeneous distribution of RBF induced by themicrotubulhypoandepith
Proinfiltereandresulstate
Recearres
As dAKI,prev
Sepsis-induced acute kidney injury revisited Zarbock et al.
1070-529I was a common condition, even in patientst severe disease. Higher cytokine levels [e.g.,kin 6 (IL-6)] were associated with severityrsening of AKI [9]. Moreover, most of thes with sepsis-induced AKI were never admit-the ICU nor suffered from hemodynamiclity [9]. Complementary to the insights fromstudies, in-vitro experiments have revealed
cubating human epithelial cells with plasmaptic patients resulted in decreased cell func-d shortened the survival of tubular cells andtes, suggesting that the plasma from septics can induce renal cell injury and dys-n absent any vasculature or circulatinge effector cells. Recent post-mortem studiested to more closely describe the pathologicals in septic kidneys [10
&&
,11]. Despite repre-
toryadapto dgenether
PATACU
Althinduare mphensinglsepsimecpoteclinimorewhictheiranismmatirespoesizekidntubuinfladysfu
RENSEP
Sepsculat
circulatory dysfunction probably causes patchyar cell injury during sepsis-induced AKI, whereasxia and hypoperfusion may amplify inflammationcontribute to an adaptive response of tubularelial cells.
flammatory cytokines released during sepsis ared in the glomerulus, enter the proximal tubuluscan directly activate tubular epithelial cellsting in a change of the metabolic and functionalof these cells.
nt clinical evidence suggests that G1 cell cyclet of tubular epithelial cells is involved in AKI.
ifferent mechanisms are involved in sepsis-inducedit is unlikely that a single treatment may be able toent or treat sepsis-induced AKI.ight Lippincott Williams & Wilkins. Unauthorize
the latest stages of the disease, these kidneysaracterized by a strikingly bland histologycal areas of tubular injury, which was alsodiscordant with the profound functionalent seen premortem. Interestingly, all these
s can occur in the presence of a normal RBF,fine the clinical phenotype characterized byced glomerular filtration rate and tubularction. Although RBF does not universallye during sepsis, some data exist suggestinge blood pressure can directly influence theon of the kidney and glomerular filtrationder some pathological conditions [12] andhigher blood pressure in patients withs hypertension can prevent AKI during sepsisese data support the hypothesis that mech-other than tissue hypoperfusion are
d in the pathogenesis of sepsis-induced
a decrebutionmicroca signif[14,15]regiona
Duwhenincreasmodelincreason sepdistribution of tubular cellular injury with apicalvacuolinecrosiin thedespiteIncreas
5 2014 Wolters Kluwer Health | Lippincott Williams & Wilkinsd reproduction of this article is prohibited.
zation, but without extensive apoptosis ors [10
&&
]. Alterations in the microcirculationrenal cortex or renal medulla can occurnormal or even increased global RBF [19].ed renal vascular resistance may represent an
www.co-criticalcare.com 589at a key event in the early dysfunction of theduring sepsis is a bioenergetic stress of theepithelial cells, in response to the amplifiedatory signal that peritubular microvascular
ction generates.
L MICROCIRCULATION DURINGS-INDUCED ACUTE KIDNEY INJURY
auses a profound alteration of the macrocir-n andmicrocirculation and is characterizedbyased peripheral vascular resistance, maldistri-of tissue blood flow, and derangement ofirculatory perfusion. These alterations causeicant decrease in functional capillary densityand an increment in the heterogeneity ofl blood flow distribution [16].ring the initial hyperdynamic stage of sepsis,AKI develops, cardiac output is usuallyed. RBF was markedly increased in a sheepof sepsis [17], and yet AKI developed despiteed RBF [18]. Similarly, post-mortem studiestic patients have shown the heterogeneoussfunction, inflammation, and bioenergetice response to injury. The aim of this review isuss the role of these mechanisms in theof sepsis-induced AKI and the potentialutic implications.
