Science;ILPAC unit I3: the periodic table...Periodic Table of the Elements 0 lliJ 2 I II III IV V VI...
Transcript of Science;ILPAC unit I3: the periodic table...Periodic Table of the Elements 0 lliJ 2 I II III IV V VI...

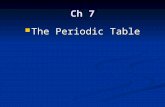
The Per iod ic Table
td-b lock
III IV V VI VII 0
p-block------')..'---~
B C N 0 F Ne5 6 7 8 9 10
A I Si P S CI Ar13 14 15 16 17 18
Ga Ge As Se Br Kr31 32 33 34 35 36
In Sn Sb Te I Xe49 50 51 52 53 54
TI Pb Bi Po At Rn8 1 82 83 84 85 86
s-block~
I II
Li Be3 4
Na Mg11 12
K Ca 21to
19 20 30
Rb Sr 3 9to
37 38 4 8
Cs Ba 57to
55 56 80
Fr Ra 89to
87 88 104

Per
iodi
cT
able
of
the
Ele
men
ts
0
lliJ2
III
III
IVV
VI
VII
He
4.0
34
56
78
910
Li
BeB
CN
0p
. Ne
6.9
9.0
10
.81
2.0
14
.01
6.0
19
.02
0.2
1112
131
415
1617
18
Na
Mg
Al
SiP
SCI
Ar
23
.02
4.3
27
.02
8.1
31
.03
2.1
35
.53
9.9
19
20
212
22
32
42
52
62
72
82
93
031
32
33
34
35
36
KC
aSc
TiV
Cr I
Mn
FeC
oN
iC
uZn
Ga
Ge
As
SeBr
Kr
39
.14
0.1
45
.04
7.9
50
.95
2.0
54
.95
5.9
58
.95
8.7
63
.56
5.4
69
.772
.67
4.9
79
.07
9.9
83
.8
37
38
39
40
414
24
34
44
54
64
74
84
95
051
52
53
54
Rb
SrY
Zr
Nb
Mo
TcR
uR
hPd
Ag
CdIn
SnSb
TeI
Xe8
5.5
87
.68
8.9
91
.29
2.9
95
.99
9.0
10
1.1
10
2.9
10
6.4
10
7.9
11
2.4
114
.81
18
.71
21
.81
27
.61
26
.91
31
.3
55
56
57
72
73
74
75
76
77
78
79
80
818
28
38
485
86
IC
sB
aLa
4~H
fTa
WRe
Os
IrPt
Au
Hg
Tl
PbBi
PoA
tRn
13
2.9
13
7.3
13
8.9
17
8.5
181.0
18
3.9
18
6.2
19
0.2
19
2.2
19
5.1
19
7.0
20
0.6
20
4.4
20
7.2
20
9.0
21
0.0
21
0.0
22
2.0
87
88
89
FrR
aA
c2
23
.02
26
.02
27
.0
58
59
60
616
26
36
46
56
66
76
86
97
071
Ce
PrN
dPm
SmEu
Gd
\fbD
yH
oE
rTm
Yb
Lu
14
0.1
14
0.9
14
4.2
(14
7)
15
0.4
15
2.0
15
7.3
15
8.9
16
2.5
16
4.9
16
7.3
16
8.9
17
3.0
17
5.0
90
919
29
39
49
59
69
79
89
91
00
10
11
02
10
3
0..
..-
ThPa
UN
pPu
kt\m
emBk
Cf
Es
Fm
Md
No
Lw2
32
.02
31
.02
38
.1(2
37
)2
39
.1(2
43
)(2
47
-)(2
47
)(2
51
)(2
54
)(2
53
)(2
56
)(2
54
)(2
57
)
Ava
lue
inb
rack
ets
de
no
tes
the
ma
ssn
um
be
ro
fth
em
ost
sta
ble
iso
top
e.
I
-1

IDThe Periodic Table
1IIIi~~~llrl~~1~11111N27909

NATIONAL
CENTRE
© Inner London Education Authority 1984
First published 1984by John Murray (Publishers) Ltd50 Albemarle StreetLondon W1X 4Bo
Reprinted 1985
All r~ghts reserved.Unauthorised duplicationcontravenes applicable laws
Printsd ~n Great Britain byMartin's of Berwick
British Library Cataloguing in Publication Data
Independent Learning Project for Advanced ChemistryThe Periodic Table. - (ILPAC; Unit 13)1. ScienceI. Title500
II. SeriesQ161.2
ISBN 0 7195 4051 8
/I

CONTENTS
PREFACE vAcknowledgements viKey to activity symbols and hazard symbols vii
INTRODUCTION 1Pre-knowledge 2PRE~TEST 3
LEVEL ONE 5PERIODICITY OF PHYSICAL PROPERTIES 5Atomic radius 5Ionic radius 7First ionization energy 8Electron affinity 8Atomic volume 9Melting-point and enthalpy of fusion 10Structure and bonding in elements of Periods 2 and 3 11Boiling-point and enthalpy of vaporization 13PERIODICITY OF CHEMICAL PROPERTIES 14LEVEL ONE CHECKLIST 18LEVEL ONE TEST 19
LEVEL TWO 25COMPOUNDS OF ELEMENTS IN PERIODS 2 AND 3 25Periodicity of formulae of Period 2 and 3 oxides, chlorides and hydrides 25CHLORIDES OF THE ELEMENTS IN PERIODS 2 AND 3 28Experiment 1 - investigating the properties of Period 3 chloridesAnhydrous chlorides of Group II elementsMethods of preparing chlorides in Period 3Experiment 2 - preparing anhydrous aluminium chlorideFormula determination of chloridesOXIDES OF THE ELEMENTS IN PERIODS 2 AND 3Experiment 3 - investigating the properties of Period 3 oxidesHYDRIDES OF THE ELEMENTS IN PERIODS 2 AND 3LEVEL TWO CHECKLIST
293435374143444852
END-OF-UNIT TEST 53
ANSWERS TO EXERCISES 59
iii


PREFACE
This volume is one of twenty Units produced by ILPAC, the Independent LearningProject for Advanced Chemistry, written for students preparing for the AdvancedLevel examinations of the G.C.E. The Project has been sponsored by the InnerLondon Education Authority"and the materials have beell extensively tested inLondon schools and colleges. In its present revised form, however, it isintended for a wider audience; the syllabuses of all the major ExaminationBoards have been taken into account and questions set by these boards have beenincluded.
Although ILPAC was initially conceived as a way of overcoming some of thedifficulties presented by uneconomically small sixth forms, it has frequentlybeen adopted because its approach to learning has certain advantages over moretraditional teaching methods. Students assume a greater responsibility fortheir own learning and can work, to some extent, at their own pace, whileteachers can devote more time to guiding individual students and to managingresources.
By providing personal guidance, and detailed solutions to the many exercises,supported by the optional use of video-cassettes, the Project allows studentsto study A-level chemistry with less teacher-contact time than a conventionalcourse demands. The extent to which this is possible must be determinedlocally; potentially hazardous practical work must, of course, be supervised.Nevertheless, flexibility in time-tabling makes ILPAC an attractive propo-sition in situations where classes are small or suitably-qualified teachersare scarce.
In addition, ILPAC can provide at least a partial solution to other problems.Students with only limited access to laboratories, for example, those studyingat evening classes, can concentrate upon ILPAC practical work in the laboratory,in the confidence that related theory can be systematically studied elsewhere.Teachers of A-level chemistry who are inexperienced, or whose main disciplineis another science, will find ILPAC very supportive. The materials can be usedeffectively where upper and lower sixth form class~s are timetabled together.ILPAC can provide 'remedial' material for students in higher education.Schools operating sixth form consortia can benefit from the cohesion that ILPACcan provide in a fragmented situation. The project can be adapted for use inparts of the world where there is a severe shortage of qualified chemistryteachers. And so on.
A more detailed introduction to ILPAC, with specific advice both to studentsand to teachers, is included in the first volume only. Details of the ProjectTeam and Trial Schools appear inside the back cover.
LONDON 1983
v

ACKNOWLEDGEMENTSThanks are due to the following examination boards for permission toreproduce questions from past A-level papers:
Joint Matriculation Board;Teacher-marked Exercise p.17(1978)
Oxford Delegacy of Local Examinations;Level One Test 13(1980)
Southern Universities Joint Board;Exercise 17(1980)
The Associated Examining Board;Exercise 16(1978)
University of Cambridge Local Examinations Syndicate;Level One Test 15(1974)
University of London Entrance and Schools Examination Council;Exercise 18(1973)Teacher-marked Exercises p.42(1978), p.48(1979), p.58(1976)Level One Test 1-4(1980), 5(N 1976), 6(1976), 7(1979), 8(1978), 9(1979),
10(1980), 11(1981), 12(1979), 14(1977) 16(1978)End-of-Unit Test 1-5(1981), 6(1980). 7(1980), 8(1980), 9(1980),
10(N 1979), 11(1979), 12(1980), 13(1977), 14(1976),15(1979)
Welsh Joint Education CommitteeLevel One Test 17(1979)End-of-Unit Test 16(1977)
Questions from papers of other examining boards appear in other Units.
Where answers to these questions are included, they are provided by ILPACand not by the examination boards.
Photographs are included by permission as follows:
J.W. O~bereiner, p7 - John Freeman (by courtesy of the Royal Institution).Circular Periodic Table, p15 - E.J. Emerson, Journal of Chemical Education,
1944, 21, 111.0.1. Mende18ev, p17 - the Royal Society of Chemistry.Photographs of students - Tony Langham.
vi

'A' Level part question
SYMBOLS USED IN ILPAC UNITS
0ITt Reading
~Exercise
\\\;l Test
oo~
~
[2]
'A' Level question
'A' Level questionSpecial paper
Worked example
Revealing exercises
® Discussion
~ Computer programme
~ Experiment
I[ )I Video programme00
[Q8 Film loop
Model-making
Teacher-marked exercise
INTERNATIONAL HAZARD SYMBOLS
1Kl Harmful
~ Flammable
...:-lit. 1.1 Corrosi ve--.
I~I Toxic
~ Explosive
I ~ I Oxidising
Radioactive
VII


INTRO DUCTIONThe Periodic Table helps you to organise your knowledge and thus makesfactual information easier to remember. In Unit S2 (Atomic Structure) youlearned how the Periodic Table is built up and divided into major blocks.In Units 11 (s-6Iock Elements) and 12 (The Halogens) you studied the extremegroups on either side of the Periodic Table with an emphasis on the verticaltrends and changes.
In this Unit, we look at the Periodic Table with particular reference to thehorizontal trends and changes that occur across Periods 2 and 3. We givethe symbols of the elements in Periods 2 and 3 in the outline Periodic Tablebelow (Fig: 1).
s-block~
~III~----fH1H;lL2l2J
p-block
III IV V VI VII 0- -
LI Be3 4
- -Na Mg11 12
B
B C N 0 F Ne5 6 7 8 9 10
d-block AI Si P S CI Ar13 14 15 16 17 18
L--
Fig.1.
In Level One, we look at the elements, concentrating on the main trends inphysical properties with increasing atomic number. We relate these to theelements' structures and reach conclusions about the overall changes acrossa period.
In Level Two, we deal with the chlorides, oxides and hydrides of the elementsof Periods 2 and 3. There are three experiments in this Unit, all in LevelTwo.
There are two ILPAC video-programmes designed to accompany this Unit. Theyare not essential, but you should try to see them at the appropriate timesif they are available.
Preparation of aluminium chloride Chlorides and oxides
1

PRE-KNOWLEDGEBefore you start work on this Unit, you should be able to:
(1) describe the periodic classification of the elements;(2) divide a Periodic Table into numbered periods and groups;(3) state the names of Groups I, II, VII and 0;(4) state the typical properties of (a) metals, and (b) non-metals;(5) explain what is meant by the following terms-
(a) element, (f) ionic bond,(b) compound, (g) molecule,(c) direct synthesis, (h) giant structure of atoms or ions,(d) atomic number, (i) acidic oxide,(e) covalent bond, (j ) basic oxide;
(6) state the properties of typical covalent and ionic compounds;(7) state the difference between covalent and van der Waals radii;(8) describe the trends in electronegativity across a period and down a
group in the Periodic Table.
PRE-TESTTo find out whether you are ready to start Level One, try thefollowing test which is based on the pre-knowledge items. You shouldnot spend more than 30 minutes on this test. Hand your answers toyour teacher for marking.
2

PRE-TEST1. Using either the group numbers or atomic numbers, identify on
outline Periodic Table below (Fig. 2) the spaces occupied by:
~\;gthe
(a) the alkali metals,(b) the halogens,(c) the noble gases,(d) carbon,(e) a metal with a coloured ion.
( 1 )
(1 )
( 1)
( 1 )
( 1)
II III IV V VI VII 0- -3 4
- -
11 12
19 20 21
37 38 39
55 56 57
87 88 89
5 6 7 8 9 10
13 14 15 16 17 18
22 23 24 25 26 27 28 29 30 31 32 33 34 35 36
40 41 42 43 44 45 46 47 48 49 50 51 52 53 54
72 73 74 75 76 77 78 79 80 81 82 83 84 85 86
104'------
Fig.2.
2. Copy and complete the table below, which shows some typical propertiesof metals and non-metals.Table 1
Property Metal Non-metal
Melting-point
Type of oxide
Malleability
Electricalconductivity
(4 )
3

3. Copy and complete the table below, which shows some typical propertiesof molecular and ionic compounds.
Table 2
Property Molecular compound Ionic compound
Melting-point
Electrical conductivitywhen molten
Solubility in hexane
Solubility in water
4. Which of the following substances will have the properties of a typicalionic compound?Na LiHNaCl
5. Write equations summarising the reactions between the followingsubstances. Include the states of each substance in the equation.(a) Zinc and hydrochloric acid.(b) Sodium and chlorine.(c) Sulphur dioxide and water.(d) Sodium oxide (Na20) and water.
6. Fig. 3 shows two molecules in a sample of solid chlorine. Identifythe radii marked as a and b in the diagram.
(Total 25 marks)Fig.3.
4
(4 )
(2)
(2)(2 )
(2 )
(2 )
(2 )

LEVEL ONE
PERIODICITY OF PHYSICAL PROPERTIESThe first section of this Unit is concerned with several physical properties.You have already studied the vertical trends in these properties for Gr~upsI, II and VII in Units 11 (The s-Block Elements) and 12 (The Halogens). Wenow look at the horizontal trends in these properties with increasing atomicnumber. In doing this we aim to give you an understanding of the term'periodic' and how to use it.
Objective. When you have finished this section, you should be able to:
(1) explain the meaning of periodicity with reference to atomic and ionicradius, first ionization energy, electron affinity and atomic volume.
The first property we consider is atomic radius.
Atomic radiusYou encountered atomic radius in Unit S4 (Structure and Bonding) and used itin studying Groups I, II and VII. Here we examine the changes in atomicradius that are apparent over the Periodic Table as a whole, with an emphasison the horizontal changes.
Fig. 4 below shows a graph of atomic (covalent) radius against atomic numberfor the first 56 elements. Fig. 5 (page 6) gives similar information on therelative sizes of the atoms of the S-, p-, and d-block elements. Study bothdiagrams and then attempt the exercise which follows.
K0.2
E Na.::Vl::J L.i"0
~f
~.~ 0.1 CIE ~.8co
H•0
Sc-Zn~Rb
Ga I
5 10 15 20 25 30 35 40 45 50 55
atomic number
Fig.4. Change in atomic (covalent) radius with atomic number
5

0 0Li Be
0 0Na Mg
00K Ca
00Rb Sr
/ '\0V. Ba/ <,0Y Ra
0 0 0 0 0 0 0 0 0 0Sc Ti V Cr Mn Fe Co Ni Cu Zn
0 0 0 0 0 0 0 0 0 0y Zr Nb Mo Tc Ru Rh Pd Ag Cd
0 0 0 0 0 0 0 0 0 0La Hf Ta W Re Os Ir Pt Au Hg
0Ac-
Fig.5. Atomic (covalent)radiiofS-, p- and d- blockelements
Exercise 1 (a)
r--
He0 0 0 0 0
B C N 0 F Ne
0 0 0 0 0AI Si P S CI Ar
0 0 0 0 0Ga Ge As Se Br Kr
0 0 0 0 0In Sn Sb Te I Xe
0 0 0 0 0TI Pb Bi Po At Rn
Describe the changes in atomic radius across ~Periods 2 and 3. Is there a recognisable trend?Explain the changes in terms of nuclear charge and ~\\\shielding.
(c) Identify and state the trend in atomic radius in Groups I,II and VII from Fig. 5. Is the same trend found in GroupsIII, IV, V and VI?
(b)
(d) What do you notice about the radii of the first and secondtransition series (Sc-Zn and Y-Cd, in the d-block)?
(e) Suggest an explanation for the fact that aluminium andgallium in Group III have the same radius.
(f) We have omitted the noble gases from Figs. 4 and 5 astheir covalent radii cannot be measured. Use yourknowledge of the trends in atomic size so far to predict:(i) the covalent radius of each noble gas compared to
other elements in its period;(ii) the trend in covalent radii down the group.
(Answers on page 59 )
We now go on to show how ionic radii relate to atomic radii for elements inPeriods 2 and 3.
6

