Self-Assembly of Amphipathic Lipids - NPTELnptel.ac.in/courses/118106019/Module 3/Lecture 1/Lecture...
Transcript of Self-Assembly of Amphipathic Lipids - NPTELnptel.ac.in/courses/118106019/Module 3/Lecture 1/Lecture...
-
NPTEL Nanotechnology - Nanobiotechnology
Joint Initiative of IITs and IISc Funded by MHRD Page 1 of 15
Self-Assembly of Amphipathic Lipids
Dr. K. Uma Maheswari
Professor, School of Chemical & Biotechnology
SASTRA University
-
NPTEL Nanotechnology - Nanobiotechnology
Joint Initiative of IITs and IISc Funded by MHRD Page 2 of 15
Table of Contents
1 WHAT ARE LIPIDS? ........................................................................................................ 3
2 SELF-ASSEMBLY OF LIPIDS ......................................................................................... 6
2.1 MICELLES ..................................................................................................................... 7
2.2 BILAYERS ..................................................................................................................... 8
2.3 PLANAR LIPID BILAYERS ........................................................................................ 10
2.4 VESICLES ................................................................................................................... 10
3 REFERENCE .................................................................................................................. 15
-
NPTEL Nanotechnology - Nanobiotechnology
Joint Initiative of IITs and IISc Funded by MHRD Page 3 of 15
Preface
The two lectures in this module provide an insight into the driving forces in lipid self-
assembly structures. This information can be extended to develop new applications using
these self-assembled structures.
This lecture introduces to the learner various lamellar structures formed by the
amphipathic lipids.
1 What are lipids? Lipids are biomolecules that comprise a huge number of compounds that are
characterized by their insolubility in aqueous and polar solvents and are soluble in
organic solvents such as chloroform or acetone. There are two broad categories of lipids
ones with linear chain arrangement such as fatty acids, phospholipids, glycolipids,
sphingolipids etc. and other with cyclic fused ring structures. This group comprises the
steroids of which salient examples are cholesterol, testosterone, bilirubin etc. Lipids are
ubiquitously distributed in the biological system. They are the major component of the
cell membrane as well as the membranes of the vesicular intracellular compartments. The
membrane lipids are amphipathic i.e., they have a hydrophilic head group and a non-polar
tail chain. The nature of the polar head group (charged or uncharged) as well as the acyl
chain (saturated or unsaturated and number of carbon atoms) will determine their
localization and function in a body. The cell membrane architecture is made up of mainly
a group of amphipathic lipids known as phospholipids. The phospholipids have a glycerol
backbone and are esterified with long chained fatty acids at the first two carbons. The
third carbon is esterified with phosphoric acid, which is then linked with a base. The two
acyl chains make up the hydrophobic portion of the phospholipid while the phosphate as
well as the base makes up the polar hydrophilic group. The following animation (Figure
1) introduces the structure and representation of a phospholipid.
Module objective
This module attempts to introduce the learner to different types of self-assembled structures
that are formed by amphipathic lipids. Both lamellar and non-lamellar structures are
discussed in detail.
-
NPTEL Nanotechnology - Nanobiotechnology
Joint Initiative of IITs and IISc Funded by MHRD Page 4 of 15
Fig. 1: Structure of phospholipid
Note: Can be viewed only in Acrobat Reader 9.0 and above
Apart from the type of base in the phospholipid, the acyl chains may also be different
depending on the cell type.
The phospholipids have a unique bilayer arrangement in the cell membrane. This
arrangement is primarily due to the amphipathic nature of the phospholipids. The
heterogeneity of the lipid composition in the cell membrane determines its permeability
characteristics and surface charge. The surface charge is an important parameter that
determines the type of interactions that can exist between the polar head groups and other
molecules at the membrane interface. Thus different cell membranes have different
compositions of the lipids. Some of the major types of phospholipids and their location in
the body are shown in the following Table 1.