PHYSIOLOGY OF SEPSIS-INDUCEDE KIDNEY INJURY
gh the functional consequences during sepsis-d AKI are dramatic, the histological changesderate and do not entirely explain the clinicalype.Recentevidence suggests that insteadof amechanism being responsible for its cause,associatedwith an entire orchestra of cellularisms, adaptive and maladaptive, whichate each other and ultimately give rise toAKI. The microcirculation is perhaps the
important physiological compartment inthese mechanisms come together and exerttegrated and deleterious action. These mech-include endothelial dysfunction, inflam-
, coagulation disturbance, and adaptive cell
-
Copyrigh
importthe dev
Ofkidneylar dysintra-abresistanresistanbeen ptitrate non theoptimaimprovsubpopage rem
Placytes tresponvasculaand lea
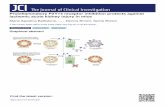
Afferent arteriole Efferent arteriole
ritu
P
FIGURE
side. Inflcan subsreleasedDAMP/PPAMPs,
Renal system
590Pe
roximal tubulet Lippincott Williams & Wilkins. Unauthorized
ant hemodynamic factor that is involved inelopment of sepsis-induced AKI.course, decreased RBF can cause injury toand when sepsis-induced renal microvascu-function is combined with an increase indominal pressure, increased renal vascularce results. Measurement of renal vascularce using renal Doppler at the bedside hasroposed by Deruddre et al. [20] as a tool toorepinephrine in septic shock patients basedrenal arterial resistance to determine thel mean arterial pressure. However, whetherement of regional blood flow, even in thisulation of patients, will prevent tubular dam-ains to be substantiated.telets, fibrin, stiff red blood cells, and leuko-ogether with endothelial cell swelling aresible for capillary occlusion [21]. Increasedr permeability is a common feature in sepsisds to interstitial edema and fluid retention
(Fig. 1)the sevedematargetin thethe kidlation adeterioalteringvenous
Endvasculaand altInjured(e.g., nresponbloodtors, vathelialmajor
1. During sepsis, DAMPs, PAMPs, and cytokines may potentiallammatory mediators derived from bacteria or immune cells are filquently injure tubular cells by binding to their respective receptorsfrom extravasated leukocytes and can also activate tubular cells fAMP receptors may induce apoptosis or cell cycle arrest. DAMPspathogen-associated molecular patterns.
www.co-criticalcare.combular capillary
Leukocyte
Cytokine/DAMPs and PAMPS
Cytokine and DAMP/PAMP-receptor
Cell cyclearrest
Apoptosisreproduction of this article is prohibited.
[22,23]. In addition to its association witherity of sepsis, fluid overload and interstitialincrease the diffusion distance for oxygen tocells [24]. Similar findings can be observedrenal microcirculation [25]. Furthermore, asney is an encapsulated organ, fluid accumu-nd tissue edema contribute to the observedration of renal microcirculatory perfusion bytransmural pressures and by aggravatingcongestion [26
&
,27].othelial cells are important determinants ofr tone, leukocyte recruitment and function,er the responsiveness of smoothmuscles [28].endothelial cells produce less vasodilators
itric oxide), resulting in a more pronouncedse to vasoconstrictors with a redistribution offlow. The imbalance between vasoconstric-sodilators, and oxidative stress at the endo-level is receiving considerable attention as acontributor to the development of AKI.
y injure tubular cells from the tubular and interstitialtered in the glomerulus, enter the tubular space and. In addition, cytokines, DAMPs, and PAMPs arerom the interstitial side. The activation of cytokine or, damage-associated molecular pattern molecules;
Volume 20 Number 6 December 2014
-
Copyr ize
Augmented vasoconstriction, small vessel occlusiondue to the interaction of leukocytes with activatedendothelial cells, and activation of the coagulationsystemcirculatpatchythe hemicroc
Nitrole in[30] anglobalthe prothase (tern, reof nitrnitricheterogated nnamicsinjurynitrogesuggestupreguproximmationration oligationinhibition of renal iNOSmight have an implicationfor the
INFLADAMA
There i(IL-6, Ifactor)[9,34],inflammsepsis,inflammof infeand orgvate a vdendritrecogn(TLR),ble genoligomengagegulatioinitiatican alsoinjuredpatternand S1
The cytokine storm during the initial phase ofsevere sepsis activates leukocytes, endothelial cells,and epithelial cells leading to leukocyte and platelet
atitissatebiltesetini
edthceimeyou[3sioitmis-iceaind),r ecesati
meabilcu0,4adisincia1lard fciamar thlarbos btoTLlianowy,titamBulaBfl
Sepsis-induced acute kidney injury revisited Zarbock et al.
1070-529ight Lippincott Williams & Wilkins. Unauthor
treatment of sepsis-induced AKI.
MMATION PROPAGATES RENALGE DURING SEPSIS
s a strong association between cytokine levelsL-10, and macrophage migration inhibitoryand the development of sepsis-induced AKIsuggesting an important role of systemicatory mediators in this process. During
infection triggers a host response, in whichatory mechanisms contribute to clearance
ction and tissue recovery on the one hand,an injury on the other [35]. Pathogens acti-ariety of cells, including renal epithelial andic cells through an interaction with pattern-ition receptors including toll-like receptorsC-type lectin receptors, retinoic acid induci-e 1-like receptors, and nucleotide-bindingerization domain-like receptors [36]. Thement of these receptors results in the upre-n of inflammatory gene transcription andon of innate immunity. The same receptorsdetect endogenous molecules released fromcells, so-called damage-associatedmoleculars, such as DNA, RNA, histones, HMGPB1,00 proteins [37].