Ionic radiusTable 3 shows the ionic radii of some elements in Periods 2 and 3. Some ofthe values given are for purely hypothetical ions. The figures in bracketsindicate the charge on the ion.Table 3 Ionic radii/nm (charges in brackets)
Period 2 Li Be B C N 0 F
0.060 0.031 0.020 0.260 0.171 0.140 0.136(+1 ) (+2) (+3) (-4) (-3) (-2) (-1 )
0.015(+4)
Period 3 Na Mg Al Si P S Cl0.095 0.065 0.050 0.271 0.212 0.184 0.181(+1 ) (+2) (+3) (-4) (-3) (-2) (-1 )
0.041(+4)
Exercise 2 (a) What is the trend in ionic radius across Periods2 and 3?
(b) How does this compare with the change in atomicradius across the same period?
(c) State two ions given in Table 3 which are purelyhypothetical.
(d) In Uni ts 11 (The a-Block Elements) and 12 (The Halogens)you studied the trend in ionic radius down Groups I, IIand VII. Using the information in the table and additionalvalues from your data book decide whether the same trendoccurs in Groups III, V and VI.
(e) With which noble gases are the ions shown in Periods 2 and3 isoelectronic?
(Answers on page 59 )
There is a close relationship between atomic size and first ionizationenergy (enthalpy of ionization). which we consider in the next section.
Johann Wolfgang Dobereiner (1780-1849)
The first person to suggest that relative atomic masscouldbe used as a basis for grouping elements.
7

First ionization energyFig. 6 shows the first ionization energies of the elements up to barium inthe Periodic Table. Compare it with Fig. 4 and then attempt the exercisewhich follows.
2500I
o~ 2000~>~ 1500c<ll
Ar Sc - Zn~
Y-CdKr~
Xec 10000.~.~c 500.2 Na In....,e
'+= 0
Ba
Cs
5 10 15 20 25 30 35 40 45 50 55 60
atomic number
Fig.6. Change in first ionization energy with atomic number
Exercise 3 (a) Excluding the noble gases, which elements are found ~at the peaks of the ionization energy graph?Where are these elements found on the atomic radius ~~graph?
(c) What does this suggest about the relationship betweenfirst ionization energy and atomic radius? Explain your
(b)
answer.(Answers on page 59 )
First ionization energy is associated with cation formation. We now considerthe electron affinities of the elements.
Electron affinityYou came across this term in Urrit S3 (Chemical Energetics) and have usedsome values in your study of Groups I, II and VII. Each value representsthe energy change (~) for the process:
X(g) + e (where X = the symbol of any element)
Table 4 gives values for Periods 2 and 3, excluding the noble gases., Studythe table and then attempt the exercise which follows.Table 4 Some electron affinities/kJ mol-1
Li 8e* 8 C N 0 F
-52 +66 -29 -120 -3 -142 -348
Na Mg* Al * Si P S C1-71 +67 -26 -180 -70 -200 -364
(Values of electron affinities can vary considerably according to the source.Values are rarely given for those elements marked *.J
8

Exercise 4 Draw a graph of electron affinity (vertical axis) ~against atomic number, for the elements lithium tochlorine (excluding the noble gases). ~~'
(b) Does the graph show a repeating pattern betweenPeriods 2 and 3?
(a)
(c) Explain why the electron affinities for beryllium andnitrogen differ from those of the other elements in theperiod.
(Answers on page 59 )
Next we consider one of the bulk properties, atomic volume.
Atomic volumeThe atomic volume of an element is the volume occupied by one mole of itsatoms. Since density = mass/volume the atomic volume is calculated usingthe formula:
molar massatomic volume = (usual unit cm3 mol-l)densityIn comparing atomic volumes we use densities at 25°C. For an element whichis not solid at this temperature, use the density of the liquid form atsome specified temperature, usually its boiling-point.
Since atomic volume depends on the molar mass of an element, you mightexpect similarities between graphs of atomic volume and atomic radius withincreasing atomic number. You compare these two properties in the nextexercise.
Figs. 7 and B below are graphs of atomic radius and atomic volume againstatomic number. Compare the two and the~ answer the exercise which follows.
0.2 K 80
Ca 'I0
E E 60c;
"'E-<,
Ul:::; U---
"D Q)
~ 0.1 E 40u :::;
E 0> Ca2 u
CD E 200'cU
0 05 10 15 20 5 10 15 20
atomic number atomic number
Fig.7. Change in atomic radius Fig.S. Change in atomic volumewith atomic number with atomic number
9

Exercise 5 (a) Compare elements between boron and fluorine andbetween aluminium and chlorine on the two graphs.How does the change in atomic volume compare withthe change in atomic radius?
(b) Suggest a reason for the difference in (a).
(Answers on page 60 )
In the last exercise you saw some evidence for structural changes takingplace in the p-block elements. We now look for further evidence byexamining another bulk property, melting-point, and its associated energychange, enthalpy of fusion.
Melting-point and enthalpy of fusionWhen a substance melts, its structure breaks down. The temperature atwhich the change takes place and the energy needed to bring it about(enthalpy of fusion) depend on the type of bonding.
In this section you compare values of these quantities for differentelements and use them to explain the changes in structure and bonding whichtake place along Periods 2 and 3.
Objective. When you have finished this section, you should be able to:
(2) explain how the changes in melting-point and enthalpy of fusion acrossa period are related to changes in structure.
Read about melting-point and enthalpy of fusion (molar enthalpy ofmelting), looking for an explanation of why they change in goingacross a period. Then attempt the following exercise.
oW
Exercise 6 (a) Using values from your data book draw a graph of ~melting-point against atomic number for the first18 elements. ~~
(b) Using a different vertical scale, add values ofenthalpy of fusion on the same paper.
(c) Suggest a reason for any similarities in the patterns ofthe two properties.
(d) In which group are the elements at the peaks of each graph?(e) What does this suggest about the structures of these
elements?(f) Are these two properties periodic, according to your graph?(Answers on page 60 )
You have seen that melting-points and enthalpies of fusion are related tobonding and structure. We now consider these properties in more detail.
10

Structure and bonding in elements of Periods 2 and 3In this section you complete a table showing the structures and bonding ofelements in Periods 2 and 3.
Objective. When you have finished this section, you should be able to:(3) state the types of structure and bonding found in the elements of
Periods 2 and 3.
In the next exercise you complete a copy of Table 5 shown on the next page.Small diagrams of the structures for the elements in Periods 2 and 3 aredrawn for you. You should be familiar with the terms 'close-packed' and'body-centred-cubic' from Unit S4 (Bonding and Structure).
Exercise 7 (a) Fill in a copy of Table 5, classifying eachstructure as one of the following:
(i) giant molecular (giant atomic),(ii) simple molecular,
(iii) metallic,(iv) atomic (non-metallic).
For (iii), specify whether the structure is close-packed(CCP or HCP) or not close-packed (BCC).
(b) Look back at your graph of melting-point against atomicnumber (Exercise 6). Explain the following by consideringdifferences in structure and/or bond strength.
(i) The steady increase in melting-point and enthalpy offusion between lithium and boron and between sodiumand aluminium.
(ii) The sharp rise in melting-point and enthalpy offusion between boron and carbon and betweenaluminium and silicon.
(iii) The sharp drop in melting-point and enthalpy offusion between carbon and nitrogen and betweensilicon and phosphorus.
(c) The enthalpy of fusion of carbon is so high that it cannotbe measured with any accuracy. Suggest a reason why theenthalpies of fusion of the elements following it, inGroups V, VI, VII and 0, are much lower.
(d) Would you say, from your completed table, that structureis a periodic property? Explain.
(Answers on page 60 )
Now that you have classified the elements according to structure we turn toanother bulk property, boiling-point, and its associated energy change,enthalpy of vaporization.
11

cc 00 0 bJJ 0OJ ~Z <:(
OJ OJC C·rl ·rl~ ~0 0:J rlrl .cLL U
~C :JOJ .cbJJ Q>J rlX :J0 en
enc :J
~
OJ ~bJJ 00 ..c OJ~ Q+J
+J en ·rl·rl o.cZ ..c 3:
D.....
E E:J :J·rl ·rlrl enrl OJ>J c~ bJJOJ m[IJ ~
E m m:J E·rl :::::J..c ·rl+J D·rl 0~ UJ
OJ OJ~ ~+J bJJ :::::J +J bJJ :J
Lfl C C +J C C +JOJ ·rl U OJ ·rl U
OJ E LJ :::::J OJ E D :J OJrl OJ C ~ Q OJ C ~ QD rl 0 +J >J rl 0 +J >Jm W [IJ (f) f--- W QJ en f---
f---
OJ+J·rl..cQ
m~bJJ
cr; 00 UD ·rl~ rl
m ·rlU (f)
E:::::J·rlC
C ·rl0 E~ :J0 rl[IJ <:(
12

Boiling-point and enthalpy of vaporizationWhen a substance boils, the bonds between its particles are completelybroken. In comparing boiling-points of different elements we get an idea ofthe forces between their particles in the liquid state. These forces varybetween different structural types as we show in this section.
Objective. When you have finished this section, you should be able to:
(4J explain, in structural terms, why the difference between melting-pointand boiling-point is greater for metals than for non-metals.
Read about boiling-point and enthalpy of vaporization. Look for anexplanation of the differences between melting-point and boiling-point in metals and non-metals.
o'If
In Fig. 9 we have superimposed the boiling-points and melting-points of thefirst 18 elements. (Some of the boiling-points are uncertain. J This willenable you to make direct comparisons in the next exercise.
c5000
~"<,
C 4000·0Q.
6c·0 3000.0
\JCcoC 20000Q.
6c~Q.J 1000E
05 10 15 20
Fig.9.atomic number
Exercise 8 Study Fig. 9 and answer the following questions.(aJ Explain why there is generally a greater
difference between melting and boilingtemperatures for metals than for non-metals.
(bJ Identify the places in Periods 2 and 3 where the sharpestchanges in melting-point and boiling-point take place.
(cJ Do the changes you have pointed out in (bJ correspond tostructural changes? Give details.
(dJ If you plotted enthalpy of vaporization values on the samegraph, how would the general shape compare with the shapeof the boiling-point plot?
(eJ Explain why enthalpy of vaporization values are greaterthan enthalpy of fusion values.
(Answers on page 61 J
13

Now that you have surveyed the main physical properties of the elements inPeriods 2 and 3 we consider, briefly, their chemical properties.
PERIODICITY OF CHEMICAL PROPERTIESSince chemical properties of elements are related to their atomic structureyou might expect a periodic relationship in chemical properties. In thissection we consider certain reactions of elements in Periods 2 and 3 todecide whether this is the case.
Objectives. When you have finished this section, you should be able to:
(5) describe the reactions of elements in Periods 2 and 3 with oxygen,chlorine, hydrogen, water, dilute acids and magnesium;
(6) state the trends in reactivity across Periods 2 and 3 in terms ofoxidizing and reducing power.
Start by reading about the reactions of elements in Periods 2 and 3bearing in mind the reactions stated in Objective 5. If possible,choose a textbook which has a section on chemical periodicity. Ifnot you will need to look up the reactions separately. Remember thatyou are looking for general patterns here, rather than a mass ofdetail. The next exercise is designed to help you organise theinformation from your reading.
oCO
Exercise 9 Complete a copy of Table 6, which appears on the nextpage, to show:(a) the ease of reaction (state whether the reaction is
violent, very vigorous, vigorous, slow, very slow orwhether there is no reaction);
(b) the compounds formed for elements in Period 3.Note that you have already studied the reactions of sodium,magnesium and chlorine. When you are completing the table youshould find that you can predict many of the others. We havefilled in some of the spaces already, as a guide.(Answers on page 61 )
14

Table 6A=Ease of reaction: violent, very vigorous, vigorous, slow, very slow, no reaction.B = Main product: (compound of given element)
Na Mg Al Si P(whitel S Cl Ar
Dry O2 A Vigorousand at firstheat
B A12D3
on sur-face
Dry C12 A Slow Slowandheat 8 SC12 and
SzCl2
Dry Hz A Very No No No Veryand vigorous reaction reaction reaction slowheat 8 NaH --- -- --
Cold A SlowHzD
8 HClD(aq)
Cold A Vigorous No No Nodilute if oxide reaction reaction reactionHCl layer
removed8 AIC13 --- --- ---
~:::::::::::::::::::::::::Mg and A NoI-lil-lllli!!i]i!l!l-l!i:ili-i iilllllll
No Slow Vigorousheat reaction reaction
B --- --- Mg2Si Mg3P21:,:,:::,:,:::,:::
Some unusual forms of the Periodic Table
15

Now use your copy of Table 6 to answer the next exercise.
How does the reactivity with oxygen, chlorine andhydrogen change in going across the period?Explain the changes in (a) in terms of reducingpower of the elements.
(c) What is the trend in reactivity with magnesiumacross the period? How does this compare with the trendyou identified in (a)?
Exercise 10 (a)
(b)
(d) Explain this trend in terms of oxidizing power.(e) Using the final two reactions in Table 6 decide at what
point the elements in Period 3 change from being typicalmetals to typical non-metals.
(f) Would you expect similar reactions in Period 2? State anygeneral differences.
(Answers on page 62 )
In the last exercise you found that elements' reducing power decreases ingoing across a period, while their oxidizing power increases. These twotrends are important in describing elements' reactivity. We use them toclassify elements as metals (electron donors with considerable reducingpower) and non-metals (electron acceptors with considerable oxidizing power).
Between the two extremes it is not easy to classify elements as metals ornon-metals. Elements which show both metallic and non-metallic propertiesare called metalloids.
Fig. 10 is an outline Periodic Table showing the approximate divisionbetween metals and non-metals. Metalloids appear near the diagonal 'stair-case' .
__ ease of electron lossINCREASING
-- reducing power
ease of electron gain __INCREASING
oxidising power---
(/l(/l
.S2c '-g Q)
~ r---r---U 0Q) Q
Q) OJ
'0 c r--- I---
'uQ) ::J(/l l:Jro ~Q)
\/<..:J~(f)
«wa::uz
Fig.l0.
CDi:.<~ C N 0 F Ne
AI! P S CI Ar
Ga !Gej As Se Br Kr
In Sn Sb! Te I Xe
TI Pb Bi Po At Rn
16

To consolidate your ideas so far, try the following Teacher-marked [2]Exercise. Before you start this question, you should look through jthe notes you have made in Level One. You may also want to re-readyour notes from Units 11 (The s-Block Elements) and 12 (The Halogens).Since this is an essay-style question, you should make a plan beforeyou start writing.
Teacher-markedExercise
Define the terms covalent and ionic radius.Give an account of the trends in the atomic radiusof the elements both along a period and down thegroups of the Periodic Table. Show how these trendshelp to explain the changes in chemical properties of theelements.
Dmitri Ivanovich Mendeleev (1839-1907).published the forerunner of the modernperiodic tables in 1869.
Extract from a book (published 1884)written by John Newlands, in which heclaims to be the discoverer of theperiodic law.
r' ;;;;ng b::::-fir~"to";bi:;th;':::':::;:;'e"1, periodic law more than nineteen years ago, I feel, under ..t existing circumstances, compelled to assert my priority in i,i this matter. ~~, That both D. Mendelejeff and Lothar Meyer have "A done a good deal to develop the periodic law is admitted, ,"
!J but this admission by no means assumes that either of '.iit these eminent chemists was the first discoverer of the law f:~ in question. As a matter of simple justice, and in the "f interest of all true workers in science, both theoretical '\ and practical, it is right that the originator of any proposal \".>, or discovery should have the credit of his labour.!i __ ., 'r-_.·..'" t,'!!f ~ ~l_ . • • Q!"'-~. . .. ' .
17

LEVEL ONE CHECKLISTYou have now reached the end of Level One of this Unit. The following is asummary of the objectives in Level One. Read carefully through them andcheck that you have adequate notes.
At this stage you should be able to:
(1) explain the meaning of periodicity with reference to atomic and ionicradius, first ionization energy, electron affinity and atomic volume;
(2) explain how the changes in melting-point and enthalpy of fusion whichtake olace across a period are related to structure;
(3) state the types of structure and bonding found in the elements ofPeriods 2 and 3;
(4) explain, in structural terms, why the difference between melting-pointand boiling-point is greater for metals than for non-metals;
(5) describe the reactio~s of elements in Periods 2 and 3 with oxygen,chlorine, hydrogen, water, dilute acids and magnesium;
(6) state the trends in reactivity across Periods 2 and 3 in terms ofoxidizing and reducing power.
LEVEL ONE TESTTo find out how well you have learned the material in Level One, try ~the test which follows. Read the notes below before starting. •1. You should spend about an hour and a half on this test. ~~2. When you have finished, hand your answers to your teacher for markin~.