-
NPTEL Nanotechnology - Nanobiotechnology
Joint Initiative of IITs and IISc Funded by MHRD Page 5 of 15
Table 1: Structure of phospholipids and their location in the body
-
NPTEL Nanotechnology - Nanobiotechnology
Joint Initiative of IITs and IISc Funded by MHRD Page 6 of 15
Apart from the membrane lipids, free lipids are also found in the biological system and
perform specific functions.For example the steroidal hormones estradione and
testosterone, inflammatory molecules leukotrienes and prostaglandins, the major
constituents of the bile juices bilirubin and biliverdin etc. are all lipidic in nature. The
major constituent of the lung surfactant is phosphatidyl choline. Though numerous
examples of lipids in biological system are available, as the present discussion is focused
on the self-assembling characteristics of lipids, further details on classification of lipids
are not provided.
2 Self-assembly of lipids The amphipathic lipids like its protein counterparts can self-assemble in aqueous medium
into a variety of assemblies. The structure of the assemblies formed is dictated by the
chemical nature of the amphipathic lipid as well as the self-assembling environment. The
major driving force for the self-assembly in aqueous medium comes from the
hydrophobic acyl chains. However, the hydrophilic head group helps in developing an
interface with the aqueous medium and also directs the inter-particle interactions as well
as the size of the self-assembled structures formed.
One of the major parameters that determines the final conformation of the self-assembled
lipid structures is a geometric factor referred to as the packing parameter, p. This
dimensionless parameter is given as the ratio of the hydrocarbon volume (v) to the
product of the area of the polar head group (a0) and the critical acyl chain length (lc)
beyond which the chain can no longer be considered fluid.
=
If the p value is less than
, then the amphipathic molecules are expected to form spherical
micellarstructures. If the value is above
but below , it may form non-sphericalmicellar
structures while p values >1 favour inverted assemblies. However, this prediction is
applicable only when a single amphipathic molecule is involved in the self-assembly
process. When more than one component is involved, due to the complex interactions
involving electrostatic interactions, hydrogen bonding and/or van der Waals forces
between the constituent molecules, deviations from the predicted structures are expected.
Novel structures such as nanodiscs, icosahedra, punctured planes etc. have been reported
in such cases.
Though the lipid self-assembled structures are not as complex or diverse in architecture
as in the case of their protein and nucleotide counterparts, the self-assembled lipid
structures have been exploited on a commercial basis by many industries. The lipid self-
assembled structures have been widely employed by the pharmaceutical industries to
-
NPTEL Nanotechnology - Nanobiotechnology
Joint Initiative of IITs and IISc
deliver therapeutic molecules. They also find
deliver anti-ageing compounds and skin nutrients. Many premier cosmetic products
contain lipid-based formulations. In the food industry, ripening of cheese and
preservation of milk-based productshave been accomplished using self
structures. The decontamination of polluted water has also been successful using self
assembled lipid structures. The spontaneous self
a template to create high ordered structures of nano
lipidic systems have also created a new paradigm in the field of nanobiotechnology.
addition, it is well known that the lipid structures play a key functional role in
biological systems. Some of the major self
lipids are discussed in the following sections.
2.1 Micelles The simplest structure formed by amphipathic lipids is a micelle structure. The fatty
acids, which possess a single fatty acyl chain and a polar head group
structures in an aqueous medium
aqueous medium and the hydrophobic acyl chains facing inwards to minimize contact
with the polar medium. The size
the acyl chain is a major determinant of the size of the
reverse micelle is formed with the polar head groups in the centre and the hydrophobic
chains facing outwards. Figure 2
Fig. 2: Pictorial representation of a
The driving force for the micelle formation is the hydrophobicity of the acy
micelle formation occurs only beyond a certain concentration of the amphipathic
molecule. This concentration is referred to as the critical micelle concentration (
Below the cmc, the amphipathic molecules e
Nanobiotechnology
Funded by MHRD
deliver therapeutic molecules. They also find applications in the cosmetic industries to
ageing compounds and skin nutrients. Many premier cosmetic products
based formulations. In the food industry, ripening of cheese and
based productshave been accomplished using self-assembled lipid
decontamination of polluted water has also been successful using self
The spontaneous self-assembly of lipids has been employed as
a template to create high ordered structures of nano-dimensions. Nano-reactors based on
lipidic systems have also created a new paradigm in the field of nanobiotechnology.