mole[6
&
,4havecanreleaasso(Fig.tubuvateassoflamentetubumetaIt habindandhaverelevunknstudconsin dHMGstimHMGproin
5 2014 Wolters Kluwer Health | Lippincott Williams & Wilkinsresults in local compromise of the micro-ion and regional ischemia [25,29]. Thetubular cell injury [10
&&
] probably reflectsterogeneous distribution of RBF caused byirculatory dysfunction.ric oxide plays a pivotal and multifacetedthe complex pathophysiology of sepsisd sepsis-induced AKI [31]. During sepsis,nitric oxide production increases, whereasducing enzyme, inducible nitric oxide syn-iNOS), has a heterogeneous expression pat-sulting in different regional concentrationsic oxide [30]. The uneven distribution ofoxide production may contribute to theeneous perfusion pattern. However, elev-itric oxide also influences renal hemody-and causes peroxynitrite-related tubularthrough the local generation of reactiven species during sepsis [32]. Evidences that this may play an important role aslation of iNOS has been associated withal tubular injury during systemic inflam-, and its selective inhibition, with amelio-f the functional impairment caused by cecaland puncture [33]. Therefore, the selective
activandactivmeagulareleaselecuponstratintoinduthekidnAlthAKIadherecrusepsinduexplgrate(ROSothestanactivd reproduction of this article is prohibited.
on, microvascular dysfunction, hypoxia,ue damage [35]. Proinflammatory mediatorsendothelial cells and increase vascular per-
ity (Fig. 1). Activated endothelial cells upre-the expression of adhesion molecules andadditional proinflammatory mediators. E-, specifically induced on the endotheliumnflammatory stimulation, has been demon-to play a major role in leukocyte recruitmente kidney during the late stages of sepsis-d AKI [38
&
]. Experimental data highlightportance of leukocyte recruitment into the[38
&
], especially in later stages of AKI.gh not seen in all models of sepsis-induced9], elimination of neutrophils or blockingn molecules that are required for neutrophilent into the kidney completely abolished
nduced AKI in a cecal ligation and puncture-d sepsis model [38
&
]. This observation can beed by the fact that adherent and transmi-neutrophils release reactive oxygen speciesproteases, elastases, myeloperoxidase, andnzymes that damage the tissue. These sub-, together with leukotriene B4 and platelet-ng factor, can both increase vascular per-ity and upregulate the expression of adhesionles that promote further inflammation1]. Leukocytes leaving peritubular capillariesclose proximity to tubular epithelial cells andrectly activate tubular epithelial cells byg proinflammatory mediators and damage-ted molecular pattern molecules (DAMPs)). On the basis of the special location ofepithelial cells, these cells can also be acti-
rom the tubular side [42]. DAMPs, pathogen-ted molecular patterns (PAMPs), and proin-tory cytokines are filtered in the glomerulus,e proximal tubulus and can directly activateepithelial cells resulting in a change of thelic and functional state of these cells (Fig. 1).een recently shown that these molecules canand activate tubular cells by binding to TLR2R4 [43,44,45
&
,46]. Although animal studiesnked TLR4 signaling to kidney injury, thece of TLR activation in human kidney wasn until recently. In a very elegant human
Kruger et al. [43] demonstrate that TLR4 isutively expressed in kidneys and that tubulesaged kidneys also stain positively for
1, a known endogenous TLR4 ligand. In-vitrotion of human tubular epithelial cells with1 confirmed that HMGB1 can stimulateammatory responses through TLR4 [43].
www.co-criticalcare.com 591
-
Copyrigh d
The released proinflammatory mediators can act inan autocrine and paracrine fashion and may con-tribute to further tubular cell damage. In agreementwith thfunctiomore htoxin itubulestress isuggestvalue i
ADAPCELLSENVIR
Tubulasequenprimaralteredthis sigparacrioccurstherefothologi
OxAKI. Poinducevacuolistress [treatedpatientproducOxidat[50].
Rectubular[10
&&
],exposeesses thThis catubularmetabo(Fig. 1)by mitprovidetion. Swbe found in humans with sepsis-induced AKI, causea reducexistinsurvivaThis isdria thkidneyand oxassocia
and organ dysfunction, and worse outcome in crit-ically ill patients [54]. Furthermore, abnormalmitophagy has also been linked to progressive renal
yguntnribit
t,ionon
eintent57larctiThartesict
ENRA
atennatebiopct kidneys at risk for injury and thus enableerheysarceicline cntt
peas
(e.g., IL[34e mcetoexptioiony
Renal system
592t Lippincott Williams & Wilkins. Unauthorize
ed tubular cell function by prioritizing theg energy to functions that are required for celll. Another important feature is mitophagy.a process that removes damaged mitochon-rough autophagy and can be induced in theby several factors including inflammationidative stress [54]. Decreased mitophagy isted with a proximal tubular dysfunction, cell
AKIthesinduendoandsorpligatstud
www.co-criticalcare.comese findings, kidneys with a TLR4 loss-of-n allele contained less TNF-a, MCP-1, andeme oxygenase 1 [43]. During sepsis, endo-n the tubule binds to TLR4 on S1 proximalcells, which subsequently causes oxidativen cells of the neighboring S2 segment [44],ing that targeting TLR4 signaling may haven preventing or treating AKI.