LEVEL ONE TESTQuestions 1 - 4 concern the following graphs showing how certainphysical properties of elements vary either across a period or downgroup of the Periodic Table:
11>.!:a.11>.s::a.
c
atomic number atomic number atomic number
II>s:a.
11>s:a.
atomic number atomic number
Fig.11.
Select from A to E, the graph which would best represent the1. electronegativities of C, N, 0 and F2. first ionization energies of Li, Na, K and Rb3. number of electrons in Ne, Na+, Mg2+ and A13+4. ionic radii of Li, Na, K and Rb
( 1 )
( 1 )
( 1 )
( 1)
In questions 5 - 7 inclusive, one, or more than one, of the suggestedresponses may be correct. Answer as follows:A if only 1 , 2 and 3 are correct8 if only 1 and 3 are correctC if only 2 and 4 are correct0 if only 4 is correctE if some other response, or combination, is correct
5. Along which of these seriesrise?1 . He, Li, 8e2. 8e, 8, C
3. Na, Mg, Al4. Si~ P, S
do the melting-points of the elements0( 1 )
19

6. A graph of the periodic variation of a property with atomic numberwas constructed for the first twenty elements of the PeriodicTable. This exhibited maxima at atomic numbers 6 and 14 and minimaat atomic numbers 2, 10 and 18.
The property plotted could be the1. melting-point2. first ionization energy3. standard enthalpy of vaporization4. atomic volume ( 1 J
7. Which of the following statements is/are true?1. The electronegativity of the alkali meta15 decrea5e5 with
increase in atomic number of the metal.2. Elements of high electronegativity usually react by gain of
electrons.3. Beryllium and aluminium have similar electronegativities.4. In a given short period of the Periodic Table, the electro-
negativity of the elements decreases from left to right. ( 1 J
Question 8 consists of an incomplete statement followed by five suggestedanswers. Select the best answer.
8. The data below refer to eight elements, lettered M to T (theseletters are NOT chemical symbols).Table 7
Element M N 0 P Q R S T- - - - - -
Atomic Number Z Z+1 Z+2 Z+3 Z+4 Z+5 Z+6 Z+7
Molar enthalpyof vaporization(kJ mol-1) 2.8 3.4 3.3 1 .8 89 129 294 377
Boiling-point (K) 73 93 83 23 1163 1373 2673 2973
From these data, it can be deduced that:A T is in the same group of the Periodic Table as heliumB S has a giant structureC N is a metallic elemento the elements are all in the same period of the Periodic TableE M is a Group 1 element in the Periodic Table (1 )
20

For questions 9 to 12 choose an answer from A to E as follows:A Both statements true: second explains first.B Both statements true: second does not explain first.C First true: second false.0 First false: second true.E Both false.
First Statement Second Statementg. The first ionization energies
of elements decrease down agroup of the Periodic Table.
In descending a group ofthe Periodic Table, theshielding effect of innerelectron shells increasesand the outermost electronshell becomes further fromthe nucleus.
10. Lithium is less electro-negative than potassium.
The outer electron in alithium atom is moretightly bound than theouter electron in apotassium atom.
11 . The first ionization energyof aluminium is greater thanthat of magnesium.
The first ionization energyof aluminium is the energyrequired to remove anelectron from an incompletep-orbital, that for magne-sium, from a complete s-orbital.
12. Fluorine has a higher firstelectron affinity than oxygen.
A fluorine atom needs onlyone electron to reach anoble gas electron config-uration while an oxygenatom needs two.
13. Explain the term electronegativity.How do the electronegativities of the elements changealong the short period lithium to fluorine, and howdo you account for this change?
(b) (i) Define the term electron affinity.
(a) Ii )
(ii)
(1 )
( 1 )
(1)
( 1 )
(4 )
(ii) Which of the elements Cl, I has the smaller numericalvalue of electron affinity and why? (4)
21

~ ~A
r--r--
B CI--- I----
0
E F G
14.
Fig.12.
The diagram shows in outline the Periodic Table, omitting the lanthanidesand actinides. You should answer the fol16wing questions by using theletters A to G as appropriate, and NOT by the usual symbols which you thinkrepresent the elements.(a) Select from the elements A to G:
(i) an element which is liquid at ordinary temperature,(ii) THREE elements which are gaseous at ordinary temperature.
(iii) THREE elements which are good electrical conductors.(iv) the element whose first four ionization energies are 740,
1500, 7700, and 10500 kJ mol-~.(v) the element with electron configuration 1s22s22p5.
(b) Select from the elements A to G:( 7 )
(i) ONE element which occurs in nature as its carbonate.(ii) TWO elements which can occur uncombined. (3 )
Radius/nm 0.095
Mg2+0.065
Si4- p3-
15. Account for the variation in the radii of the following ions.Ion
0.050 0.271 0.212 0.184 0.181(5 J
What would you predict about the size of(a ) the K + ion,(b) the Si4+ ion?Give your reasons. (4)
22

1 E • The graph shows a plot of atomic volume (i.e., relative atomic mass/density) against atomic number for ten successive elements, all withatomic number less than 20. The densities used are of the solid atroom temperature, or, if the element is not a solid under thoseconditions, then at its melting-point.
o>
K
.~Eoro
B
(j)
E
F
c H
A G
atomic number
Ca) (i) To which periodic group do each of the elements A and Bbelong?
Cii) Which other element(s) of the ten shown is or are in thesame group as element B?
(iii) Which of the elements 0 or K will have the higher melting-point? Give your reason. (5)
(b) The atomic volume is a rough measure of the size of the atoms.On what other factor or factors might atomic volume depend? (2)
Explain the variations in the first ionization energies of theseelements including particular reference to a comparison of thefirst ionization energies of the following pairs of atoms:beryllium and boron: nitrogen and oxygen.
17 . Consider the elements of the second period of the Periodic Table(lithium to neon).
(4 )(Total 50 marks)
23


LEVEL TWO
COMPOUNDS OF ELEMENTS IN PERIODS 2 AND 3In Level One you studied the elements in Periods 2 and 3. You examined keyphysical and chemical properties and built up a picture of the PeriodicTable as a whole. In Level Two you use this knowledge as a basis forstudying three important groups of compounds. the chlorides. oxides andhydrides of these elements. We begin with a survey of their formulae.
Periodicity of formulae of Period 2 and 3 oxides, chloridesand hydridesIn Units 11 (The a-Block Elements) and 12 (The Halogens) you learned thatGroups I and II show oxidation states of +1 and +2 respectively in all theircompounds, and that Group VII elements show a range of oxidation states intheir compounds. In this section you extend your knowledge to include theother members of Periods 2 and 3.
First. you predict the formulae of the other members of Periods 2 and 3 anddecide whether there is a periodic pattern.
Objectives. When you have finished this section. you should be able to:
(7) state the formulae of the oxides, chlorides and hydrides of the elementsin Periods 2 and 3;
(8) identify the periodic pattern in the formulae of oxides, chlorides andhydrides.
Start by reading about the compounds of the elements in Periods 2 and <=23 in a dteoxt-boOk. Look for a chapter on periodicity which will help ~,you to the next exercise. ~
Exercise 11 (a) Complete copies of Tables 8, 9 and 10 on the next ~page with the formulae of the oxides, chlorides andhydrides respecti vely. ~\\\Describe the pattern shown by each group ofcompounds. Would you say it is periodic in each case?Explain.
(c) Explain why phosphorus, sulphur and chlorine in Period 3show several oxidation states in their oxides and chlorides.
(b)
(Answers on page 62 )
25

r-,
(Y)
1]0
'rl CD~OJo,4-0
Lilen0.::J0~~
<:TC'rl
enOJ1]'rl (Y)
X04-0
OJ Nrorl
::JE~0u.. <-
r-,
N
1]0
-r-i CD~OJn,
4-0
Lilen0.::J0~~
<:TC·rl
enOJ1]-r-i (Y)
X0
4-0
OJ Nrorl
::JE~0u.. <-
Lil 0 in 0 Lil 0 Lil
+- (Y) (Y) N N <- ..- 0
rl rom rl~ ::JOJ ~ '" il'I CTl
C 0 CTl 0 !'j 0 0OJ 0 !'j 0 !'j 0 !'j 0 !'j~ 4- X X X X X X X
(Y) r-,
D0
·rl~OJo, CD
4-0
en0.::J Lil0~~C·rl
<:TenOJD-r-li-.
0rl (Y).r::u4-0
OJ Nmrl:::JE~0u.. e--'
N r-,
D0
-r-ii-.OJo. CD
4-0
U10.:::J Lil0i-.~C
-r-l<:T
U1OJD-r-i~0rl (Y).r::u4-0
OJ Nrorl
:::JEc,0u.. ..-
rorl x::J'-..Erl~U Lil <:T (Y) N ..-04- 0
-r-irl +-'m ro'-< '-<OJ il'I ~ CTl !'jC D rl rl rl rl rlOJ C U U U U U~ ro x x x x x
r-,(Y)
""'J0
-r-li-.OJ CDn,
4-0
U10. Lil:::J0'-<~C·rl <:T
enOJD·rl'-<D (Y)
>..r::4-0
OJ Nrorl:::JE'-<0u.. ..-
r-,N
D0
-r-l'-<OJ CDo,4-0
U10. Lil:::J0'-<~C·rl <:T
enQJD-r-i'-<D (Y)
>..c
4-0
OJ Nrorl
:::JE'-<0u.. ..-
rorl
:::JXE'-..
'-<I <:T (Y) N .,-04- 0
-r-lrl +-'m roc, '-<OJC D ~ '" !'jOJ c I I I I~ m x x x x
0CO mQ) Q) Q)
r-! r-! r-!..0 ..0 DCO CO COf-.- f-.- f-.-
26
X4-oEo
+-'ro
'-<OJ0.
o4-oenED
+-'ro4-oc,OJ.0E:::JC
OJ.c+-'en3o.cen
cE:::Jrl
oU
en-r-i.cf-
+-

Having considered the formulae of oxides. chlorides and hydrides. you cannow compare the oxidation states of the elements in each compound. You dothis in the next exercise.
Exercise 12 (a) Make a copy of the oxidation number chart shown in ~Fig. 14 and fill in the oxidation numbers of theelerneri t.s in Periods 2 and 3. (Ignore the hydrid es ~\\\of C and Si except for CH4 and SiH4 since thepresence ofC-C and Si-Si bonds give confusingoxidation numbers.)We have inserted three symbols as a guide, usingx for oxides, 0 for hydrides. and D for ch lorides.
+7
+6
+5
+4+3
¢ +2.0
E::l +1cc0
''::; 0co"C
Li Be B C N 0 F Ne Na Mg AI Si'x0 -1
-2-3-4-5
-6
P S CI Ar
Fig,14,
(b) As you noticed in part fa), many of the elementsshow more than one oxidation state in theircompounds. How do you account for the fact thatphosphorus, sulphur and chlorine show theirhighest oxidation states in oxides or chloridesbut not hydrides? (Hint: compare the electro-negativities of 0, Cl and H.J
(Answers on page 63 )
We now consider the physical and chemical properties of the oxides. chloridesand hydrides. We start with the chlorides.
27

Chlorides of the elements in Periods 2 and 3In this section you use electronegativity values to predict the bond typein the chlorides of the elements in Period 3. You then test these predict-ions by experiment.
Objectives. When you have finished this section, you should be able to:
(9) explain the variation in structure and bonding which is evident in thechlorides of Periods 2 and 3;
(10) identify a periodic pattern in the hydrolysis of Period 2 and 3chlorides;
(11) write equations for the hydrolysis reactions shown by Period 2 and 3chlorides.
In Unit S4 (Structure and Bonding) you learned that differences in electro-negativity values between elements in a compound can be used as a rough guideto identify the type of bonding found between them4 You use this idea againin the next exercise.
Exercise 13 Use electronegativity values from your data book toidentify the trend in bond character in the chloridesPeriod 3.(Answers on page 63 )
of /)~
Now test your predictions by doing Experiment 1, in which youexamine the chlorides of the elements in Period 3.
There is an ILPAC video-programme entitled 'Chlorides and Oxides'which covers similar ground to the experiment you are about toperform. Ask your teacher if it is available and whether you shouldwatch it at this point.
28

EXPERIMENT 1Investigating the propertiesof Period 3 chlorides
AimThe purpose of this experiment is to studythe chlorides of Period 3 elements andclassify them according to structural typeand bonding.
IntroductionYou first examine the appearance of each compound and then you find outwhether it dissolves in water and/or hexane. If it does dissolve you maydetect a temperature change. In general, a small temperature changeindicates a physical process and a large one a chemical process. This willhelp you to distinguish between the physical process of dissolving and thechemical one of hydrolysis when you add these substances to water.You also determine any pH changes that take place when you mix the chlorideswith water. A decrease in pH indicates that hydrolysis has taken place.Finally, you consider physical data for each compound and reach a conclusionabout its structure and bonding.
Requirementssafety spectaclesprotective glovesaccess to fume-cupboard14 test-tubes (6 must be dry)test-tube rack2 measuring cylinders, 10 cm3 (1 must be dry)distilled waterthermometer, 0-100 °cspatulauniversal indicator solution and colour chartpH paper to cover the range 1 to 7ammonia solution, 0.880 NH3- - - - - - - - - - - - - - -
sodium chloride, NaClmagnesium chloride, MgCl2aluminium chloride, AICl3 (anhydrous if4 teat-pipettessilicon tetrachloride, SiC14- - - - - - - - - - - - - - - - - ~
;::;;.. Iphosphorus trichloride, PCI3---------------------~_disulphur dichloride, S2C12- - - - - - - - - - - - - - - - - - -, ,.---_hexane, C6H14------------------------ I I ~organic residues bottle -- - ~ •••
I
~---[!]
~-~--- I ;III ~_
I) Ipossible) - - -- --
29

Hazard warningMany of th8 chlorid8s in this 8xp8rim8nt r8act vigorouslywith wat8r. SiC14,PC13 and S2C12 ar8 corrosive and harmful.Th8r8for8 you MUST:DO THIS EXPERIMENT IN A FUME CUPBOARDKEEP STOPPERS ON BOTTLES AS MUCH AS POSSIBLEWEAR GLOVES AND SAFETY SPECTACLESOld stock of silicon tetrachloride often has hydrogen chlorid8 trapp8dat th8 n8ck of th8 bott18. Ensur8 th8 bott18 has b88n ch8ck8d by yourt8ach8r b8for8 you att8mpt to op8n it.Hexan8 is very flammable. Ther8fore you MUST:KEEP THE STOPPER ON THE BOTTLE AS MUCH AS POSSIBLEKEEP THE BOTTLE AWAY FROM FLAMES
Proc8dur8
A. App8aranc8Examin8 the chloride samples provided and, in a larger copy of ResultsTable 1, note for each:(a) wh8th8r it is solid, liquid or gas80us,(b) its colour (if any).
B. On mixing with wat8r1. Set up S8V8n test-tubes, side by side.2. Into each test-tub8 pour about 5 cm3 of distil18d water.3. In the first test-tub8 plac8 a th8rmom8t8r.
(a) Note th8 t8mperature.(b) Add half a spatula-tip of sodium chloride and v8ry car8fully
stir with th8 th8rmometer.(c) Note, after about on8 minute, (i) the temperatur8, (ii) whether
the solid has dissolv8d and (iii) anything 81s8 you S88. For8xamp18, is gas 8volv8d at any time? If so, if th8 gas acidic?Can you id8ntify it using B simp18 t8St?
Cd) Add 2-4 droos of univ8rsal indicator solution, or US8 a pi8c8 ofpH paper, compare the colour with the chart provided, and notethe pH indicated.
Repeat (but with more care!) the above steps 3. (a) - (d) ~uSing. in turn. magnesium chloride. aluminium chloride. silicon ~t8trachlorid8 (2 drops), phosphorus trichlorid8 (2 drops), and ~disulphur dichloride (2 drops).
4.
5. M8asur8 the oH of the water in the sev8nth t8st-tube byadding 2-4 drops of universal indicator solution or byusing pH pap8r, for comparison with th8 above.
C. On mixing with hexan81. S8t up anoth8r six t8st-tub8s, side by sid8. Th8se must be dry.2. Into each test-tube pour about 5 cm3 of hexane.3. In th8 first test-tub8 plac8 a thermomet8r.
(a) Note the temperature.
30

(b) Add half a spatula-tip of sodium chloride and stir very carefullywith the thermometer,
(c) Note, after about one minute, (i) the temperature, (Ii) whetherthe solid dissolves and (iii) anything else you see.(Dispose of hexane by pouring into the residue bottle provided.)
4. Repeat the above steps 3.(a) - (c) using, in turn, magnesiumchloride, aluminium chloride, silicon tetrachloride (2 drops),phosphorus trichloride (2 drops), and disulphur dichloride(2 drops).
Results Table 1
NaCl MgC12 AlC13 SiC14 PC13 S2C12
Appearance
On mixing with waterInitial temperatureFinal temperatureDoes it dissolve?pH of solutionOther observation(s)(if any)
On mixing with hexaneInitial temperatureFinal temperatureDoes it dissolve?Other observation(s)(if any)
(Specimen results on page 63 )
Questions
2.
1. Complete a copy of Table 11 on the next page using your experimentalresults and your data book. Then decide on the structure of thesechlorides and the bonding found in them and fill in the last part ofyour table.In the experiment you discovered that some of the chlorides 0are hydrolysed by water. Look up the equations for these ca. .......• ..
reactions in your text~book(s). In the case of S2C12 you may •.' ...find that most books do not give an equation for its hydrolysis.This is because a mixture of products is obtained. We suggestthe following equation:2S2C12(1) + 2H20(1) ~ S02(aq) + 3S(s) + 4HC1(aq)
3. Compare your conclusions from this experiment with the predictions youmade in Exercise 13. Did the experiment confirm your predictions?
(Answers on page 64 )
31

CY)
Do'rl'-<QJD....
'+-o
Nr-I
U
Nr-I
UN
en
rtlr-I
UD....
:tr-I
U'rlen
rtlr-I
U.--1.::(
Nr-I
UtJ.O~
r-I
UCDZ
MI
*r-I
00. E cD 0-t-l 'J -r-l
·rll :J QJQJ '-< <, IT
-t-lC
D r-I 'rl ::J CD-r--l -t-l U r-I r-I
X'-< U U CD 0 QJ0 0 0 '+- 4- '-< (f) ..cr-I <, <, QJ 0 0 QJ..c -t-l -t-l -t-l +J (f) CU C C CD M QJ >, CD ::J 'rl
·rl ·rl -t-l I r-I -t-l 3: 04- 0 0 (f) r-I 0 -r-i OJ >,0 D- D- o E > 4- ::J +J QJ
I I r-I E ·rl 0 IT ·rl '-<CD tJ.O b.D CD '-< +J CD r-I ::J tJ.Or-I c: c: U 'J QJ U c: -r-i -t-l C:J 'rl -r-i 'rl s: D.. :J 0 4- D U -r-i
E -t-l r-I (J) <, D ·rl 0 :J :J D'-< r-I ·rl >,
~4- ~4- c: -t-l r-I '-< c0 QJ 0 .r; 0 u I 0 -t-l 0
LL ~ OJ D- U <C 0. en en OJ
(f)QJD-r-l
'-<or-I
s:U
4-o(f)
QJ'rl-t-l
'-<QJ0.o'-<D-
QJr-I
DCDI-
32
E-t-lCD
DCCD
Uo
oN
QJ
-r-l
QJ'-<::J(f)(f)QJ'-<0.
Dc:CD
QJ~:J
-t-lCD~QJD..
EQJ
-t-l
Eoo'-<
D..
'-<*