ion, it is well known that the lipid structures play a key functional role in
Some of the major self-assembled structures formed by amphipathic
lipids are discussed in the following sections.
formed by amphipathic lipids is a micelle structure. The fatty
which possess a single fatty acyl chain and a polar head group, form micellar
in an aqueous medium with the polar head group facing outwards towards the
hydrophobic acyl chains facing inwards to minimize contact
The sizes of the micelles range between 2-20 nm. The length of
the acyl chain is a major determinant of the size of the micelle. In organic solvents, a
formed with the polar head groups in the centre and the hydrophobic
hains facing outwards. Figure 2 represents the structure of a micelle and reverse micelle.
: Pictorial representation of a reverse micelle (left panel) and micelle (right panel)
The driving force for the micelle formation is the hydrophobicity of the acy
micelle formation occurs only beyond a certain concentration of the amphipathic
molecule. This concentration is referred to as the critical micelle concentration (
Below the cmc, the amphipathic molecules exist as monolayers at the air
Page 7 of 15
cosmetic industries to
ageing compounds and skin nutrients. Many premier cosmetic products
based formulations. In the food industry, ripening of cheese and
assembled lipid
decontamination of polluted water has also been successful using self-
of lipids has been employed as
reactors based on
lipidic systems have also created a new paradigm in the field of nanobiotechnology.In
ion, it is well known that the lipid structures play a key functional role in all
assembled structures formed by amphipathic
formed by amphipathic lipids is a micelle structure. The fatty
form micellar
with the polar head group facing outwards towards the
hydrophobic acyl chains facing inwards to minimize contact
20 nm. The length of
In organic solvents, a
formed with the polar head groups in the centre and the hydrophobic
represents the structure of a micelle and reverse micelle.
micelle (left panel) and micelle (right panel)
The driving force for the micelle formation is the hydrophobicity of the acyl chains. The
micelle formation occurs only beyond a certain concentration of the amphipathic
molecule. This concentration is referred to as the critical micelle concentration (cmc).
xist as monolayers at the air-liquid
-
NPTEL Nanotechnology - Nanobiotechnology
Joint Initiative of IITs and IISc Funded by MHRD Page 8 of 15
interface.As the concentration of the amphipathic molecule increases, the hydrophobic
forces drive them to aggregate. At the same time, a repulsive force develops between the
charged/polar head groups. These opposing forces balance each other in the self-
assembled micellar structure that is formed.
Aggregation number refers to the number of amphipathic molecules present in each
micellar structure. As there will be size variations, the aggregation number can be
presented only as a range of values that follow the Gaussian distribution. Micelles
containing charged head groups wouldbe associated with counter ions from the aqueous
medium. As the concentration of the surfactant increases, deviations from the spherical
morphology increase, resulting in formation of rod-like, worm-like and mesophase
structures. These structures contain a greater aggregation number. The rod-like structures
have similar diameter as the spherical micelle but has a length scale that is two to five
fold higher than its spherical counterpart. The worm-like micelles have length scales in
the micron range while the mesophase structures can adopt a hexagonal or cubic phase
arrangements.
A micelle containing more than one type of amphipathic lipid is known as a multi-
component micelle or a mixed micelle. The presence of additional components can
either decrease the cmc by promoting faster aggregation at much lower concentrations or
increase the cmc. The decrease in cmc values in a multi-component system is referred to
as synergism while increased cmc values due to the additional components is known as
antagonism. One of the major commercial applications of micelles is in detergents.