TIVE RESPONSES OF TUBULARTO CHANGES IN THE LOCALONMENT
r cells exposed to inflammation and the con-ces of microcirculatory dysfunction act asy targets and respond by adaptation to thetubular environment. They may also spreadnal and shutdown other tubular cells in ane fashion [43]. Microvascular dysfunctionin heterogeneous regions of the kidney and,re, may explain the heterogeneous histopa-c changes of tubular epithelial cells.idative stress is a hallmark of sepsis-inducedst-mortem studies in humans with sepsis-d AKI show apical epithelial tubular cellzation, which has been linked to oxidative47]. Cultured tubular cells and podocyteswith components of bacteria or plasma froms with severe burns and sepsis-associated AKIe ROS [48] or undergo apoptosis [48,49].ive stress is also linked to tubular dysfunction
ent studies demonstrate that apoptosis ofcells is rare during sepsis-induced AKIsuggesting that tubular epithelial cells
d to hypoxia and inflammation limit proc-at can result in apoptosis or necrosis (Fig. 1).n be achieved by an adaptive response ofcells characterized by downregulating
lism and undergoing cell cycle arrest[5153]. This response may be orchestratedochondria and it limits further damage ands cells with the opportunity to recover func-ollen and injured mitochondria, which can
injuruprepatievatiocont
Marresdivisdemcycleprotlopropatie[56
&
,tuburedu[59].cycleundeInterpred
POTTHE
To dprevreasotoo lnewpathdeteearli
Tlar dcellsindupeutinterthesponegereddamincrereproduction of this article is prohibited.
initiation of interventions [56&
,57,58&&
].knowledge that inflammation, microvascu-
function, and adaptive responses of tubulare involved in the development of sepsis-d AKI provides new diagnostic and thera-avenues. As these mechanisms are closelyked with each other, modulating one ofomponents simultaneously alters other com-s. The recognition of inflammation has trig-he investigation of therapeutic strategies ton inflammation to prevent/treat AKI. Ased levels of proinflammatory mediators-6) are associated with the development of], it is tempting to speculate that eliminatingediators or endotoxin can prevent sepsis-
d AKI. Indeed, elimination of cytokines andxin is feasible by hemoadsorption [61,62]erimentally it has been shown that hemoad-n completely protects against AKI in a cecaland puncturemodel of sepsis [63]. A clinical
demonstrated that reducing endotoxin by
Volume 20 Number 6 December 2014[55]. However, mitophagy was significantlylated in septic kidneys [10
&&
]. As most of thes had an already established AKI, this obser-let us speculate that increased mitophagyutes to renal recovery.ochondria are also involved in cell cyclewhich is a quality control process of cell. Recent clinical studies have independentlystrated that two markers involved in G1 cellarrest, insulin-like growth factor-binding7 (IGFBP7) and tissue inhibitor of metal-inase 2 (TIMP-2), predict AKI in critically ills and in patients undergoing cardiac surgery,58
&&
], suggesting that cell cycle arrest ofepithelial cells is involved in AKI. The
on in ATP production triggers cell cycle arresterefore, a reduced ATP level may induce cellrrest in these cells and prevent the cell fromaking a process that could end in cell death.tingly, these cell cycle arrest markers can alsorenal recovery [56
&
,60].
TIAL FOR DIAGNOSTIC ANDPEUTIC TARGETS
e, no therapeutic measures are available tot or treat sepsis-induced AKI. A potentialfor this may be that often therapy is startedin the disease process. The development of
omarkers, which also provide insights in thehysiology of the disease, makes it possible to
-
Copyr ize
polymyxin-B hemoperfusion reduced RIFLE scoresand urine tubular enzymes [62]. Another option tointerfere with cytokines and endotoxin is the appli-cationphosphdetoxifendotoATP anHeemsphosphdecreastubulenuatedmarker. In a small, randomized trial, Pickkers et al.[65] shalkalinendogerequiretherapyanothein theated wdistantimporttory mand bacan lateof inju
Micinitiatethe misettingincludiistratio[30,32]on theropoietinjurythasemeabilmechaninterrelunlikelemergetreatme
CONC
In concAKI ismacrovquestioence ocontrasis charaepithel
by healthy or reversibly injured renal tubular epi-thelial cells.