You have seen how the physical and chemical properties of the chloridesch~nge in going across Period 3. You should now be able to make somepredictions about the chlorides of the elements in Period 2. Bear in mindthat the first three elements in Period 2 resemble those diagonally belowthem.
You make these predictions in the next exercise.
Exercise 14 (a) Complete a larger copy of Table 12. Some of thetable has been filled in for you.
(b) Using your textbook(s) if necessary, write anequation for the reaction of each chloridewhich is hydrolysed by water.
(Answers on page 64 )
Table 12
Formula ofchloride LiC1 BeC12 BC13 CC14 GJC13 OC12 FCI
State atat r.t.p.Conductivityof liquid
Action of Reactswater
Structure Chainpolymer
Bonding
33

From what you know about a-block compounds you might expect thatcalcium chloride would be resistant to hydrolysis. This is not thecase, however, as the next exercise shows.
Exercise 15 Calcium chloride hydrolyses slightly, as shown by theequation:
This indicates some degree of covalent character. Suggest anexplanation for this using your knowledge of polarizing powersof cations and the polarizability of anions from Unit 11(a-Block Elements).
We now briefly consider the anhydrous chlorides of Group II elements.(Answer on page 64 )
Anhydrous chlorides of Group II elementsObjective. When you have finished this section, you should be able to:
(12) explain why the chlorides of certain Group II elements cannot beobtained simply by heating the hydrated salts.
Find out from your text-book(s) why a sample of hydrated magnesiumchloride cannot be made anhydrous directly by heating it. Read about c:>.the indirect method of making the anhydrous salt. This will enable ~. ,.......•..you to do the next exercise. ~
Exercise 16 The salt MgCl2'6H20 when heated alone does not yieldanhydrous MgCI2• Anhydrous MgCl2 can, however, beobtained by heating the salt in a stream of hydrogenchloride gas. Explain.(Answer on page 64 )
Now attempt the next exercise, again using your knowledge of polarizingpower from Unit I1 (s-Block Elements).
Exercise 17 Beryllium(II) chloride hydrolyses with complete removalof chlorine, but magnesium(II) chloride forms anoxychloride on hydrolysis. Explain.(Answer on page 64 )
You should now be able to answer the following A-level question.
34

Exercise 18 (a) Copy and complete the following table:Table 13
Formula of Physical state of Type of bondingElement chloride chloride at s.t.p. found in chloride
Hydrogen
Barium
Nitrogen
Silicon
(b) Give the equations describing the reaction of thechlorides of hydrogen and silicon with water.(i) chloride of hydrogen
(ii) chloride of silicon(Answers on page 64
We end the work on chlorides by considering methods of preparing them. Themethods used depend on the physical states of the elements and on thenature of the chlorides formed. This section draws on ideas both from yourpre-A-level course and from the work you have just done on chlorides.
Methods of preparing chlorides in Period 3We focus on the preparation of aluminium chloride AlCls' which you carryout in Experiment 2. You then suggest adaptations of this method, oralternative methods, for preparing other chlorides in these periods.
Objectives. When you have finished this section, you should be able to:
(13) prepare a sample of a solid covalent chloride, e.g. aluminium chloride;(14) describe the apparatus suitable for preparing a solid covalent
chloride;(15) explai~ how the apparatus for preparing a solid chloride needs to be
modified for preparing a liquid or gaseous chloride;(16) calculate the percentage yield of a product.
In the next experiment you prepare a sample of aluminium chloride. You usea known amount of aluminium and weigh your product at the end. You thencalculate the theoretical mass of aluminium chloride from the chemicalequation and compare this with the actual mass. This enables you to obtainthe percentage yield of the product.
35

We briefly consider the method for such a calculation in the followingworked example. This will enable you to calculate your percentage yieldat the end of the experimeni.
Worked Example In an experiment to prepare anhydrous aluminiumchloride, 0.80 g of aluminium foil were heated in astream of dry chlorine. This gave 2.38 g of product.What is the percentage yield of AICI3?
Solution1. Write the equation for this reaction:
AI(s) + 1~CI2(g) ~ AICI3(s)2. Use the equation to calculate the mass of aluminium chloride that is
formed from 0.8 g aluminium.1 mol of Al produces 1 mol of AICl3
i.e. 27 g of Al produces 133.5 g of AICl3
133.5 g1.0 g of Al produces 27 of AICl3
133.5 g0.80 g of Al produces x 0.80 = 4.0 g of AICl3273. Express the actual mass formed in the experiment as a percentage of
the theoretical mass.o • ld actual mass of product~ Yle = theoretical mass of product x 100
. ld 2.38 g% Yle = x4.0 g 100 = 160% I
You need to use expensive ground-glass-joint apparatus in thisexperiment. If you have not already used this apparatus in Unit 02(Some Functional Groups) ask your t8acher for advice, or if it isavailable watch the ILPAC video-programme 'Organic Techniques I'.Alternatively, you could watch the complete experiment on the ILPACvideo-programme 'Preparation of aluminium chloride' either as anintroduction or, if your teacher prefers, instead of carrying itout yourself.
36

EXPERIMENT 2Preparing anhydrous aluminium chloride
AimIn this experiment you gain experience insetting up an assembly of glassware inorder to carry out an inorganic synthesis.You also calculate the percentage yield ofyour product.
IntroductionYou prepare chlorine by adding concentrated hydrochloric acid to potassiummanganate(VII), dry it using anhydrous calcium chloride and then pass itover heated aluminium foil in a combustion tube. When the reaction iscomplete you weich your collected product and calculate the percentageyield.
Requirementssafety spectaclesaccess to fume cupboardforcepsglass rodcalcium chloride, anhydrous, CaClzlong spatulacombustion tubeceramic woolbung fitted with a short piece of glass tubingaccess to a balance capable of weighing to within 0.01 galuminium foil, Alabsorption tubesoda-limereceiver bottle with two holed bung (connected to absorption tube and
combustion tUbe)rulerrubber tubing for connections3 clamps, bosses and standspotassium manganate (VII), KMn0
4- - - - - - - - - - _
pear-shaped flask with ground-glass joint, 50 cm3
ground-glass adapter with T-connectioncylindrical funnel with ground-glass jointhydrochloric acid. concentrated, HCl- - - - - - -- - - - - - _-Bunsen burnerspecimen tube with lidlabelsaccess to a desiccator
37

Hazard WarningChlorine is a toxic gas. Therefore you must:00 THE EXPERIMENT AT THE FUME CUPBOARD
Concentrated hydrochloric acid is a corrosive liquid, and its vapouris harmful to eyes, lungs and skin. Therefore you must:WEAR GLOVESWEAR SAFETY SPECTACLESKEEP THE HYDROCHLORIC ACID IN A FUME CUPBOARDKEEP THE STOPPER ON THE BOTTLE AS MUCH AS POSSIBLE.
ProcedureThere are several steps in assembling the apparatus for this experiment.The diagram below (Fig. 15) gives you an idea of what you are aiming for.Details of the separate stages are given at appropriate points in theprocedure.
receiver bottleanhydrous
CaCI2aluminium
foil I
HEATcombustion tube
Fig.15. Preparation of aluminium chloride
1. Using forceps and a glass rod, put some granular anhydrous calciumchloride between two loose plugs of ceramic wool near the entrance ofthe combustion tube. (Make sure that the calcium chloride fills thecross-section of the tube without preventing the free flow of chlorine.)Attach the bung fitted with glass tubing at the entrance of thecombustion tube.
2. Weigh about 0.25 g of aluminium foil, crumple it loosely, and put itin the combustion tube as shown in Fig. 16.
anhydrous CaCI2
2cm
ceramicwool plugs
crumpledaluminium foil
Fig.16.
38

3. Loosely pack the absorption tube as follows.(a) Using forceps. push in a plug of ceramic wool.(b) Fill with soda-lime.(c) Using forceps again. close with a second plug of ceramic wool.
Both plugs must be loose to avoid blockage.
4. Fit the combustion tube and absorption tube to the receiver bottle asshown in Fig. 15. Clamp the apparatus so that the combustion tube isabout 15 cm above the base of the fume-cupboard.
5. Weigh about 5 g of potassium manganate(VII) and place it in the pear-shaped flask.
6. Fit the pear-shaped flask with the adapter and dropping funnel. Thenclamp the flask and connect it to the combustion tube. as shown inFig. 15.
You are now ready to start the preparation.7. Get your teacher to check the apparatus.B. Make sure that the tap of the dropping funnel is closed.9. Pour 10 cm3 of concentrated hydrochloric acid into the dropping
funnel.10. Allow a few drops of acid to trickle on to the potassium manganate(VII)
and allow chlorine to displace air from the apparatus. Then let theacid continue dripping slowly into the flask.
11. Heat the aluminium gently near the calcium chloride until a bright glowshows that the chlorine is reacting exothermically with the aluminium.If the glow is very bright, remove the heat till it subsides.
12. Continue heating. all around the tube. moving the flame slowly towardsthe receiver bottle. until reaction. is complete.
13. When reaction is complete, stop the trickle of acid and let thecombustion tube cool (about 10 minutes). Meanwhile weigh the emptyspecimen tube and top.
14. Remove the receiver bottle and. using your spatUla. quickly scrapethe product into the specimen tube. Place the top on the specimentube and weigh it.
15. Record your results in a copy of Results Table 2.16. Label the specimen tube with your name and the name of the product and
store it in a desiccator.
Results Table 2
Mass of aluminium c 2 C;;::t-~,"g
\ ~:, :.-It.'!,
1--- ~C'>Mass of empty specimen tube, m1 -J.~~, ~gMass of specimen tube and product " m2 (~~)b gMass of product, m = (m2-m1) 1 ~) t g% yield _-7/;$AI,
;;
() /
't) /
(Specimen results on page 65 )
39

Questions1. Why is it important to use dry chlorine in this experiment?2. What impurity would be present in the product if damp chlorine were
used? Write an equation for the reaction giving this impurity.3. Calculate and comment on the % yield of your product.4. Why is your product stored in a desiccator?(Answers on page 65 )
The next exercise is about modifications to the method you have just used.
Exercise 19 (a) Figs. 17 and 1B below show two assemblies forpreparing the chlorides of the elements in PeriodWhich apparatus would be more suitable for:
3. /)
~
(i) SiC14, (ii) PC13,
In making your choice, you should consider both the stateof each chloride at room temperature (20 °C) and themelting-point of the element.
anhydrousCaCI2
HEAT
soda lime
.~
ceramicwool plugs
Fig.17.
concentrated HCI
ceramicwool pluqs
HEATFig.18
40

(b) How would you heat each of the three elements in part (a)in preparing its chloride? We suggest you choose fromthe following alternatives -I strongly I, Ienough to start the reaction', 'enough tomelt the element'.In making your choice, consider the melting-point of eachelement and its reactivity with chlorine.
(c) Bearing in mind the melting-point and boiling-point ofsulphur, what would you have to avoid in the preparationof disulphur dichloride?
(Answers on page 65 )
So far, you have learned about preparing chlorides by direct synthesis.Although ionic chlorides may also be prepared in this way, there is analternative method, which you should know from your pre-A-level work.
If necessary, consult a textbook on the preparation of ionic salts.Then go on to do the next exercise. o
WExercise 20 Outline a method, other than direct synthesis, by ~
which you could prepare a sample of lithiumchloride or sodium chloride, starting with a sample ~~of the metal.
(b) Why would this method not be suitable for anhydrous
(a)
magnesium chloride?(Answers on page 65 )
In addition to knowing how to prepare chlorides of various elements youneed to be able to determine their composition. We deal with this in thenext section.
Formula determination of chloridesThe most straight-forward way to determine the composition of a chloride,given simple laboratory apparatus, is to use a titration method.
Objective. When you have finished this section, you should be able to:
(17) calculate the formula of a chloride using data from a silver nitratetitration.
You have already performed a silver nitrate titration in Unit S1 (The Mole)in order to determine the number of molecules of water of hydration inhydrated barium chloiide. You should therefore be able to calculate anempirical formula using titration data. In Unit S1 (The Mole) you met thecalculation in several stages. To give you practice in following it throughas a single process, try the exercises which follow. The first one isbroken down into separate steps as a guide.
41

Exercise 21 0.767 g of a chloride of phosphorus was dissolved in ~water and made up to 250 cm3 of solution. After neutra-lization, 25.0 cm3 of this solution required 18.4 cm3 of ~~0.100 M AgN03 for complete precipitation of the chloride.(a) Calculate the amount of chloride ion in 25.0 cm3 of
solution.(b) Calculate the amount and hence the mass bf chloride ion in
the whole sample.(c) Calculate the mass and hence the amount of phosphorus in
the whole sample.(d) Calculate the amount of chlorine combined with one mole of
phosphorus.(e) Hence state the simplest formula of the chloride.
(Answers on page 65 )
Now try a similar calculation by doing the next exercise.
Exercise 22 1.420 g of a chloride of sulphur was dissolved in water ~and the solution made up to a v~lume of 250 cm3•25.0 cm3 of this solution, after neutralization, needed ~~.21.0 cm3 of 0.100 M AgN03 for complete precipitation ofthe chloride.(a) Calculate the number of moles of chloride combined with
one mole of sulphur.(b) If the relative molar mass of the chloride is 135.2 what
is its molecular formula?(Answers on page 66 )
Now that you have finished the work on chlorides you should be ableto answer the following Teacher-marked Exercise which is an A-levelessay question. Look through your notes and make a rough plan beforeyou start writing.
Teacher-markedExercise
Write an essay on the chlorides of the elements,sodium, magnesium, aluminium, silicon, phosphorusand sulphur. You may like to consider theircomposition, preparation. physical nature and theirreactions with water. You should also try torelate these considerations to such properties ofthe atoms as size and electronic structure.Describe how you would determine the formula of one ofthese chlorides in the laboratory, if you were given therelative atomic mass of t~e parent element.
We now move on to consider the oxides of Periods 2 and 3.
42

Oxides of the elements in Periods 2 and 3You have already studied the oxides of Groups I, II and VII in some detail,in Units 11 (The s-Block Elements) and 12 (The Halogens). In this sectionyou extend your knowledge to include the other elements of Periods 2 and 3.
Objectives. When you have finished this section, you should be able to:
(18) state the changes in the acid-base nature of the oxides of theelements across Periods 2 and 3;
(19) explain the change in structure and bonding ir, the oxides of theelements in Periods 2 and 3;
(20) identify a periodic pattern in the reactions of the oxides of theelements in Periods 2 and 3 with water.
We start with an exercise similar to the one you did for the chlorides ofthe elements in Period 3 where you use electronegativity differences topredict the type of bonding found in these compounds.
Exercise 23 Use the electronegativity values from your data book to ~identify:(a) the type of bonding found in Period 3 oxides; ~\\(b) the change in bond type that takes place with
increasing atomic number across the period.(Answers on page 66 )
You could, as an alternative, watch part 2 of the ILPAC video-programme entitled 'Chlorides and Oxides' if it is available.
Now test your predictions by doing Experiment 3, in which you examinethe oxides of the elements in Period 3.
43

EXPERIMENT 3Investigating the propertiesof Period 3 oxides
AimThe purpose of this experiment is to examinethe oxides of Period 3 elements and describetheir structure and bonding.
IntroductionYou carry out.an investigation along similar lines to the work you didon the chlorides of the elements in Period 3. However, you will not beasked to test the oxides with hexane because unlike the covalent chlorides,most of the oxides are not composed of discrete molecules. Therefore theyare unlikely to dissolve in hexane and simple experiments cannot distinguishbetween insolubility and slight solubility.
Requirementssafety spectaclesaccess to a fume cupboard6 test-tubestest-tube rack1 measuring cylinder, 10 cm3
1 measuring cylinder, 100 cm3
distilled waterthermometer, 0-100 °c1 spatulauniversal indicator solution and colour chartteat-pipette
~~d~~~e~erOXide, NaZ02-------------------.- I ~ I
splints ~magnesium oxide, MgO ~.~--~~aluminium oxide, A1203 I ~phosphorus(V) oxide, P4010-------------------
silicon(IV) oxide, Si02 [i]access to sulphur dioxide cylinder or generator, S02 -- - - - - - - - ,(~Drechsel bottle ~glass tubing with right-angled beDdrubber tubing for connections.
Hazard WarningPhosphorus(V) oxide is corrosive and irritates eyes, skin and lungs.Sodium peroxide is also corrosive and a powerful oxidant. Sulphurdioxide is a toxic gas with a choking smell. Therefore you must:DO THE EXPERIMENT AT THE FUME-CUPBOARDWEAR SAFETY SPECTACLESAVOID CONTACT WITH SKIN
44

Procedure
A. AppearanceExamjne your oxide samples, and in a larger copy of Results Table 3note fGr each:(aJ whether it is solid, liquid or gaseous,(bJ its colour (if anyJ.
B. On mixing with water1. Set uo six test-tubes. side by side.2. Into each tube pour about 5 cm3 of distilled water.3. In the first test-tube place a thermometer.
(aJ Note the temperature.(bJ Add half a spatula-tip of sodium peroxide and stir carefully
with the thermometer.(cJ Note after about one minute, (iJ the temperature, (ii J whether
the solid has dissolved and (iiiJ anything else you see. Forexample. is gas evolved at any time? If so. is the gas acidic?Can you identify it using a simple test?
(dJ Add 2-4 drops of universal indicator solution. compare thecolour with the chart provided, and note the pH indicated, oruse a piece of pH paper.
4. Repeat the above steps 3. (aJ-(dJ using, in turn, magnesium oxide.aluminium oxide, silicon(IVJ oxide and phosphorus(VJ oxide.
5. Measure the pH of the water in the sixth test-tube by adding 2-4drops of universal indicator solution for comparison with theabove.
6. Bubble sulphur dioxide slowly through the water in the sixth test-tube until there is no further change in the colour of theindicator. Note the final pH of the solution. (You will probablybe given sulphur dioxide in liquid form in a cylinder. To obtainthe gas you carefully open the valve and the sudden decrease inoressure inside the cylinder causes the surface liquid to vaporize.Make sure there is a Drechsel bottle between the cylinder and thewater in case of suck-back. Alternatively, your teacher may suggestother ways of generating the gas. J
7. To test the solubility of sulphur dioxide lower the delivery tubefrom your generator to the bottom of the 100 cm3 measuring cylinderfilled with water. Pass a slow steady stream of gas through thewater and when the air has been expelled from your apparatus lookfor a change in the size of the sulphur dioxide gas bubbles as theyrise up through the water.
45