Apart from this, catalysis, drug delivery, stabilizers all have incorporated micellar
assemblies for better performance.
2.2 Bilayers Lipid bilayers are the most common assembly encountered in nature. A bilayer contains
two leaflets each made of phospholipids with the acyl chains in each leaflet associated in
a tail-to-tail manner. The polar head groups of the phospholipids in the outer and inner
leaflets are in contact with the aqueous medium. The presence of bilayer offers maximum
shielding to the two acyl chains in each phospholipid in both leaflets of the bilayer from
the aqueous environment. The cell membrane contains the lipid bilayer construct, which
is nearly flat. Vesicles and tubular constructs that have a curvature are also encountered
in the biological system as intracellular transport vehicles. The bilayer architecture
confers stability to the amphipathic molecules that make the bilayer. If only a single
bilayer is present in the lipid structure, then it is known as a unilamellarlipid bilayer. If
multiple bilayers are stacked upon each other in the lipid structure, they are known as
multilamellar lipid bilayers. Figure 3 shows the arrangement of lipid molecules in
unilamellar and multilamellar lipid bilayers.
-
NPTEL Nanotechnology - Nanobiotechnology
Joint Initiative of IITs and IISc Funded by MHRD Page 9 of 15
Fig. 3: Unilamellar and multilamellar lipid bilayers
In the biological system, the composition of the phospholipids is diverse and hence is
referred to as heterogenous bilayer. The lipids are capable of moving within the same
leaflet (lateral mobility). They can also move from one leaflet to the other in rare
circumstances and this movement is referred to as the flip-flop motion. The packing of
the lipid bilayer depends on the nature of the acyl chains in the phospholipids forming the
bilayer. Acyl chains with higher percentage of unsaturation lead to lesser packing density
compared to saturated acyl chains. In biological system, the packing density is a critical
factor as it determines the permeability characteristics of the membrane. The
phospholipids have a characteristic temperature at which they undergo gel-to-liquid
crystalline phase transition, which also determines the fluidity of the bilayer membrane
for a given temperature.
Recently, the existence of lipid rafts in the lipid bilayers have been identified. Lipid rafts
are microdomains that have more disordered packing of lipids that are in the liquid
crystalline phase when compared with the more gel-like packing in the rest of the lipid
bilayer. These regions are believed to be associated with enhanced permeability to
molecules as well as respond quickly to external stimuli in cell membranes. A single lipid
bilayer membrane might possess multiple lipid rafts.
-
NPTEL Nanotechnology - Nanobiotechnology
Joint Initiative of IITs and IISc
2.3 Planar lipid bilayersFlat lamellar bilayer structures are known as planar lipid bilayers. Such assemblies have
been spontaneously formed as an experimental system and used to investigate the
interactions of single molecules on the bilayer architecture.
the bilayer construct of the cell membrane and helps to understand the role of lipid
molecule interactions in altering membrane properties. The formation of a planar lipid
bilayer is carried out by introducing a small amount of phospholi
bifurcating two chambers filled with aqueous medium. The phospholipids spontaneously
self-assemble to form a bilayer structure acr
and any residual organic solvent during the process. The shape
it is chamfered to ensure a reservoir of phospholipids remains at the edges of the aperture.
This facilitates dynamic exchange of phospholipids as is observed in biological
membranes. Figure 4 represents the various stages involved in the formation of a planar
lipid bilayer through self-assembly.
Fig. 4: Stages involved in the formation of planar lipid bilayer
The process of thinning and formation of the planar lipid bilayer takes between 10
minutes. The formation of lipid bilayer can also be carried out at the tip of a metal
(supported bilayer) as well as through covalent linking to the metal.