New evidence suggests that the inflammatorynhecer tsursimmtivsio
ment owaonntegyce
no
wanerBI 6802 awarded to
fli
authors have no conflicts of interest.
ERDofighlspeout
llomeasuconitiatistermcordngbn, asteoblehrie1:1omeneye tunt reof dDouteowlenal furugnsewerkassfun7:5a c
ction
Sepsis-induced acute kidney injury revisited Zarbock et al.
1070-529ight Lippincott Williams & Wilkins. Unauthor
owed that the administration of exogenouse phosphatase in septic patients improvednous creatinine clearance and reduced thement and duration of renal replacement. Modulating TNF-a signaling might ber therapy option because a polymorphismpromoter region of the TNFA gene is associ-ith markers of kidney disease severity andorgan dysfunction [66]. However, it is
ant to keep in mind that these proinflamma-ediators are required for the host responsecterial clearance during sepsis and that theyr provide necessary signals for the resolutionry.rocirculatory dysfunction during AKIs hypoxia and inflammation. To improvecrocirculatory perfusion, vasodilators in theof sepsis are currently under investigationng nitroglycerin [14,67], nitric oxide admin-n, andmodulation of nitric oxide production. Furthermore, drugs with pleiotropic effectsvasculature, such as statins [68] and eryth-in [69], have the potential to prevent kidneyby enhancing endothelial nitric oxide syn-expression and decreasing vascular per-ity. However, on the basis of the differentisms involved in sepsis-induced AKI and theationship among these mechanisms, it isy that a single treatment modality mayas a magic bullet in the prevention and/ornt of sepsis-induced AKI.
LUSION
lusion, the old paradigm that sepsis-inducedinitiated by renal ischemia as a result ofascular dysfunction has been called inton because AKI can also develop in the pres-f normal or increased RBF. Furthermore, int to renal ischemia reperfusion injury, whichcterized by apoptosis or necrosis of tubularial cells, sepsis-induced AKI is characterized
pathmatiponestratindu
Ack
ThisGermKronNHLH.G.
Con
The
REFREAPapersbeen h& of&& of
1. BemSeIn
2. Oac
3. Sitio
4. Hopr
5. Sc35
6.&
Gkidth
Excellereview7. Le
ac8. Pr
re9. M
nolo
10.&&
Tady18
This isdysfun
5 2014 Wolters Kluwer Health | Lippincott Williams & Wilkinsof exogenous alkaline phosphatase. Alkalineatase is an endogenous enzyme that exertsying effects through dephosphorylation ofxins and proinflammatory extracellulard is reduced during systemic inflammation.kerk et al. [64] demonstrated that alkalineatase application was associated with aed expression of iNOS synthase in proximalcells isolated from urine related to an atte-urinary excretion of a proximal tubule injury
respoof tinduordecellTheandadapriousis alsd reproduction of this article is prohibited.
ENCES AND RECOMMENDEDINGparticular interest, published within the annual period of review, haveighted as:cial intereststanding interest
o R, Ronco C, Kellum JA, et al. Acute renal failure: definition, outcomeres, animal models, fluid therapy and information technology needs: thed International Consensus Conference of the Acute Dialysis Qualityve (ADQI) Group. Crit care 2004; 8:R204R212.ann M, Chang RW. Acute kidney injury in the intensive care uniting to RIFLE. Crit Care Med 2007; 35:18371843.artl K, Kellum JA. AKI in the ICU: definition, epidemiology, risk stratifica-nd outcomes. Kidney Int 2012; 81:819825.EA, Schurgers M. Epidemiology of acute kidney injury: how big is them? Crit Care Med 2008; 36:S146S151.r RW, Wang W. Acute renal failure and sepsis. N Engl J Med 2004;59169.z H, Ince C, De Backer D, et al. A unified theory of sepsis-induced acuteinjury: inflammation, microcirculatory dysfunction, bioenergetics, and
bular cell adaptation to injury. Shock 2014; 41:311.view on the pathophysiology of sepsis-induced AKI. A comprehensiveifferent mechanims causing AKI during sepsis are presented.rze M, Legrand M, Payen D, Ince C. The role of the microcirculation inkidney injury. Curr Opin Crit Care 2009; 15:503508.JR, Ishikawa K, May CN, Bellomo R. Renal blood flow during acute
ailure in man. Blood Purif 2009; 28:216225.an R, Karajala-Subramanyam V, Lee M, et al. Acute kidney injury invere pneumonia is associated with an increased immune response andsurvival. Kidney Int 2010; 77:527535.u O, Gaut JP, Watanabe E, et al. Mechanisms of cardiac and renalction in patients dying of sepsis. Am J Respir Crit Care Med 2013;09517.linical trial to investigate the pathophysiology of cardiac and renalin patients dying of sepsis.