Results Table 3
Na202 MgO A1203 Si02 P4010 S02
Appearance
On mixing with waterInitial temperatureFinal temperatureDoes it dissolve?pH of solutionOther observation(s)(if any)
(Specimen results on page 66 )
1 •o
Use your experimental results, your data book and your ~text-book(s) if necessary to complete a larger copy of Table 14. ~
Questions
Table 14
Formula of oxide Na202* MgO A1203 Si02 P4010* S02* C12O*
Melting-point/oC
Boiling-point/oC
State at s.t.p.
Action of water
pH of aq. solution
Acid/base nature
Conductivity ofliquid
Solubility inhexane
Structure
Bonding
Those substances marked * represent the most familiar or readilyavailable oxides of that element. In general the other oxides ofthat element have similar properties.
46

2. Write equations for any reactions which took place when you added theoxides to water.
3. Comment on the change in structure and bonding in the oxides betweensodium and chlorine.
4. How does the acid-base nature of the oxides of the elements in Period 3change with increasing atomic number?
5. Can you relate this change to the change in structure and bonding thattakes place along the period?
6. 00 your conclusions about the bonding of the oxides of the elements inPeriod 3 confirm your predictions in Exercise 23?
(Answers on page 67 )
You are now in a position to predict the structure and bonding of Period 2oxides and relate these to their acid-base nature. You do this in the nextexercise.
Exercise 24 Complete a larger copy of Table 15. Remember that Li20, ~BeD and B203 are likely to have much in common withthose diagonally below them in Period 3. ~~(Answer on page 67 J
Table 15
Formula Li20 BeD 8203 CO2 * N204 * O2 F2O*of oxide
state ats.t.p.
Conduct- Fairly Veryivity of poor poorliquid
Acid-basenature
Structure
Bonding
Those substances marked * represent the most familiar or readily availableoxides of that element.
You should now be able to answer the next exercise.
47

Exercise 25 (a) state the name and formula of the main productformed when each of the following elements reactswith an eXCESS of dry oxygen in the absence of acatalyst.(i) Na (ii) Al (iii) S
(b) Briefly state the bonding in, and the structure of, thethree compounds formed in (a).
(c) Which one of these oxides has the lowest melting-point?(d) \~hich one of these oxides dissolves in water to form an
alkaline solution?(e) Write an equation for the reaction in (d).(Answers on page 67 )
To help you consolidate your knowledge of the oxides of Periods 23 you should now answer the following Teacher-marked Exercise,which is an A-level essay question. Look through your notes andmake a rough plan before you start writing.
anci [2JTeacher-marked
Exercise :::e0more than one well-characterized oxide. You may liketo consider their composition, structure, thermal stability,and behaviour with water and/or acids and alkalis, but youraccount need not be concerned with all these points norconfined to them.
Write an account of the more important oxides ofelements from sodium (Na; Z = 11) to chlorine(Cl; Z = 17J, remember that some of the elements
Hydrides of the elements in Periods 2 and 3In Units 11 (The a-Block Elements) and 12 (The HalogensJ you studied thehydrides of Groups I, II and VII. You learned that most of the a-blockhydrides are ionic whereas the halogen hydrides (hydrogen halides) arecovalent. In this section we consider the hydrides of all the elements inPeriods 2 and 3.
Objectives. When you have finished this section you should be able to:
(21) state which hydrides of Periods 2 and 3 react with water and writethe appropriate equations;
(22) describe the trend in acid-base nature of the hydrides across Periods2 and 3;
(23) classify the hydrides of the elements in Periods 2 and 3 as ionic,covalent or borderline.
Start by reading about the hydrides of Periods 2 and 3. In parti-cular look for the reaction of each one with water. Find a textbookwith a section on hydrides if possible, otherwisE you will have tolook up each compound according to its group in the Periodic Table.Then do the next exercise.
oat48

(i) give the physical states of the missinghydrides at room temperature and pressurewith the aid of your data book;
Exercise 26 (a) In a larger copy of Table 16 which you will findbelow:
(ii) say whether the missing hydrides react with waterand, if so, whether hydrogen is given off in thereaction;
(iii) classify the missing hydrides as acidic, basic orneutral depending on whether they are a source ofH+ or OH- ions in water.
(b) \~rite an equation for the reactions with water of:(i) NaH, (ii) MgH2, (iii) AIH3.
(c) Describe the trend in acid-base nature across each period.(Answers on page 67 )
Table 16
Empiricalformula ofhydrides LiH BeH2 BH3 * CH4 ** NH3 OH2 FH
state at Gasr.t.p. Solid (B2H6)
Behaviour Reacts Reactswith H2 H2v!ater evolved evolved
Acid/basenature Basic Neutral
Empiricalformula ofhydrides NaH MgH2 AIH3 SiH4*** PH3 SH2 CIH
"State atr.t.p. Solid Solid
Behaviour Reacts Reacts Virtuallywith H2 H2 insoluble,water evolved evolved no reaction
Acid/base Essentially****nature Basic Basic neutral
* A series of boron hydrides is formed, the simolest being B2H6(diborane).** A series of hydrocarbons is formed (see Unit 01 (Hydrocarbons)).
*** A series of silicon hydrides is formed (silanes).**** Could be interpreted as amphoteric.
49

The hydrides of Periods 2 and 3 can be classified in relation to their bond-type and structure. and to the electronegativity of the element concerned.The change in acid-base character of the hydrides can also help us in thisclassification just as the change in the acid-base nature of the oxidescorrelated with the change in bond-type. As with the oxides. borderlinecases exist between the extremes of the periods.
Exercise 27 (a) In a copy of Table 17. which you will find on the ~next page. fill in the electronegativity differencebetween each element in Periods 2 and 3 and ~\\\hydrogen. The electronegativity values are in yourdata book.
(b) With the aid of the rest of the table classify thehydrides of Periods 2 and 3 as ionic, covalent orborderline and enter your decisions in the appropriatespaces. Don't rely entirely on electronegativity.
(cl State the ions present in each of the hydrides which youhave classified as ionic. Give reasons for your answer.
(d) You may be surprised by the small electronegativitydifferences for LiH and NaH which have NaCI type lattices.What do these values suggest about the bonding in LiHand NaH? Explain your answer by considering the size ofthe H- ion shown in Fig. 19 below.
(Answers on page 68 )
G G G0.136 0.181 0.195
000.208 0.216
Fig.19. Relative sizes of the halide & hydride ions/nm
You have now completed your main work on hydrides. We suggest yourevise this topic and answer the following Teacher-marked Exercise.
Teacher marf<.edExercise
Compare: and contrast the physical and chemical 0properties of the simple hydrides of each of Athe following elements:sodium. calcium, carbon, nitrogen, oxygen. fluorine. I
In your answer, attempt to relate these propertiesto the type of bonding encountered in these hydrides.
To end this section, we give an exercise to summarise the evidence from thehydrides for diagonal r81ationships in the Periodic Table.
Exercise 28 In what ways do the hydrides of Li and Mg, Be and Al and ~Band Si illustrate the diagonal relationships between .•..these elements? ~~(Answers on page 68 )
50

0)rl.Dco1-
~ ~CO +J >. CO +Jrl C rl rl C
U 0) :::J 0) b.O U 0) :::J 0)·rl rl U rl C ·rl rl U rl"'0 0. 0) CO 0 "'0 0. 0) CO·rl E rl > I ~ ·rl Erl >
I U ·rl 0 0 rl +J U ·rl 0 0LL c::( Cf) E u u Cf) CO Cf) E u
~ ~CO +J CO +J
rl rl C rl CCO 0) :::J 0) >. o 0) :::J 0)~ rl U rl rl ·rl rl U rl+J 0.. 0) CO .::L "'0 0.. 0) Ctl
N :::J E rl > N m ·rl E rl >I 0) ·rl 0 0 I 0) U ·rl 0 00 Z Cf) E u Cf) 3: m Cf) E u
~ ~Ctl +J >. m +Jrl C rl rl C
0) :::J 0) +J 0) :::J 0)U rl U rl ..c U rl U rl·rl 0. 0) CO >. b.O ·rl 0. 0) CO
1'1\ UJ E rl > 1'1\ ~ ·rl UJ Erl >I m ·rl 0 0 I 0) rl Ctl ·rl 0 0Z OJ Cf) E u D.... > UJD Cf) E u
>.rl~ rl ~
m +J m m +Jrl rl C ·rl rl rl CCtl 0) :::J 0) +J m 0) :::J 0)~ rl U rl C ~ rl U rl+J 0.. OJ Ctl .;t Q) +J 0..Q) Ctl
.::t :::J E rl > I UJ :::J Erl >I 0) ·rl 0 0 ·rl UJ Q) ·rl 0 0U Z Cf) E u Cf) w c Cf) E U
km +J >. +-'
rl rl C rl 0) CCtl Q) :::J Q) rl U >'Q)k rl U rl Ctl U +-' ·rl rl rl
-I-' 0. Q) m 1'1\ E ·rl C +J C m1'1\ :::J E rl > I ~ UJ m +J ·rl >
I 0) ·rl 0 0 rl 0 Ctl ·rl Ctl m 0OJ Z Cf) E u c::( Z D t!) rl :E U
U.,-t +J +J~ C Q) CQ) >. Q) o >'Q)
U E rl rl U +J ·rl rl rlN ·rl >. C Ctl N ·rl C +J C Ctl
I UJ rl ·rl > I UJ m +J •..-t >0) Ctl 0 Ctl 0 b.O m ·rl m m 0rn OJ D.... :E U :E rn t!) rl :E U
>. >.rl rlD Dm Ctl·rl ·rlU U
U +J U 0) U U +J U 0) U-rl C ·rl ~ .,-t ·rl C ·rl ~ ·rl
I UJ Ctl C 0.. C I UJ Ctl C 0. C·rl- Ctl ·rl 0 0.. 0 m CO ·rl 0 0.0~ OJ ~- ·rl c::( ·rl Z rn t!) ·rl c::( ·rl
m >. m >.rl +J 0) rl +J OJ::::J ·rl ~ :::J ·rl ~E > :::J C E > :::J C~ .r! +J 0 ~ ·rl -I-' 00 -I-' CO ·rl 0 +J CO ·rl4- m C +J 4- m C +J
Q) b.O OJ- CO 0) on 0) IIIrl "'0 0) U 0) 0) U rl 1:) 0) U 0) OJ 0Ctl ·rl C C UJ ~ ·rl CO ·rl C C U} ~ ·rlU ~ 0 Q) CO :::J b.O 4- U ~ 0 Q) Ctl :::J b.O 4-·rl "'0 ~ ~ D +J C ·rl ·rl "'0 ~ ~ D -f.J C ·rl~ >. +J Q) <, U ·rl (J) ~ >. +J Q) <, 0 ·rl UJ·rl ..c U 4- "'0 :::J "'0 UJ ·rl ..c U 4- "'0 :::J "'0 (J)0.. Q) 4- .r-! ~ C Ctl 0.. Q) 4- ·rl ~ C CtlE4- rl ·rl U +J 0 rl E 4- rl -rl 0 +J 0 rlW 0 W "'0 c::( Cf) OJ U W 0 W "'0 c:( (f) OJ U
51

LEVEL TWO CHECKLISTYou have now reached the end of this Unit. Look again at the checklist atthe end of Level One. In addition, you should be able to:
(7) state the formulae of the oxides, chlorides and hydrides of the elementsin Periods 2 and 3;
(8) identify the periodic pattern in the formulae of oxides, chlorides andhydrides;
(9) explain the variation in structure and bonding which is evident in thechlorides of Periods 2 and 3;
(10) identify a periodic pattern in the hydrolysis of Period 2 and 3chlorides;
(11) write equations for the hydrolysis reactions shown by Period 2 and 3chlorides;
(12) explain why the chlorides of certain Group II elements cannot beobtained simply by heating the hydrated salts;
(13) prepare a sample of a solid covalent chloride, e.g. aluminium chloride;(14) describe the apparatus suitable for preparing a solid covalent
chloride;(15) explain how the apparatus for preparing a solid chloride needs to be
modified for preparing a liquid or a gaseous chloride;(16) calculate the percentage yield of a product;(17) calculate the formula of a chloride using data from a silver nitrate
titration;(18) state the changes in the acid-base nature of the oxides of the elements
across Periods 2 and 3;(19) explain the change in .structure and bonding in the oxides of the
elements in Periods 2 and 3;(20) identify a periodic pattern in the reactions of the oxides of the
elements in Periods 2 and 3 with water;(21) state which hydrides of Periods 2 and 3 react with water and write the
appropriate equations;(22) describe the trend in acid-base nature of the hydrides across Periods
2 and 3;(23) classify the hydrides of the elements in Periods 2 and 3 as ionic,
covalent or borderline.
END~OF-UNIT TESTTo find out how well you have learned the material in this Unit,try the test which follows. Read the notes below before starting.1. You should spend about 1~ hours on this test.2. Hand your answers to your teacher for marking.
52

~\~lthe properties of a number of chlorides as shown 0END-OF-UNIT TEST
Questions 1-5 concernin the table below.Table 18
state at room Effect of Action of watertemperature gentle heat
A Solid Stable Dissolves, no hydrolysis
B Solid Stable Partially hydrolysed
C Liquid StElble Not soluble, no hydrolysis
D Liquid Stable Completely hydrolysed
E Liquid Decomposes Completely hydrolysed
Select, from A to E, the set of properties which best fits each of thefollowing:
1. Phosphorus trichloride2. Tetrachloromethane3. Barium chloride4. Silicon tetrachlorides. Aluminium chloride.
In questions 6-8, one, or more than one, of the suggested responsesmay be correct. Answer as follows:A if only 1, 2 and 3 are correctB if only and 3 are correctC if only 2 and 4 are correctD if only 4 is correctE if some other response, or combination, is correct.
6. In which of the following compounds are the atoms predominantlycovalently bonded?1. Hydrogen iodide2. Calcium oxide3. Aluminium chloride4. Sodium hydride
( 1)
( 1)
( 1 )
( 1 )
( 1 )
( 1)
Consider the elements from sodium to chlorine in the third: periodof the Periodic Table. Which of the following changes occur inthis period with increasing atomic number?1. A change from ionic to covalent hydrides2. An increase in the acidity of the oxides3. A change from ionic to covalent chlorides4. A steady decrease in the first ionization energy of the elements (1)
7.
53

8. Elements having a hydride which reacts with water to give analkaline solution include:1. sodium2. carbon3. nitrogen4.' chlorine ( 1 )
In questions 9 - 14 each of the questions or incomplete statements isfollowed by five suggested answers. Suggest the best answer in each case.
9. The following diagram shows the first ionization energies ofeight consecutive elementsl I to VIllI in the Periodic T~ble.Their atomic numbers lie between 3 and 20.
II>C>iiic::Cll
c0
.~
.~c.2-~;;:
VII
IIIV
atomic number
Which of the following statements is INCORRECT?A II is an alkali metalB VI forms a liquid chlorideC VIII forms a hydride of general formula H2Xo III forms a basic oxideE V forms a chloride which reacts with water to give an acidic
solution. (1 )
54

10.
to fumecupboard
solidelement
drygaseous ----.-element
tHEATreceiver to
collect product
The above diagram shows the apparatus that a student proposed as beingsuitable for combining various solid elements with various gaseouselements and collecting the products.
Which of the following pairs of elements would be the MOST suitable foruse in this apparatus?A potassium and. oxygenB sulphur and oxygenC silicon and chlorine0 magnesium and chlorineE aluminium and chlorine (1 )
11 . 0.28 g of a basic oxide 1"10 was dissolved in 150 cm3 of 0.1 M HCl. ~The excess acid required 50 cm3 of 0.1 M NaOH for neutralization .•,'A "What is the relative atomic mass of the element 1"1? (0 = 16). '
A 12
B 24
C 28o 40E 56 ( 1 )
12. An element forms a hydride which may be hydrolysed to give ahydrated form of an acidic oxide. The element could be:A lithiumB calciumC nitrogenD siliconE iodine
55

13. In the graph below, the axes are not stated:
y
x
Which pair of labels for the axes would be correct?y X
A Melting-point of the hydrides N, P, As, SbB First ionization energies Li, Be, B, cc Radius of the atom F, [1, Br, I0 Boiling-points Ne, Ne, Ar, KrE First ionization energies Li, Na, K, Rb
14. The chloride of an element X is a fuming liquid of boiling-point 76 O[ at atmospheric pressure. Exactly 0.01 mol of thischloride was mixed with water and the resulting solutionrequired 100 cm3 of 0.3 M aqueous silver nitrate for completeprecipitation of the chloride. To which group of the PeriodicTable does the element X belong?A Group IVB Group V[ Group VIo Group VIIE Group VIII
56
( 1)
( 1)