2.4 Vesicles When the amphipathic lipids are dispersed in a large amount of aqueous medium, they do
not form monolayers but tend to form spherical structures made of lipid bilayers,
especially if the packing parameter values are in between and 1. The bilayer structure
ensures that the hydrophobic chains do not come into direct contact with the aqueous
Bulk lipid droplet
Nanobiotechnology
Funded by MHRD
lipid bilayers lat lamellar bilayer structures are known as planar lipid bilayers. Such assemblies have
been spontaneously formed as an experimental system and used to investigate the
interactions of single molecules on the bilayer architecture. This system serves to mimic
the bilayer construct of the cell membrane and helps to understand the role of lipid
molecule interactions in altering membrane properties. The formation of a planar lipid
bilayer is carried out by introducing a small amount of phospholipids in an aperture
bifurcating two chambers filled with aqueous medium. The phospholipids spontaneously
assemble to form a bilayer structure across the membrane, thereby eliminating wat
and any residual organic solvent during the process. The shape of the aperture is such that
it is chamfered to ensure a reservoir of phospholipids remains at the edges of the aperture.
This facilitates dynamic exchange of phospholipids as is observed in biological
represents the various stages involved in the formation of a planar
assembly.
: Stages involved in the formation of planar lipid bilayer
of thinning and formation of the planar lipid bilayer takes between 10
The formation of lipid bilayer can also be carried out at the tip of a metal
(supported bilayer) as well as through covalent linking to the metal.
mphipathic lipids are dispersed in a large amount of aqueous medium, they do
not form monolayers but tend to form spherical structures made of lipid bilayers,
especially if the packing parameter values are in between and 1. The bilayer structure
that the hydrophobic chains do not come into direct contact with the aqueous
Bulk lipid droplet Thinning lipid layer Planar lipid bilayer
Page 10 of 15
lat lamellar bilayer structures are known as planar lipid bilayers. Such assemblies have
been spontaneously formed as an experimental system and used to investigate the
em serves to mimic
the bilayer construct of the cell membrane and helps to understand the role of lipid-
molecule interactions in altering membrane properties. The formation of a planar lipid
pids in an aperture
bifurcating two chambers filled with aqueous medium. The phospholipids spontaneously
eliminating water
of the aperture is such that
it is chamfered to ensure a reservoir of phospholipids remains at the edges of the aperture.
This facilitates dynamic exchange of phospholipids as is observed in biological
represents the various stages involved in the formation of a planar
of thinning and formation of the planar lipid bilayer takes between 10-30
The formation of lipid bilayer can also be carried out at the tip of a metal
mphipathic lipids are dispersed in a large amount of aqueous medium, they do
not form monolayers but tend to form spherical structures made of lipid bilayers,
especially if the packing parameter values are in between and 1. The bilayer structure
that the hydrophobic chains do not come into direct contact with the aqueous
Planar lipid bilayer
-
NPTEL Nanotechnology - Nanobiotechnology
Joint Initiative of IITs and IISc Funded by MHRD Page 11 of 15
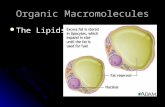
phase. These spherical structures are termed as vesicles and as they are composed on
lipids, they are also commonly referred to as liposomes. The liposomes enclose an
aqueous core that is surrounded by a hydrophobic region comprising the acyl chains. The
periphery of the liposomes contains the polar head groups that interact with the aqueous
medium surrounding it. Liposomes literally translate to fat body, which is a misnomer.
Hence, most literatures still refer to these structures as lipid vesicles. There are a large
number of vesicles that vary in the type and composition of the amphipathic molecule
forming these structures. These are listed in the following Table 2.