www.co-criticalcare.com 593cts of interestFresenius Stiftung awardedgrant number 1K12HL1090se during sepsis causes an adaptive responsetubular epithelial cells. These alterationsa downregulation of the cell function ino minimize energy demand and to ensurevival. The result is reduced kidney function.ultaneous occurrence of renal inflammationicrovascular dysfunction exacerbates thee response of tubular epithelial cells to inju-gnals. In addition, the endothelial cell injuryof importance in the initiation and develop-f sepsis-induced AKI through the nitric oxidey, leukocyte adhesion, ROS, and inflam-. Targeting tubular epithelial cells and com-s of the microcirculation may be an effective
in preventing and/or treating sepsis-d AKI.
wledgements
ork was funded by a research grant from theresearch foundation (ZA428/61) and Else-
to A.Z., and NIH/
-
Copyrigh d
11. Lerolle N, Nochy D, Guerot E, et al. Histopathology of septic shock inducedacute kidney injury: apoptosis and leukocyte infiltration. Intensive Care Med2010; 36:471478.
12. Redfors B, Bragadottir G, Sellgren J, et al. Effects of norepinephrine on renalperfusion, filtration and oxygenation in vasodilatory shock and acute kidneyinjury. Intensive Care Med 2011; 37:6067.
13. Asfarpatien
14. Spronintrava
15. Donatcircula13:25
16. De Bapatien
17. Di Giahyperd
18. Chvojklatory,tic sho
19. Bezemmicrovimagin
20. Derudshockrenal rMed 2
21. De Bapotent1:27.
22. Prowleinjury.
23. Bagshimpacinjury.
24. Hollendynam2004;
25. Holthotion, psepsis
26.&
Prowleattenu
Excellent reAKI.27. Rajend
injury28. Sprag
vascul29. Seely
a pediPhysio
30. Trzecisepsisand ch
31. Aksu Utoxic tNephr
32. Heemtion fo2009;
33. Tiwariflow icaspa
34. Payenkidneypheno
35. Angus2013;
36. Takeu2010;
37. ChanClin In
38.&
HerterneutroImmun
Experimentneutrophil39. Singb
talk du80:63
40. Brownorgan
41. Zarbock A, Ley K. Mechanisms and consequences of neutrophil interactionwith the endothelium. Am J Pathol 2008; 172:17.
42. El-Achkar TM, Hosein M, Dagher PC. Pathways of renal injury in systemicgram-negative sepsis. Eur J Clin Invest 2008; 38:3944.
43. Kruger B, Krick S, Dhillon N, et al. Donor toll-like receptor 4 contributes toischemia and reperfusion injury following human kidney transplantation. Proc
tl Alakebulac NudalLR)nalentepd DtubM,n inu Lecien anancouce97;arianbula:R4orrering19.ngedocationealesfun0:2ealeng-teompan Ms a1.nsallula3.eersrly brgerinica7 sesur
horaomarit Cshacle5.ulticFBPs. Ber candall cyn ineggoteo13;llummov:26antactiv:16ngediaperiement iare Mckkeent oind p
Renal system
594t Lippincott Williams & Wilkins. Unauthorize
P, Meziani F, Hamel JF, et al. High versus low blood-pressure target ints with septic shock. N Engl J Med 2014; 370:15831593.k PE, Ince C, Gardien MJ, et al. Nitroglycerin in septic shock afterscular volume resuscitation. Lancet 2002; 360:13951396.i A, Damiani E, Botticelli L, et al. The aPC treatment improves micro-tion in severe sepsis/septic shock syndrome. BMC Anesthesiol 2013;.cker D, Creteur J, Preiser JC, et al.Microvascular blood flow is altered ints with sepsis. Am J Respir Crit Care Med 2002; 166:98104.ntomasso D, May CN, Bellomo R. Vital organ blood flow duringynamic sepsis. Chest 2003; 124:10531059.a J, Sykora R, Krouzecky A, et al. Renal haemodynamic, microcircu-metabolic and histopathological responses to peritonitis-induced sep-ck in pigs. Crit Care 2008; 12:R164.er R, Legrand M, Klijn E, et al. Real-time assessment of renal corticalascular perfusion heterogeneities using near-infrared laser speckleg. Opt Express 2010; 18:1505415061.dre S, Cheisson G, Mazoit JX, et al. Renal arterial resistance in septic: effects of increasing mean arterial pressure with norepinephrine on theesistive index assessed with Doppler ultrasonography. Intensive Care007; 33:15571562.cker D, Donadello K, Taccone FS, et al. Microcirculatory alterations:ial mechanisms and implications for therapy. Ann Intensive Care 2011;