15. The table gives some information about eight elements A to H,with consecutive atomic numbers; these letters are not the usualchemical symbols for the elements.
Table 19
Configuration First ioniza- Formula of Formula ofof outer tion energy typical typical
Element electrons /kJ mol -1 oxide chloride
A 3s23p1 580
B 790
C 1010
0 1000
E 1260
F 1520
G 420
H 3s23p64s2 590
(a) Fill in the blank spaces in a copy of the table, whereappropriate.
(b) Select from the elements A to H(6 )
(i) a noble gas,(ii) an alkaline earth metal,
(iii) a Group IV element.(c) Select from the elements A to H
( 3)
(i) one element which forms a basic oxide,(ii) one which has an acidic oxide,
(iii) one which might form an amphoteric oxide. (3 )
(a) Which element most readily forms a peroxide? Give theformula of the peroxide.
16. The second short period contains the elements Na, Mg, AI, Si, P,S, ci , Ar.
( 1 )
(b) Which element has an amphoteric hydroxide? Give two equations toillustrate your answer. (2)
(c) (i) Which element forms the most stable hydride containing thehydride ion?
(ii) Give the reason for your answer in (c) (i).(iii) Give the reaction of the hydride with water.
(d) Which element forms a weakly acidic hydride, and two oxidesgiving acids with water?
(4 )
( 1 )
57

(e) Li ) Which element has a slightly basic hydride and twoacidic oxides?
(ii) Give the formula of the hydride.(iii) Is the hydride more, or less, basic than ammonia?
(f) (i) Which element has the largest atomic radius?(ii) Which element has the smallest ionic radius?
(2)
(2)
17. (a) Arrange the ions Na+, Mg2+ and A13+ in order of increasingionic radius. (1)Which of the chlorides of the three elements in (a) is themost covalent? Explain your answer. (2)
(b)
(c) What species are present in solution when the chlorides of(i) sodium, (ii) magnesium and (iii) aluminium are added toseparate portions of water? (3)
(d) Briefly describe how anhydrous MgC12 can be obtained fromMgC12·6H20. (2)
(e) Give two examples of the similarity in the chemistry ofberyllium and elumdruurn. (2)
(f) Why are there similarities between beryllium and aluminium? (2)(Total 50 marks)
58

QJQJ Ul'-' -r!i:
.;..> QJ
'-'.;..> QJUl..c'-'I--r!4-
QJ';">..c C.;..> QJ
E'-' QJ0'--<4- QJ
Ul..c:::J.;..>
-r-i '-'D :::J '-'(1J 0 C'-' 4- QJ
EU QJ QJ-r!..c .--<C .;..> QJo-r!..c H
.;..> H
C -r! >-r-i 3:
0.QJ QJ :::Jen Ul 0(1J -r! '-'QJ '-' l:J
~ 0. QJQJ ,-,..cD ro.;..>
..cro Ul 0.;..>Ul ro-r! QJ
>'UlQJ .0 ro'-' QJQJ D '-'..c QJ U
.;..> 3: OJOD
D'--<o .--< >,-r-l 0 D'-' 4- roQJ QJ0. -.;..>
Ul Ul..c.;..>U C ro
N ro QJQJ E C
QJ QJ QJUl C.--<..c
-r-i H QJ';">U'-'~ -;;;
W
Ql..c >U Ol -r!m QJ >.;..>OJ bO -r-i ro
C Ul .;..> bOUl ro.;..> -e+ QJUl ..c C Ul Co U QJ 0'-' E 0. EU QJ QJ '-'ro s: rl E 0
I- QJ '-' 4-QJ 0Ul QJ 4- 0ro • .r: .;..>QJ Ul .;..> 0'-':::J .;..> UlU -r! QJ CQJ D '-' Ul 0D m QJ C '-'~'-'..cO';">
3: '-' UUl U .;..> QJ:::J -r-i .;..> U .--<-r! C C Ql QlD 0 -r!'--<ro -r! 0 QJ bO'-' 0. C
C bO-r!U -r-i QJ C C-r! ..c -r!-r!E Ul';"> Ul roo D 0 bO.;..>C.;..>.--<ro Ol ro
'-' UlC 4-) (f) tn r-I-r! '-'.--< roeo :::J ro .;..>D C U.;..> QlC -r! U QJ EQl Ul 0 E ,'-'0 C.;..> 0. '-' .--< 0
0. Ql (IJ COl o..c U.--< .;..> -r!'--<bOOOo.roC3:C>,U-r!';"> ro';">-r!Ul 0.
Ql 0 I)(] >,(IJ '-' .;..> C +-'
ro -r!Ul D Ql I)(]-r! Ql CD C
'-' QJ -r-iQl Ql '-' E Ql,-,..c.;..> 0.0Ql.;..> '-'
..c Ql 4- 0
.;..>~C .;..>D 0 Ql
Ql 0 bO.--< -r! E C Ul Ul-e+ '-' 0 (IJ C Cs: Ql '-' s: 0 03: 0. 4- U -r! -r!
.0
~-r!Ul
DC(IJ
I'~U
s,o
~QJ'-':::J0.
QJ
'-'(IJ
i:U-r!..c3:
UlCo-r!~ ,O~3: -r!+-'Ul
Ql '-'..c 0I- ~
QJ'-'roU-r! UlC Co 0-r! '-' -r-t
Ql C'.0 (IJ
ED:::J QlC C i:Ql .;..>
'-'U.;..> -r! .;..>E :::J
'-' 0 .0(IJ';">
r-I iT)-r!E bO-r! C ..cUl -r-i +-'
Ul -r-l(IJ (IJ 3:
QJ3: '-'o U.r: CUl -r!
QlI
o..cD+-'
-r-lH 3:> 0.D :::JC 0(IJ '-'
bO
U U-r! -r-iC Co 0'-' '-'.;..> .;..>U UQJ Ql
.--< .--<QJ Qlo 0Ul Ul-r! '-r!
Ql '-'QlZQle!:'-' '-'(IJ.r: (IJ..c
.;..> +-'Ul -e+ Ul-r!C 3: C 3:o 0-r! U -r! U+-' -r! +-'-r!(IJ C (IJ CU 0 U 0'-' '-'N+-'[<)';">
U UD Ql D Ql0'--< 0'--<-r! Ql -r-i Ql'-'0 '-'0Ql Ul Ol UlQ.. -r! Q..-r!
Ol'-'(IJ
UlCo-r!C(IJ
Ols:+-'.;..>:::J.0
QlZ
s:.;..>-r!3:
Qls:+-'4-oUl
.>C.(IJQl0.
Qls:+-'~
Ul+-' Ql(IJ Ul
(IJD bOC:::J QJ0'--<4-.0
oQl C'-'ro Ql
s:Ul+-'CQl bObOCo -r-irlD(I) :::J
[<) ..c rlU
QJ QJ XUl s: QJ-r! I- ~U'-'~ -;;;
W
s:U(IJ
W
s:0.ro
'-'en>,bOs,QJCQJ
Co-r!+-'(IJN
-r-iCo-r!
..c0.ro
'-'bODo
. Ul-r!:::J '-'-r! QlDO.(IJ'-' Ul.;..>U -r!
-r+E Co -r!
+-'(IJ Ul
+-'Ql C
..c Ol+-' E
QJ4-'--<o QJ
s:U-r! C..c -r!3:
Ul >,:::J QJ - bO
D -r!..c C '-'CD';">OQlro ro '-' C
'-' >, +-' OJUl .0 U:::J'--< Ol C-r!'--< D'--< 0D (IJ Ql QJ -r!roE.;..> +-''-' Ul U C ro
(IJ ro NU Ol '-' -r!-r!..c.;..> QJ CE.;..>+,> 0o (IJ 0 -r!+' Ul Em C >, Ql +-'
Ql'--< '-' UlCbObO '-'Ql 0 C 0 -r!QlrlO+'4-3: ro '-'+'..c+-'D+-'QJ Ul Ql Ul.0 Ql D Ql
..c Ql QJ..c Ul0. +-' '-' QJ eo Ql-r! ro C -r! Uls: C s: roUl -r! Ul Ql bOC C '-'Olo - 0 o..c QJ-r!Ql'-'4-+,rl+-''--<+'Ql .0ro 0. U '-' Ul 0.--< E Ql Ql (IJ CQlro.--<..c..c'-' X Ol +' QlQJ c..cQl '-'UlOl+,Ul '-' Ql -r! bO'-'o+' OEOlLL:::J>''--<O> 0 en (IJ '-'C '-' s: 4-
-r-i Ql Ql>,..c c..c+'
C en +-' QJ (J '-'(IJ '-' (IJ :u
Ql +' Ol Ol 0.Ul C !1J '-' (IJ+'Ql..cO>'Ul +-,:E..cQl C 3: DbO 0 Ul 0bO -r-i Ql Ul -r!:::J+'+'UlC'-'Ul !1J"(IJ :::J -r! Ql
N U QJ !1J 0.Ul -r!-r!.--<.--<-r!CDUo.Ul..cOC:::JX+,I- -r! -r-i C Ql -r!
srQJUl-r!U'-'OlX
W
'"00"
oS2
o o52
ooN,
ooc;'oo1
> s:U
H !1JH OlH
CUl 3:0. 0:::JDo'-' Qll:J Ul
!1J, Ql
'-'Ul UQJ C>- -r!
Ul QJ..c.r:bO+-':::J04-
'-'0+'Ul
QJ E..c 0+-'+-'~
([J Ul+-' Qlm +' Ul
Ul (IJD Ql eoC'--<:::J'--< Qlo !1J .--<4-ED
Ul 0CQl
'-' Ol!1J..c Ql+'.r:
Ul .;..>C UlQJ (IJ eoen r; Co -r-i'--<CD(IJ QJ :::J..c bO'--<
o UQl .--< X
..c (IJ QlI- .r: ~
.0
Ql '-'ui Ol ..c Ul -r!
QJ 0 ,> Ul I- -r! C QJ-r-i -r! -r!.r: D Ol s: +' +' +'D C O+' s: Ol +-' s: c.r: +-' Ql C 0 ~
Ol •..(IJ +' -r! Ul+' Ul r-. >,u Ol co+-, s: r-. ~ +' 0'-' co
en :::J o.(IJ QJ Ul >, bO rl C -r! ~..c c+' QJ QJ 0.co 0 DD 0 -r! 4- :::J QJ DD Ol C QJ QJ co.r: COl- M4- Ul -r! en co -r! s: 0aJ ..c C QJ 0. D 0 0 '-' E ~.r: -r! ..c s: 3: QJ C 0 !1J E 0 > -r! +-' '-'Ul (J (lJD ;, co '-' U :::J co+' Ul+' C Ul C .--< -n QJ 0'--< 0 D..c 0.'-' C (IJ D !1J '-' +-' btC C 3: QJ !1J co 3: - U ·D '-' - r... r... U U !1J+' EOl 0 Ol Ul ro r...rl r... -e+ OD '-' OlD ..c >,0 en :::J Ulri Ol en CO 4- '-' -r! 0 Ol0. -r!
:::J +-' 0. (J !1J QJ U+-' C 0 r... C +-' bO D ri C :::J OlD C en +-' 3: r...D(IJ+'
- Ol Ul X r... or! o...c D -r! III E U CO ~ ri Ol -r! E 0 Ol Ul -r! Ul C 4-0.(IJ +-' ri C QJ (IJ E +' Ol E Ol C Ul Ol Ul QJDri..c :::J r... Ul C QJ -r! Ol DC s: U 0 s: 0 r... .>C. 0 Ul r... -r! :::J C :::J..c Ql U Ul C+-' QJ Ori ri Ul Ulr-iC -r-i bO:::J r... Ol Ul +-' Ol4- r... +-' (lJr-i Ol D -r-i Ol -r! en en :J U.r: '-'ri ri D III Ql :::J0 E -r! C+-'..c (IJ +-' 0 (IJ !1J Qlri..c Ql QlD D D !1J C >,D Ql+-'+-' Ql !1J C Ql E 0-r-i (IJ ~ U+-' Ul !1J E ~ Ol+-'..c+-' Ol !1J '-' CO C QJ .0 Cri U.r: E Ql r... 0 3:+-' X ~ Ul QJ Ol C Ql Ul bOU..c !1J1- !1J+-' r-. Ql r... 0 r... QJ !1JQJ 4- Ql Ul Ul ~ U UCOW O+-'ri E Ul -r! '-'+'+-' C C en r... en r... s: r... U..cD Ori +' C .r:C
Ql +' -r! QJ 0 III bOC Ul -r!-r! .0 C Ol U bOU+, C+-' Ql Ql III Ql r... Ql -r! en U-r! D ~ QJ Ql QJ 0 Ul :::J Ul Ql > -r! -r!-r! U -r! U '-' r...O , Ql i: ~ -r!-r!E..cC +-' C QJ4- '-' Ul Ql E E !1Jri Ul :JD 0. III E..c E QJ 0 :J :J+-'..- I:I..+-' +' (lJD..c.r: --!1J+-' (IJ 4- -r-i ..c U Ill..c Ol QJ (IJ , Olri E 0 Or-i Ol+-'D+-' X <:r- :J ri r-i +-' 3: QJ
X >, Ol I- >,Ol Ol.;..>ri QJ en r... :::J '"tl ri :::J 0 C +-' Ill+' QJ.r: QJ III QlD 0 QJ -r! :::J '-'Ol .0 N ri C riD ~ QJ ..c +' U+-' U 0 U QJ III !1J +-' '-' r... C C QJ > E 0 r... - (IJ0 .:L U +' +' C C U '-' :::J 3: ..c C r-i OJ CO CO.r: OJ III -r! 3: 0 en
E Ul E+-' CO UJ C ::JD Q) -r! CO Q) C Q)+-' •.•• -r! C CO • en Q)..- +-' .c .c UJ 4- Q) OJO+-' D 0 Or-i QJ ::J -r! .0 r...+-, E C Ul r... Ol -r! C >,0 Ol..c +-' -r! Ul Ul•.. 0 0 •.. r...ri 3: -rl -r! :::J Ol .r: C C Ol C OD +' OJ Oriri Ul+' OJ ED 0 Ill-r! Ol III OJ4- C -r! 4- o.Ol D U • .r: .0 rl +-' !1J -r!..c 04- C CO •.•• D -r! D U •... •... ::Jrl+-' +-' D U bO..c
UlU '-' ..c >,!1J Ol Ul+' QJ -r! +' r... QJ III Ol III C+-' C :::J C !1J -r! Ol III 3: CO C I-Ol Ulr-i enr-i r... .:L+' 3: Ul C +' r... r... QJ -r! 0 en 0 0 C -r! .r: U 0 ~ Ol OlC C Q.. D CO C •... CDHD -r! !1J E U OlU bOUl r...D Url U Ol -r!..c +-' QJ ..c Uri
0 !1J 0 C';"> 0 U III Ol C> C ul 0 Ol..c Ol C+'D OJri en '-' E ul +' ul Ol -r! .0-r! ..c -r! 0 Ul -r! E E CO 0 ::J Q) 0 •.•• rl +-' C !1J 0 10 Ul Q)4-D QJ ::J 0 U .c > o -r!+-'U +-' r... -r! 0 r... E QJ 0 U -r! '-'+-'4- QJ Ol r... Ol ..c 0 c..cri >,UlU QJ O+-'W C -r!Ule!: 0 QJ+-' E QJ 0 Illrl+-'+' QJ U QJ en ul Ol+-'..c4- • ul Ol+' COr-i Ol 0. +-' DQJQ.. .0 0. -r! r....r:+' QJ Ul ul co..cU ul rl -r! en > u+' OU - 0. rl QJ+-' X QJ III:::J..J D Ol+-' CO ul •.•• H r...+, Ol Cri Ol III Ol C Ol ul Ol -D III bOU Ol D • ..c •...crH Ul QJD';"> 0 -r! +-' -r! H QJ D 0 QJ enri ..c rl QJ C co..c -r! D Ol C -r! •... Ol QJ Ul ~+-'
0 >, Ul en III ::J C Ol Ul4-H.r: CDD r....r: :::J OJ U 0 +' en CO+-' CO 0. QJ +-' o.Ol +'0 QJ 0 co..c QJ CO +-' -r! C CO+' ul QJ en 0 C -r! en Ol C !1J r.....c X .0 U :::JD4- C+-,.0 r... ..c C +-' r...rl QJ ul CO U I .--< -r! +' •... Ol+-' :::J 3: bOO U "C 10 U QJ Ol 0 E 0 QJ
enDU +' !1J en Ol QJ ..c o.D Ol tlOQJ r... U Ol :::J !1J -r! !1J r... -r! 0 0. DD 0. •.•• :::J r-iCO +' C 0 .r: QJ+' :::J C ul - C .--< Ol :::J en D+'ri o.D r... CO+' en r... r... Ol r-i 0 X tlO C -r! IIIr... QJ 4- en -r! 0 Ul +' ..c 0 (IJ III en -r! QJ+' C ::J Ol ::J4- ::J !1JD..c U -r! 0 >'!1JD ::J -r! QJ -r! >QJD en 0 C +' C r... QJ Ol Ol :J -r! +-' 0 C U r... U ::J.r:Dri Ol 0 •... r... UU 03: -r! Ol -r! CD H Oll:J+-' r... -r! .0 ri 0 OlD U -rl U 0 D+' Cri+' 3: OJ Ol Ol -r! (IJ U
Ul > Ul ..c (IJ -r! rl ul H D QJ Ul U r... ri ..c (IJ QJ Ul 0 QJ+' r... QJ C 0 u..c 0. .0 ..c E r...C 0 ro U bO QJ :::J > C 3: '-' r... OJ Ol Ol Ol 3+-' r... o.+' en ..c III r... C Ol :J bO ul +' 0 '-'~ '-' QJ (IJ C -r! Ol (IJ+' o -r! D Ul r....r: Ol X -r! r... Ol+-' til Ol H Ol >, C -r! (IJ ul D O+-' til -r!0. r... QJ+-' o..crl D QJ4-4- (IJ til C4- U QJ o.r: Uri til 3: QJ E bO+-' r-i !1Jrl Ol
U C '-' ul U C+'D..c C o -r! Ol+-'+' Ol C U -r! +'..cD -r! ::J C (IJ.r:
Ol til Ol+-' ::J (IJ til r... Ol U 0 til •... C E QJ ul U til Ol ::J..c OlI- Ol C Ol o -r! bO!1J+-'U til E u.--< C r... til (IJ..c ::J -r! C QJ (IJ QJ 0 .0 ::J (IJ • III +-' c+' enD til (IJ ri C 3: C3:
C/) 0 QJ QJri H -r! '-'ri+-' E+-' o+-'.r: bO+-' III 4- >'Ol C !1J..c .0. -r! ul -r! 0til r...riri QJ Ol H4- ::J -r! -r! r... ::J+-' '-' (IJ +' U ri •.•• ..c Ol4- 00 QJ+-' 0 O+-' ul r...+-,::J U QJ QJ..c..c U E C +' til+' 0 (IJ ..c Ol bO+' U u..c 0 '-'..-r-i r... C ul C (IJ QJc:: -r! !1J ul+-' - Ol U -r!" QJ 0 C U >,..c C bOD C til C -r! +-' .;..> rl U til ::J o.Ol QJDrlU QJri H ..c 0 Ul QJ C ro QJ Olri U -r! -r! -r! r... -r!.r: +-' U 0 Ol C ::J .r: -r! ::J E '-' (IJ
Wro bO> (IJ '-' C +-' 3: '-'ri..c QJ E E Ul or! 34- C QJ +-'..c-r! QJ UD 0 Ol (J C C'-' Cor! C QJ 0 O. QJ ul+-' Ul+-'W+' > Ol Ol ::J 04- 0 0 Olri en ri (IJ (IJ r...ri C !1J 0
-r! ul O+-' ::J C Ul -r! Ol or! -r! > Ul E -r! 0. +' +-' OlD I >,U W '-' l:J Ol -r! > -r!
~U > en -r! ::J QJ 0 QJ (IJ .0 r... D +' -r! (IJ ::J C 0. •• OJ+' X OlDri :J-r! 0 Ol+-' 0 bO r... QJ QJD QJ QJ • ri U+-' QJ -r! -r! 0 o.ul bOU QJ r... til ::J+-' CE E U -r! r... l:J 3: r... c+-' r.....c til QJ QJ -r! r... ri E ::JD r... Ol QJ 0 ul (IJ
Ol 0 UD QJ (IJ +' U OJ (IJ Ol +' D -r! 4- ul U ri :J a a C III 4- Ol C 0. I Ol Ol -r! -r!
C/) til +-' C ::JD.c..c C Ol C '-'.c ..c or! c.c4- 0 QJ IOri 3 r... Q)..c4-.c C 0.'"13 r....c -r!-r! e!: H til ro+, U H .0 or! +-' +' I- Ol Ol ul Ol o.D l:J Illl- bO+' U OJ +' -r! 0[<) bll+'
Z U'-'Ol '7iJ 'ZJ« X .0 ::; ~ 4-W
59
EC
"'-Ul::J'r!D!1Jr...
Ulrlroro3:
r...Ol
0U N I'-. I'-.to m m ~ IC ..- ..- ..-
!1J c:i c:i c:i c:i>
ulrobO
~D0 Ol '-' '-' Ol CZ Z e!: ~ X 0::