Table 2. Different types of vesicles
Name of vesicle Meaning and use of the term in the literature
Algosome Vesicle prepared on the basis of 1-O-alkylglycerol
Archaesome Vesicles prepared from archaebacterial and bolaamphiphiliclipids
Bilosome Vesicles prepared form a mixture of non-ionic surfactants (1-
monopalmitoyl-glycerol), cholesterol anddihexadecylphosphate in the
molar ratio of 5:4:1 and deoxycholate
Catanionic vesicle Vesicles prepared from a mixture of anionic and cationic surfactants
Cerosome Vesicles with a silicate framework on its surface
Ethosome Vesicles that contains a considerable amount of ethanol in the final
preparation
Fluorosome Single unilamellar vesicle containing a fluorescent dye embedded in the
lipid bilayer to monitor the entry of molecules into the bilayer
Hemosome Hemoglobin containing vesicle
Immunoliposome Vesicles contains antibodies specific for a particular antigen on its surface
Lipid vesicle,
Liposomes
Vesicles prepared from amphiphilic lipids
Magnetoliposomes Vesicles containing magnetic nanoparticles (eg. Magnetite Fe3O4)
Marinosome Vesicles based on marine lipid extract containing high amount of
polyunsaturated acyl chains
Niosome Vesicles prepared form non-ionic surfactants
Novasome Oligo- or multilamellar vesicle prepared by the addition of vesicle-
forming surfactants in liquid state to an aqueous solution
Phospholipid
vesicles
Vesicles prepared from amphiphilic phospholipids
-
NPTEL Nanotechnology - Nanobiotechnology
Joint Initiative of IITs and IISc Funded by MHRD Page 12 of 15
PLARosome Phospholipid containing resorcinolic lipids or their derivatives
Polymer vesicle,
Polymersome
Vesicles prepared from di- or tri- block copolymers
Polymerized vesicle Vesicles prepared from polymerizableamphiphiles that were partially
polymerized after vesicle formation
Proliposomes Dry (ethanol-free) granular preparations of vesicle-forming amphiphiles,
which upon hydration lead to vesicle formation
Proniosomes A dry, granular product containing mainly non-ionic surfactants which on
addition of water disperse to form multilamellar vesicles
Reversed vesicle Inverted micelle formed in apolar solvent in the presence of small amount
of water
Spherulite Onion-like vesicle prepared using shear forces
Sphingosome Vesicles prepared from sphingolipids present in human skin
Stealth liposome Sterically stabilized vesicle achieved through the use of co-amphiphiles
that have PEG. Sometimes polysaccharides can also be used
Synthetic vesicle Vesicles prepared from synthetic surfactants that are not present in the
biological membranes
Toposome Vesicles that has a surface that is site-selectively modified in a stable
manner at specific and deliberate locations
Transferosome Ultradeformable ethanol-containing mixed lipid/detergent vesicle used to
transfer water soluble molecules across human skin
Ufasome Vesicles prepared from unsaturated fatty acid/soap mixtures
Vesicle A small, membrane-bounded, spherical organelle in the cytoplasm of an
eukaryotic cell
Virosome Vesicles containing viral proteins and viral membranes reconstituted from
viral envelope
Lipid vesicles can be classified into different types based on their size as well as number
of layers of bilayers found in the structure. The small unilamellar vesicles (SUV) have
sizes below 50 nm and consist of a single bilayer surrounding an aqueous core. The large
unilamellar vesicles (LUV) still possess a single bilayer surrounding the aqueous core but
the sizes are below 1000 nm. In the case of giant vesicles (GV), the sizes of the vesicles
are well above 1 m but the number of bilayers is still restricted to one. Multi-lamellar
vesicles (MLV) have multiple concentric bilayers while multi-vesicular vesicles (MVV)
-
NPTEL Nanotechnology - Nanobiotechnology
Joint Initiative of IITs and IISc Funded by MHRD Page 13 of 15
or vesosomes have non-concentric vesicles inside a large vesicle. Figure 5 shows the
pictorial representation of a few classes of lipid vesicles.
Fig. 5: Different types of lipid vesicles
Lipid vesicles like their planar counterparts have characteristic gel-to-liquid crystalline
phase transition temperature that is influenced by the length of the acyl chains, the
unsaturation status of the acyl chains, the nature of the head group as well as the
intermolecular interactions. A higher phase transition temperature indicates greater
associative forces between the lipids and closer packing. A lower phase transition
temperature indicates greater percentage of fluidity and looser packing of the lipids.