JR, Echeverri JE, Ligabo EV, et al. Fluid balance and acute kidneyNat Rev Nephrol 2010; 6:107115.aw SM, Brophy PD, Cruz D, Ronco C. Fluid balance as a biomarker:t of fluid overload on outcome in critically ill patients with acute kidneyCrit Care 2008; 12:169.berg SM, Ahrens TS, Annane D, et al. Practice parameters for hemo-ic support of sepsis in adult patients: 2004 update. Crit Care Med32:19281948.ff JH, Wang Z, Seely KA, et al. Resveratrol improves renal microcircula-rotects the tubular epithelium, and prolongs survival in a mouse model of-induced acute kidney injury. Kidney Int 2012; 81:370378.JR, Kirwan CJ, Bellomo R. Fluid management for the prevention and
ation of acute kidney injury. Nat Rev Nephrol 2014; 10:3747.view on the crucial role of optimal volume management in patients with
ram R, Prowle JR. Venous congestion: are we adding insult to kidneyin sepsis? Crit Care 2014; 18:104.ue AH, Khalil RA. Inflammatory cytokines in vascular dysfunction andar disease. Biochem Pharmacol 2009; 78:539552.KA, Holthoff JH, Burns ST, et al.Hemodynamic changes in the kidney inatric rat model of sepsis-induced acute kidney injury. Am J Physiol Renall 2011; 301:F209F217.ak S, Cinel I, Phillip Dellinger R, et al.Resuscitating themicrocirculation in: the central role of nitric oxide, emerging concepts for novel therapies,allenges for clinical trials. Acad Emerg Med 2008; 15:399413., Demirci C, Ince C. The pathogenesis of acute kidney injury and theriangle of oxygen, reactive oxygen species and nitric oxide. Contribol 2011; 174:119128.skerk S, Masereeuw R, Russel FG, Pickkers P. Selective iNOS inhibi-r the treatment of sepsis-induced acute kidney injury. Nat Rev Nephrol5:629640.MM, Brock RW, Megyesi JK, et al. Disruption of renal peritubular bloodn lipopolysaccharide-induced renal failure: role of nitric oxide andses. Am J Physiol Renal Physiol 2005; 289:F1324F1332.D, Lukaszewicz AC, Legrand M, et al. A multicentre study of acuteinjury in severe sepsis and septic shock: association with inflammatorytype and HLA genotype. PLoS One 2012; 7:e35838.DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med369:840851.chi O, Akira S. Pattern recognition receptors and inflammation. Cell140:805820.JK, Roth J, Oppenheim JJ, et al. Alarmins: awaiting a clinical response. Jvest 2012; 122:27112719.JM, Rossaint J, Spieker T, Zarbock A. Adhesion molecules involved inphil recruitment during sepsis-induced acute kidney injury. J Innate2014; 6:597606.al study investigating the different adhesion molecules required forrecruitment into the kidney during sepsis-induced AKI.artl K, Bishop JV, Wen X, et al. Differential effects of kidney-lung cross-ring acute kidney injury and bacterial pneumonia. Kidney Int 2011;3644.KA, Brain SD, Pearson JD, et al.Neutrophils in development of multiplefailure in sepsis. Lancet 2006; 368:157169.
Na44. Ka
tuSo
45.&
M(TRe
Experimtubularreleaseactivate46. Lin
tio47. W
sptio
48. Biind19
49. Mtu12
50. Mdu13
51. Sienm
52. Brdy36
53. BrloC
54. Zhism58
55. Sace28
56.&
Measu
This clIGFBPcardiac57. Bi
biC
58.&&
KacyR2
This mand IGpatienttion ov59. M
cetio
60. Arpr20
61. Kere36
62. Cina34
63. Pemex
64. HemC
65. Pimbl
www.co-criticalcare.comreproduction of this article is prohibited.