en0 IlJ h
.j-J0 C
OJ OJ en IlJ
IE :>, CL en OJ L:0 tllj OJ OJ IlJ E o OJ .j-J
0 0 0 0 0 0U h U rl L: 0 h L: Z Z Z Z Z zh OJ IlJ CL en +-, 0 .j-J :>,OJ OJ C rl E I1J '+- b.(J
Ii> > OJ CL ·rl rl C h
+' C Ol0 -r-i en IlJ OJ OJ OJ s: IT 0 :JblJ h OJ h L: > 0 C DD OJ 0+' <-<OJ OJ s: 0 OJ .j-J ·rl -r-l OJ
~0 u 0.0 0 .c IlJ .j-J C .j-J .j-J
on cU U
OJ on U.j-J .j-J .j-J OJ U <U OJU
0 -r< C :J 0 OJ DD0 h h b.(J 0 I1J N h z > -r< tn I I Z <-< > L.j-J .j-J 0 .j-J IlJ .0 h -r-l 0rl '+- C rl OJ -i--l h E
'0 Cen OJ 'rl 0 E D +' 0C 0 S :JIlJ E OJ 0 U I1J OJ I1J CL en OJ 0s: rl CL OJ en +' I1J OJ +' +' c.,
~ ~Nrl ~~ U U 0en +' I rl CLD > h
~-rlU Ul <1J <1J DD UlD rl +' b.(J 0 OJ 0 C 'rl
Ul0 U N
I0 OJ 0 OJ onC IlJ -r-l C E L: h ro c 0 Ul UlUl '> tn z <-< z <-< > LIlJ .j-J rl ·rl .j-J en rl 0-
OJ rl +' 'rl OJ .>, tn '0 '0 C COJ E D ·rl C OJ D D h <-< :J C C 0 0 0 :J+' I OJ 0 ro > -r-t
OJ 0 f1J m .r< .r< -r< 0ro c OJ LJ ·rl ro Ul :::J OJ en > <-< .r.>U U
<-<n,
.j-J 0 C b.(J .c -r-i o-+' 'rl 0 '" ~~
m ~ U 0on 00 OJ m <1J eoUl D ·rl ro .c
A o, "-"- uu 0 OJ 0 OJ 0 OJ DD.r: c E D OJ rl +' .j-J > 0.."- Z '-- Z <-< Z '-- > LD +' U IlJ 0 rl h en'rl en 'rl h :::J 0 OJ D tn0 0 L: .j-J '+- 0 .j-J .c en c
UlC C C0- E :s c D- :s u +' :::J IlJ 0 0 0'C' -r-l en 0 0 :J+'
.r<0rl
h'en 0 D OJ C h C OJ D <-<
A 0 ~ UU U U
~UlOJ CL C b.(J OJ 0 .j-J 'rl en OJ 0
.(Jjro
0m
0OJ
onDD 0 OJ OJ OJOJ OJ rl I ro c en -r-i Ul IlJ +' 'r< Ul 01 Ul Z <-< z <-< z <-< L.r: > 0 b.(J ro IlJ -i--l h b.(J ro >+' OJ U C 'rl i: U IlJ D OJ h:s OJ 'rl (f) U N -r-i L: OJ IlJ tn +' tn C C tn OJ cC 0 rl +' .c -r-t rl .j-J .c CL :J C0
0 0 :J '0 0·rl :r:: 0 rl C 0 U h 0 OJ +' OJ 0 OJ+'
.r{ o ·ri .r<
-~ ~ .~2; ; ~ +'
IE OJ OJ +' IlJ 0 en b.(J en 0 u u uen E OJ OJ D_ o C U
0OJ
0<1J
'~4-- ~U
0m.j-J h 3: D IlJ OJ .j-J -r-i :>, ;;: OJ OJ CDUJ OJ 0 C +' C C > .c rl
C> <1J « > -c z <-< z r,
>'rl rl « Z <-<'rl N -r-l OJ 0 ·rl +' +' OJ C OJ c-, OJ
!iUJ -r-i UJ :0 CL '+- 0 U) IlJ .j-J <-< >
Ul Ul Ol tnh h E UJ UJ UJ 0 rl 0 .c OJ OJ> oo :J :J :J OJOJ 0 0 OJ D OJ Q) CL ';;D rl +' rl
'+- 0 0 0I
c, 0CL CL +' b.(J C h h :>, U D. <-< <-< <-< :J <-<+' 0
~.~0 0
~o OJ », 0ro I1J C IlJ h :::J CL+' C h E C 0 DD U on :r:: ~ u <-< DD Ub.(J > I1J 0 .j-J rl C ·rl C OJ 0 OJ '0 t! DD DD DIJ DD C rn CD -r< DDC OJ .c z o U IlJ -r-l +' -r-t b.(J U C > > L > L > L L 0 '+- > > L'rl 0 OJ U 0 i: 0 rl IlJ C 0 :JE 0D +' h D UJ h +' CL OJ E 0 OJ > Q
tn CC '+- OJ .j-J C OJ -i--l C I E OJ h h
Btn
:J :J:J :J 00 rl .c UJ I1J b.(J UJ OJ b.(J h +' IlJ0 0 o ' 0.0 IlJ 2;'-i--l OJ C C UJ C <-< <-< <-< <-< OJ +'+' CLU IlJ h '+- ·rl C UJ en 0 i-~ 0 >, 0
U>, 0
I>, 0 I
0 UUU OJ OJ OJ h .c ro 0 rl 0 OJ .c OJ ~ <-< biJ <-< DD '-- DD 0
0 '"+' m OJ·r< '" OJ-r< m CD·r< m m CD·rl E > N ro C U rl 'rl ·rl rl U rl U +' Z > > Z > .> z > .> Z > Z Z <-<rl -r-i 'rl .c OJ 0 +' 0 UJ 0 0 U C <1J Uco rl ro -i--l r.,UJ OJ OJ U 0 .0 0 o E ·rl 0 m .0 OJ :J
<-< '0m
IlJ ro 0 :s en OJ rl '+- OJ +' -r-i 0 « m « ro « « rn .s. rn « mOJ +' h rl 0 OJ .j-J OJ rl CL OJ rl OJ h UJ OJ '+- <-<Ul OJ 0 OJ ro s: OJ L: 0 i: C 0 h ro 0 Ul D 0 Q
-r-t ~ '+- h > I- .0 l- E <r: +' H E IlJ CL'+- -r-i COJ OJ C N IN '0U U ro .r< 0 U Ch h rl '" <1J +' +' '0 '0 ru +'OJ OJ C;U
.0 .u L
~~~ >''0 <1J >''0 m rl 00 U
<1Jro II II r., c; OJ <-< C CD o N DDOJX IlJ .0 U D OJ X Omr: o m.c UI U I LL:W W I- « rn
Exercise 7 (continued).ra) Table 5_
Element Li thium Beryllium Boron Carbon [graphite) Nitrogen Oxygen Fluorine Neon
Bonding Metallic Metallic Covalent Covalent Covalent Covalent Covalent --
Structure~r--<ro=-M=>m / ~1--0-;::j --0--, I~ 0--0 0-0 0---0 0
~ tl~y .~,
.nIJ 0-v- ~
.lj.J
Type 6.C.C. H.C.P. Giant Giant molecular Simple Simple Simple Atomicmolecular molecular molecular molecularElement Sodium Magnesium Aluminium Silicon Phosphorus Sulphur Chlorine Argon[white)
Bonding Metallic Metallic Metallic Covalent Covalent Covalent Covalent --
Structure Q
m , I
~ ~0--0 0~
~. - r,mE e-,
~"nIJ
5::::::0
F. C. C. Giant molecular Simple Simple Simple AtomicType B.C.C. H.C.P. (C. C. P.) molecular molecular molecular
61

Table BGeneralformula
Xa07 3.5
XO. 3.0
XaO• 2.5
XOa 2.0
XaO• 1.5
XO 1.0
XaO 0.5
Teb l e- 9General formulaand ratio CI/X
XCl.
XCI_
XCI.
xcr,
XCI
Table 10
Formulae of chlorides in Groups of Period 3Formulae of chlorides in Groups of Period
General formula ~ __ -.r-__~ -. -. -. -. ~ .- ~ ~ r- r- r-__~
and ratio H/X
XH.
XH'."-:.:~tjt~i.
.... :-:.:.;. .
Formulae of hydrides in Groups of Period 2 Formulae of hydrides in Groups of Period 3
t This column shows the number of atoms of ° per atom of X •
• In some contexts the dime ric formulae are more appropriate.
~ QJ ~ ~ rl UJ CQJ 0 QJ D 0 mC > IU +"' ~ QJ C -r-l rl » ~ m·rl ·rl 4- C QJ ....,
E UJ » s: s: ::JD E fll .r::.D QJ rl ::J bO C ~~+"' s, ~ fll C rl ...., +J bOO 0 +J c.r::. +J 0 fll 0 0 ::J UJ
0 0 ::J QJ IU rl UJ 0 en -r-i D C 4- -r-i ::J~ QJ -r-i fll+J s: 0 0 ~ ·rl .r::. mrl fll UJ en ·rl D C 3 0 .r::. 'rl ~ ·rl D.r::. 3 QJ 0 QJ 0 UJD rn +J o.D
.r::. QJ 0 c.r::. QJ C ~ 0 'rl .r::. +J N QJ m D QJ+J C • .r::. UJ m s, 'rl »c.r::. fll rl -r-i
0 ~rl ~ QJ+J.r::. fll m ~ » ~+J ~ ·rl 'rl ~o... 0 > D 0 C+J QJ QJ -r-i rl £ -r-i ~ E ::J X
'rl D bO·rl ~.r::. 0 +J QJ ~+J f)J -r+ 0 3 D fll Ul 0 C C 0 QJ 0 D m UJ ~ UJ 0
~ UJ +"' »0 3 0 0 UJ£ rn -r-i 0.3 -r-i QJ E ·rl ::J 'rl s: 0 C C +J D ~ E 0
UJ c.r::. ~ m ~ +J 3 > .D X UJ£ QJ Orl+J+J QJ -r-i bOrn C 0 ::J .4- C
QJ QJ ::J D UJ C ED £ o 'rl rn C UJ C O+J+J .: ~ UJ 0. 'rl ~ C E ::J EH UJ ·rl
tl() rl ~ .r::. »::J » +J 3 0.+J s: 0 :::J ::J C +J E UJ E QJ +J'rl I 0 QJ 'rl H D ::J
» +J£ ~ QJrlI 'M 0 0 +"' 'M ~ 0 Q) QJ4- QJ C C 0 QJ ::J 4- C+' C o.C x> C ~ UJ
X Q) C 'M 0 o.lJlU~ ~rl bOlU +J 04- > E 0 +J QJ QJ o.r::. o -r-l 't- QJ 0 0 E 'M m ::J 0 m
0 ~ QJ 3 D £ 0 0 rl C QJ UJ U£ m QJ I1j Q) E +"' C C 'M E IU C 0.... E O. 0 £ +'
IU en C 0. ~ 'M '""" » IU 'rl ~ UJ IU o.D .r::. rl QJ 3D QJ fll -r-t QJ -r-l D Ql Ql 0 0 ::J o.o.fll
£ » +J fll UJDDD rl 0 0 m UJ C QJD rl 3D E rl ~ 0 rl IU 0 E LD+'
+J UJ X 0 0 »(1J UJD ::J ~ ~ ~ 0 QJ £ -e+ D UJ QJ E 'M ::J .r::. QJ +J 4-.r::. ~ 0 0 UJ
'M +"' 0 C QJ£.r::. ~+"' ::J CD 'M U .c ~ U£ LD C (1J ~ UJ >rl ea +J 0 0 tl()l9 o.r::.
3 C C 0. 'M 0 (1J QJ QJ (1J 3 0.+J 'M U (1J£ bOO ~ QJ (1J ::J o.Orl C o.C
QJ £ +"' 'M s: QJ C ~ ,~ £ 0 .r::. ::JD C4- ::J 0 0 C (1J 0. • 'rl C 0 0
D E+J ::J ~4-+J QJ::J 0 UJ +J UJrl.r::. m 3Ul C +J ~ 'M o't-r-! .r::.+J +J ::J C £ ·rl 'M » 'M
QJ Q)'M D 0 o -r-i ~ bO C - Q) UJ+J£ (1J rl Q) C 0 0 0 (1J +J ai QJ 0 Q) 0 't-D+J
...., rl 3 rl 34- QJ ·rl 0 UJ UJ UJ ·rl +J UJ .r::. (1J »'rl +J 0 +J rl Q) E ~ bOlU E ·rl IU
(1J Q) • .r::. UJ QJ > ~+J Q) (1J (1J 3 +J UJ (1J (1J UJ Q) (1J£rl tl()>>QJ ::Jrl(y)D
QJ o~ U C QJ (1J UJ +J C 0 Q) 4- C UJ +' rl ED C Q) E +J o.E x ~ E 0 ·rl
.c ~ UJ C 0 C +J 0 Q)D ~ ·D 0 Q) C£ (1J Q) Q) 0 U E 0 s: 0 -r-i ~D X
QJrl 0 "rl -r--i » ~ UJ QJ E U UJ QJ E 0 eo m ~ +J ·rl rnH N4- (1J ~ U C 0.0 0
c.r::. (1J -r-i C+J ~D Orl Q) 0 Crl+J Q) QJ ~ ·rl ED U +' ·rl H 0 X4- (1J 4- +' -r-i -r-i
rn+J +J rn U 0 0 E rnrl UJ 'rl (1J (1J+J rl +J ~ ~ -r-i m m 0 0. H D rn rn 0 C E rn ~ ~s: OD U tl()(1Jrl UJ ·rl rn +J rn ·rl C Q) U 0 X£ 0.(1J 0 ~ Q) Q) s: Q) Q)
3 C (1J »rn£D+J +J 0. ~ E QJ.r::. UJ rn rn rn 't- O+J X QJ 0 0. -r-i ::J ~ en »UJ E (1J+J0....r::.
m (1J rn x ~ 0 Q) U 0 ::J UJ rn ::J E 0 eo £rl£ rn ~ 3 ::J ~ 0 0 C D E rn tl()
».r::. ~ 0 rn (1J (1J eCD -r-i C 0.0 +J QJ+J 0 UJD +J 0 QJ 'rl 4- (1J Orl.r::. » C ·rl
rl~ Q) Q)D U Q) rn rn E UJ rn rn o.~ ·rl +J"'" C Ol UJ ~ 0... > s: UJ +, QJ o D 'rl .c
UJ C ~£.r::. C 0 ~ ~ > ::J Q).r::..r::.OD 3+J LD -r-i (1J .0 -r-i Q)l9 (1J ~ U LD IU IU
::J -r-i Q)+J+J IU ~ ·rl C C+J 3 » o.D D £ UJ c..c QJ Q) rnNDrl
0 ui ~ ..c -e-i o.'t- rl tl()UJ en Ol.£: »Ol ~ ·rl D CD~ Q) Q) 'rl Q) ·rl 4- rl't-£ ~ C (1J
~ o+", 3 E Q) 0 rl C U 1U4- C£ rl U (1J 0 Q) Orl .r::. s: D 0 O+J D (1J C
ODrl ~ 0 CD (1J 0 o ·rl E 0 O+JD m 0 3 (1JD U ::J +J +J ~ rl N ~ 0 0 0