Lipid vesicles have been extensively investigated as drug and gene delivery carriers. This
is because of their ability to load both hydrophilic as well as hydrophobic moieties as
well as the ease with which they can be self-assembled. The polar groups on the outer
surface makes them easily modifiable with chemical functionalities that will impart
specific properties to it such as target specificity, optical visibility, long circulating nature
etc. Also, the lipid vesicles exhibit excellent cell uptake making them the preferred choice
in many drug delivery applications. Figure 6 depicts a surface-modified liposomal drug
carrier that has poly(ethylene glycol) chains on its surface to enhance its lifetime within
the biological system as well as a targeting ligand to specifically enter desired cells.
-
NPTEL Nanotechnology - Nanobiotechnology
Joint Initiative of IITs and IISc Funded by MHRD Page 14 of 15
Fig. 6: Pictorial representation of a surface modified liposomal drug delivery system
The lipid vesicles can beformed by different techniques. The method chosen for
preparing the lipid vesicles will determine the size and stability of the final product.
Though many techniques such as thin film hydration, freeze-thaw, solvent exchange,
detergent depletion, proliposome method etc. are available, the most widely used method
is the thin film hydration technique.Figure 7 shows the various stages involved in the
preparation of a liposome by thin film hydration method.
Fig. 7: Stages involved in the formation of liposomes by thin film hydration process
-
NPTEL Nanotechnology - Nanobiotechnology
Joint Initiative of IITs and IISc Funded by MHRD Page 15 of 15
Initially, the phospholipid solution is taken in a container and the solvent is evaporated
under reduced pressure or by purging with argon or nitrogen. As phospholipids are
extremely sensitive to thermal and oxidative degradation, the solvent evaporation
iscarried at low temperatures in the absence of air. Argon purging is preferable to
nitrogen purging as argon is denser than oxygen and hence the chances of being displaced
by oxygen are less. In the case of nitrogen that is less denser than oxygen, the chances of
it being displaced by the denser oxygen is more. Contact with oxygen may promote the
oxidative degradation of the lipids and is to be avoided at all times. The phospholipids are
soluble in chloroform, which is volatile and hence can be easily evaporated by purging or
under reduced pressure.After the solvent is evaporated completely, a thin film of the
phospholipids remains in the system. Now, a buffer is added to the system and warmed to
about 60-65oC. The addition of the buffer is to drive the amphipathic lipid molecules to
self assemble into a vesicle. The temperature of 60-65oC is slightly above the phase
transition temperature of most phospholipids and hence they are all in the liquid
crystalline phase at this temperature. These conditions will cause greater degree of
mobility of the acyl chains, which will further drive them to form vesicles in an effort to
minimize contact with the aqueous medium. The buffer solution also provides a highly
polar environment that will drive the hydrophobic acyl chains to form vesicles. The
vesicles thus formed are not of uniform size and in many cases they might possess multi-
lamellar arrangement. Hence, a final step known as extrusion is carried out. In this stage,
the liposomes are placed in an extruder that consists of two compartments separated by a
polycarbonate membrane with specific pore dimensions. The lipid vesicle dispersion is
forced from the first compartment to the second through the membrane using a syringe.
Once the lipid vesicle dispersion is in the second compartment, it is forced back to the
first compartment through the membrane using a syringe. This completes one cycle of
extrusion. The extrusion cycles are repeated for 10-25 times to achieve nearly mono-
disperse lipid vesicles with sizes below the pore size of the membrane used. The process
of extrusion squeezes off the additional lamellae that tend to increase the size of the
vesicles and repeated cycles ensures that the lipid vesicles remain in the same size.
3 Reference The multiple faces of self-assembled lipidic systems, Guillaume Tresset, PMC
Biophysics, 2(3), (2009)