cad Sci U S A 2009; 106:33903395.che R, Hato T, Rhodes G, et al. Endotoxin uptake by S1 proximalr segment causes oxidative stress in the downstream S2 segment. J Amephrol 2011; 22:15051516.iar H, Pollock C, Komala MG, et al. The role of Toll-like receptor proteins2 and 4 in mediating inflammation in proximal tubules. Am J PhysiolPhysiol 2013; 305:F143F154.al study showing that the binding of DAMPs to TLR2 and TLR4 onithelial cells induces a dysfunction of these cells, suggesting thatAMPs during sepsis are filtered in the glomerulus and can directlyular epithelial cells.Yiu WH, Wu HJ, et al. Toll-like receptor 4 promotes tubular inflamma-diabetic nephropathy. J Am Soc Nephrol 2012; 23:86102., Gokden N, Mayeux PR. Evidence for the role of reactive nitrogens in polymicrobial sepsis-induced renal peritubular capillary dysfunc-d tubular injury. J Am Soc Nephrol 2007; 18:18071815.ne L, Conaldi PG, Toniolo A, Camussi G. Escherichia coli porins proinflammatory alterations in renal tubular cells. Exp Nephrol5:330336.o F, Cantaluppi V, Stella M, et al. Circulating plasma factors inducer and glomerular alterations in septic burns patients. Crit Care 2008;2.ll ED, Kellum JA, Hallows KR, Pastor-Soler NM. Epithelial transportseptic acute kidney injury. Nephrol Dial Transplant 2013; 29:1312
r M, De Santis V, Vitale D, Jeffcoate W. Multiorgan failure is an adaptive,rine-mediated, metabolic response to overwhelming systemic inflam-. Lancet 2004; 364:545548.y D, Brand M, Hargreaves I, et al. Association between mitochondrialction and severity and outcome of septic shock. Lancet 2002;19223.y D, Karyampudi S, Jacques TS, et al. Mitochondrial dysfunction in arm rodent model of sepsis and organ failure. Am J Physiol Regul IntegrPhysiol 2004; 286:R491R497., Brooks C, Liu F, et al. Mitochondrial dynamics: regulatory mechan-
nd emerging role in renal pathophysiology. Kidney Int 2013; 83:568
nwal P, Yen B, Gahl WA, et al. Mitochondrial autophagy promotesr injury in nephropathic cystinosis. J Am Soc Nephrol 2010; 21:272
ch M, Schmidt C, Van Aken H, et al. Urinary TIMP-2 and IGFBP7 asiomarkers of acute kidney injury and renal recovery following cardiacy. PLoS One 2014; 9:e93460.l study demonstrates that the cell cycle arrest markers TIMP-2 andrve as sensitive and specific biomarkers to predict AKI early aftergery and to predict renal recovery.c A, Chawla LS, Shaw AD, et al. Validation of cell-cycle arrestrkers for acute kidney injury using clinical adjudication. Am J Respirare Med 2014; 189:932939.ni K, Al-Khafaji A, Ardiles T, et al. Discovery and validation of cellarrest biomarkers in human acute kidney injury. Crit Care 2013; 17:
enter observational trial identified two new biomarkers for AKI. TIMP-27, both inducers of G1 cell cycle arrest, can predict AKI in critically illoth markers are superior to existing markers, provide additional informa-linical variables and add mechanistic insight into AKI.l S, Guptan P, Owusu-Ansah E, Banerjee U.Mitochondrial regulation ofcle progression during development as revealed by the tenured muta-Drosophila. Dev Cell 2005; 9:843854.er F, Uehlinger DE,Witowski J, et al. Identification of IGFBP-7 by urinarymics as a novel prognostic marker in early acute kidney injury. Kidney Int85:909919.JA, Venkataraman R, Powner D, et al. Feasibility study of cytokine
al by hemoadsorption in brain-dead humans. Crit Care Med 2008;8272.luppi V, Assenzio B, Pasero D, et al. Polymyxin-B hemoperfusionates circulating proapoptotic factors. Intensive Care Medicine 2008;381645.ZY, Wang HZ, Carter MJ, et al. Acute removal of common sepsistors does not explain the effects of extracorporeal blood purification inmental sepsis. Kidney Int 2012; 81:363369.skerk S, Masereeuw R, Moesker O, et al. Alkaline phosphatase treat-mproves renal function in severe sepsis or septic shock patients. Crited 2009; 37:417423.rs P, Heemskerk S, Schouten J, et al. Alkaline phosphatase for treat-f sepsis-induced acute kidney injury: a prospective randomized double-lacebo-controlled trial. Crit Care 2012; 16:R14.
Volume 20 Number 6 December 2014
-
Copyr ize
66. Susantitaphong P, Perianayagam MC, Tighiouart H, et al. Tumor necrosisfactor alpha promoter polymorphism and severity of acute kidney injury.Nephron Clin Pract 2013; 123:6773.
67. Boerma EC, Koopmans M, Konijn A, et al. Effects of nitroglycerin on sub-lingual microcirculatory blood flow in patients with severe sepsis/septic shockafter a strict resuscitation protocol: a double-blind randomized placebocontrolled trial. Crit Care Med 2010; 38:93100.
68. Liakopoulos OJ, Choi YH, Haldenwang PL, et al. Impact of preoperative statintherapy on adverse postoperative outcomes in patients undergoing cardiacsurgery: a meta-analysis of over 30,000 patients. Eur Heart J 2008;29:15481559.
69. Song YR, Lee T, You SJ, et al. Prevention of acute kidney injury by erythro-poietin in patients undergoing coronary artery bypass grafting: a pilot study.Am J Nephrol 2009; 30:253260.
Sepsis-induced acute kidney injury revisited Zarbock et al.
1070-529ight Lippincott Williams & Wilkins. Unauthor5 2014 Wolters Kluwer Health | Lippincott Williams & Wilkinsd reproduction of this article is prohibited.www.co-criticalcare.com 595