tl()'rl £ ::J UJ ~ ·rl C rn +J 'rl E C -r+ C > (1J 0 ·rl -r-i 0 E ::JrleC+J 0 ~ 4- +J ·rl C 'rl
·rl rl U 't- C4- ~ (1J 0. C 0 £ »+J UJ (1J -r-i +J QJ >rl 3 E E 0 (1J CD m 0 ~ rn+J
> 0 0 0 » QJ D I +J Q) +"' C U ·rl +J 0 +J o -r-i ::J 0 0 0 U GJ ·rl 0 rnD UJ Ol tu;'rl
UJ£ UJ -r-i +J rl +J +J tl()cI (1J eo 'rl (1J (1J m O+J ::J ~ UJ rl ·rl ~ ~ o ·rl rl o ·rl ~ F- E QJo... »D
+J +J +J +J ~.r::...c 0 m 0 3 Ol UJ C Ol Ol rl 0. (1J UJlL.4- C o.D o.~ 0 ::J 0 UJ XD
0 o -r-i C U IU 0 tl()UJ ~+JD tl()~ £ ~ ·rl -r-l 4-D 'M~ QJ+J m 0 »»E Ol E C+J (1J C 0 (1J
(1J ·rl 3 m m 0. ·rl -r-i D C CD »+J s: ~ 4- C -r-i D rl m C tl()U rl +J Ol Olo... (1J QJ ·rl
rn C > Ol (1J£rl£ ».r::. IU ·rl » +J 'M UJ~ 0 Ol Ol E -r+ E en C -r-l rl (1J UJ.r::. C Ol ~ D 3
~ 0 UJ Ol ~ +J C+J i: 0 UJ£ -r-i 3 ::J rl +J ~ s: ::J.r::. I (1J +' ·rl rl (1J QJ+' C 3£ QJ UD C 0
·rl +"' ~ .·rl ::J 'rl I IU > 0 s: +' +"' 'rl 3 C E 'rl UJ ·rl ::J Ol ~ -r-i a+' C CJ Ol (1J.r::.
UJ 0 o.tIl+"' 3 UJ4- D.r::.rl OlD ·rl C ~ 0 UJ£D 'rl C a try UJ UJ£ UJ £ aD+"' UJ
+"' E (1J D C a C 3U ~ C +' a a Ol a 0 ~ 3 -r-i ~ C OlD (1J ::J+", a :J+", UJ C IU C
C ~ Ol ".. ~ Ol UJ C (1J 0 (1J U ·rl tl()C E 'M (1J E .x: C o.rl +'£4- 'rl £ C U Ol.r::.
Ol a ~D (1J +J ::J ·rl UJ UJ C (1J +J ·rl·rl C s: 3 +J ::J o eC -r-i 0 (1J UJ4- ~ rn C +J QJ 'rl Cl'0 ~E4- "13 UJ ~ rl QJ +J I ·rl rn Ol 0 > ~ Ol UJ 3 C o rl ·rl rl »C+J c a UJ rl ~ QJ 'rl £ rl o 'rl C')
Ol Erl C ·rl a UJ -r-i C CN C ~ (1J a eo +"' a (1J (1J+J QJ a rn 0 D QJ UJ 3+J o.~£ CDrl a ::JeC a X£ ~ IU 'M Ol 0 s: 'M Ol IUrl » U"O Ol 0 • rl D E ·rl Ol C Ol +' IU E+' 3:
Ol +J·rl Ol o.::J+", ~ E +J ~ - ~ s: x (1J C> ~D (1J+", (1J UJ I +"' UJ Ol c.r::. +J Ol UJ+", a ·rl Ol
sc C a c I UJ 0 Ol a Ol 4- 'rl a UJ D 0 0 Ol (1JH C Ol o+"' UJ ~ C 0 ::J+J Ol+J (1J ~ QJH 0 C rn eoC'rl +J 0 C 0 OD rl rl 0 3rl +J 0 C ~ UJ (1J ~ C Ol+", ::J a (1J (1J bO D. U C C LD IU
0 QJ E U a.r::. a £ OJ £ C C (1J ~ UJ UJ 0. rl E C 0 C rn 0 QJ 0 0 -:;:; C 'rl UJ .·rl QJ 0.
0 tl()::J C ·rl C 0. QJ 0 C UJ U m a rl E Ol :J IU E LD a QJ U .... QJCO ....+J £ U 'M .0 • 'M C ~ "0
rl Orl Orl C ~ tl()a QJ E UJ ~ (1J ::J ·rl a +J ::J'rl D QJ E 0> D D tl() ·rl E> 0 o ·rl C
.0 ~ (1J'rl 'rl ~ a a C 'M UJ ~ QJ 'rl ~+' 'rl +J ~ QJ'rl .r::. > QJ H ~ »~ 'M UJ ~ aH CDrlrl 0
0 I D +"' LD a C 'M E C 'M +' IU rl a :J QJ LD ~l9 E LD+'"O'Mrl QJ ~ QJ .£ QJ '""" .... IU IU U ~ ~£ IU
'""" co » (1J QJ+J QJ 0 (1J QJ QJ QJ QJ o.r::. E QJ QJ QJ QJ O+' QJ tl()o. tl()o. £ or Drl o.QJ (1J o.r::. D
.r::. UJD E 3 bOU ~ tl()::J E ~ tl() ~ 'M 0. I C o. E rl »C 3 ·rl 0 C :J 'M ~ ::J.r::.+"' QJ :J+' :J+' U Ol
Ol QJ ::J 'M 0 0 »(1J a »D ~ 0 » QJ QJrlrl C tl()0 OD C tl()0 ~ (1J QJ (1J a E QJ O+J ~ QJ QJ QJ tl()(1J O+' DD ::J
UJ £ I-..r::. x ~rl X QJlL. X QJ 0 QJ X .r::. £ 'M ::J 0 (1J ~ ~ (1J (1J (1J.r::. QJ QJ££ ~ ·rl E ~ 'rl ::J LD UJ.r::. QJ.r::. ~ (1J4- C C C
'rl ~ O~ 0lL. UJ a I-.~ 0 ~4-"o 0 r-r- UJ UJ C :E 0. 4- r- :E E UJ 0.~+J Ul9 Ul QJ tl()3:4- 'M ~ ~ ~+'l9 0. 0 IU IU ·rl
U0 +J
~ ~ C
QJ ~ ~ ~ ~ ~ ~ rn q] ~ 0
x ~ D ~ D ~ 4- X D 0
W ~ ~ ~ w ~ ~ ~
62

uUl
U
::Jtr....• "'Q)
>-
mcoz
U11.
mm'"'u::J ••..•o "•..• t:T0 .•..•U ...•
"'m>-
Q)>-
OJCoZ
uUl
OJ
'"'U" ....•o "•..• t:T0 .•..•U ...•
o~
u-c
"'Q)
'"'::JU0 ••..•
o 0
u "'
3o
"'m m>- <Il
'"..,'"
'"'Q)'>,
'"
Q)
CoZ
"'Q)
'"'::JU0 .•..•U
~ 8 £
(/)
+'r-i
:::J(/)Q)~CCJE"rlUQ)0.
rJJ
"'Q)>-
"o'"' mo -a~..cDO ...•.••...•.•• +J
u.c .•..•> <33
oz
Q)coz
u
'"z
"'onm
'"'::JTI0 .•..•
o 0u on
m>-
Q)
c:oz
oz
Q)
coz
Q)r-i.0
+' COC t-Q)E (/)"rl +'~ rlQ) :::J0. (/)X Q)W 0::
muc
i-c
onQ)
>-
Q)
coz
'"'Q)
s:o
+'CQ)
E"rl~Q)0.X
W
(Y)
Llo"rl~Q)0..
4-o(/)Q)Ll"rl~orl.eu4-o
'"-e
a u ~ ~
c c '"o0. 0.
to bIl b{) 10C c
1 " s a.
'"to
"0
to
c:'"to
'"c
i
(/)
Co
-r-i
+'(/)
Ol:::JG
ua.
"0
o
'"a: '"
0.
'" 0
~ ~ ~ ~ ~ ~ ~ & N 0
"''' '"•..• u
!i 8
o >0.
'"'" '"> a:: N 0
I-.
'" ::J0.'"E~.•.• 0 0
UlE U
U 0
Ul
I-.
o0.
cI-. m
> a: '"
I-.
'">"''' '"o0.'" '"E •..• >
o UJ E u
co..,::Joon
4- '"'o Q)s:J: ..,
0. 0
c-,c
'"4-
E~'" .I---+--+-+-+--+-+--+--+-+-+--+---j ~«"0'"C C
mu'-'
o00
I-.
I---+--+-t--+--+-+--+--+-+-~+--+---j ~!o C
1-.",,..."H t:) 0.'-'f-+--+-t--+--+-t-+--+-t-+---t---! ~~
] ~ 5 '" Ii~ 6 0.c ;pi~ ~ : - ~
;~; i; J i:~
"0
o~ Ul '"o
a. a: '"
.",'" ::J
0.'" '"E ~ >.•.•0 0UlE u
(/) OlOl s: (/)+'Ll +''\:lLlLlco Ol (Y) C Q)+'(/) 4- O+'(/):::J o+,.ou
c C C OlC :::J "rl Q) co +' rlo C U C Ol
-r-l Q) bOO co Q] rJJ+,.e C >rl~co +' -r-i coLl D oQ]>lD"rlbOC(/).eOHXCO:::J+'Uo -r-i .0 ~ +'
(/) 0 4- 11l "rl4-"rl~.co cOrlOo. E::::J
"rl 4- Ul Ol ~Ol+' acoOlbO:::JOl.ea4-Q]C rlo. (/)co».o ac~.oco -+'CO
rl Q) C U (/)Ol (/) -r-i rl"rl r-iLl +' co 0. .e"rl"rl Q] > E Ll U co3:+'COCOOl"rl+'
U x+,.eQ)'ll a Ol Ol "rl 3: Ll~ U3: ~ co ~ X (/) ~a"rl 0 Q] C Q).e Q].e4- a.e(/).eu Q]~+,
+'"rl -.0-1-';'"Q] .e ~ U:::JCD3:OlCQ]4-
-r-i CD'll rl~CUrl"rlUQ)~a 0. r-i (/) 0rlXQ]CrlLlLL.eQ].ea'llQ]U (/)u+' ~
o "rl"rl 0
Ll+'D .oCU(/)C ~ 0 ~ 0.-1-'m Q] -r-l (/) 0 C C
rl s: Q) :::J Q]~.o+'CJCO E:::J CU C (Y) 11l Q) •.e Ol co r-i ~0.Q].e+, Q) bOOl (/)rl~+'(/).ec +':::J co E +' "rl eo C(/) C:::J > C Q)
»"rl CJ C co "rl E_ Q] ~"rl Q) C Ol
(/) .e (/)·rl rl ·rl r-i:::J+'rlCJ(/) .ow~ co C (/) Eo Ol+'COr-i 0'>::.e (/) ·rl ·rl ~ co CJ CJo.:::J.o co+'+' 0(/) m ~+' CJ"rl.erlaCJo~Ol.o+,(!J.eQ)IOJrl~"rllo...o~CJalO3:~
*-""""*"--""*"-----+1----+ LiB
----+*+---+-+-+-+--+ U)---f)
lft--,...t+----+a..~.-..-....-8
1(><lII-----+<i:
I(,"*---+~
roZ
Q)
Z
I(~I------+ CO
I>t-l---M----+u------<O
1(><lI---+~
N CD+
LO+
(")
+N+
NI
(")
ILO CDI I
.;tI
63
(/)
Ol·M
+'~OJ0.o~0..
OJrl.0'llt-
'"D
"o<D
+'rl 0
E 'll C~~+'Ol Ca ·rl -rl > cu4- Ol .0 -r-i OJ CJ
.e~-I-'>Ll+'OCO·rlCC en +' Q)co ~~ Ol m eno (Y) C bO 0(/) 4- 0 OJ ~+' +'~CLlOlLlC+'O»+'OJCOCJ~.eU+'UOJ+'o CJ co rl CJ Ul
OJ > OJ OJ 'll'-t a, r-i Q)-e+ X OJ » OJ ~OJOJ.er-i Ol.c +'.eQ).e-I-' Gl ee ~ 3
Ul a·M aLlo+,.eECc.e C a'll +' ·rl co .e ·rl0. CJ +'X C Ul 4- :::J mGl co caE +'s: 0 ·rlrl+,~.eGlUrl +'CJ~Xco ~ CJ co CU Gl
Ol Gl 0c.erl~Q)OJco en tn o.c.eU ·rl o.·rl +'.e ~ co ~Gl Gl 0 +'C(/)+'OlrlCJ
-r-i Ol :::J .e s: Ol~+,a+'U4-o co 4-r-i+' Gl »Ll m.eUl.e.oCCJ +' co C
C Ll CULlO4-GlCCJC ·rl 0 0. OJ'll+' rl bO»
m C Gl » Gl'-tLl o.e x.c:::J ·rl·M 0 +'.eX+'Ul0. 0 co "rl drl +' • Ul:::J s: ·rl c EUl+'uaoc
"rl x -r-i +' Ol• 3 Gl +' co bO
Ul co 0:::J(/)>>+'bO~~ Ll.o -r-l C Llo C CJ "rl ».e:::J(/)XC:.eo.oo.Gl·rlUlo.:::J .ocOED Gl E 'll.ea~.ea.e0.. CJ bO I- CJ +'
.0
(/)3:orlrla4-
~Gl+'U'll~co.eCJ
+'CQ)rlco>aCJ
enC-e+tncoOl~UCH
(/)CO
Gl~CU
mrl'llCJUl
eoC
·Mrl:::J'll0..
rns:+'Co
UlGl co N eng N 0Q)~Gl4-4-·rlLl en N l/l a:J
» 0+'·rl>·rl 0 0 0 0~ (Y) c<l c<l c<l c<lbOGlCa~+' N '"tl r-i r-t r-t
~ ~ ~ ~w Z E «
OlUl·rlU~mXW
~Gl+'UCU~cos:CJ
CJ-r-i
Ca·rl
enC·rlUlCUQ)~UGlo
'* Nrl '" rlU rl U·M U NU) a.. U)
o
11l
N
o

DOJ C C£ H ::J+' 0
OJ OJ Q[J] h . E E·rl 0 cooE 0 [J] UD -r-lCDC 0 OJ:::JC<1J+'.co <1J +'0. OJ CEh.cO[J]o OJ +' ·rl Q)Url -'L
rl OJ OJ <1J<1J <1J N D E
E -r-l .,~C [J] h h D·rl <1J 0 C
Q) rl rl <1Jh.c O.cQ)f-o.Uh+' Q)U 0 Q) +'<1J +'.cUh C +' <1J<1J 0 [J] h.c ·rl 'rl DD <1JU+' c.c
<1J +' ·rl UU U ·rl N·rl 'rl UC Q) :>, h 'rlo ...c rl m c·rl +' OJ rl 0
-'L 0 ·rl4- :>"rl 0.O.orl 4-
4- 0Q) C Q) 0OJ 0 r.., Q)h -r-l 0 Q) Q)DD C E °rl hQ) <1J .0 DDD OJ <1J Q)
Q) .c Q DQ) .c +' <1J
.c +' U W+' .c4-C[J]+'[J]
[J] 0 O'rl 'rl+' ·rl [J] [J]UC+'CQ):>'Q) 0 <1J 0 [J] rl4- 'rl U 'rl <1J 04-+' OJh<1J <1J OJ E r.., D
N .c :::J U :>,.c ·rl +' ·rl OJ .cU h U D·rl <1JD rl 0.crlQ)<1J[J]+'3 0 DD U 'H
o.h .cOJh <1J Q)f-rl04-£.c .0
+' 0 U +' ·rlU I • +'<1J+':>"+'o.
4-Crl OICQ)W£rlOJU
Q)+'DDU+'[J]C X 'rl <1J X :::Jo Q) s: U Q) [J]
Er..,o4-
o+'[J]r..,:::JUUoCo'rl+'U<1JOJr.,
DDC·rl3orlrlo4-
OJ.c+'Q)
Co
rl<1J
DOJ+'<1JQ).c
[J]'rl
o01 OJIDCD ·rl. h01 0
rl rlu.cDDUL
UC -r-iQ) [J]
.c <1J3.0
o01
IUl
+
rlUI
+
DQ)Eho4-
[J] :>,:::IDoh [J]D ·rl:>,[J].c :>,Crl<1J 0
hOJDU >-:::J £Do [J]
h +'Q·rl
Drl -r-irl .c'rl C3 -r-l
DDC'rlD:::JrlUC
-r-i
Q) DD OJ·rl [J]h :Jorl Q).c >U <1J
s:U
-r-i W[J] 3<1J
.0 Q)
C[J] 0'rl.c OJ+' s:
+'o+' [J]<1J
DQ)rlCrlDDOJ
·rl 3[J][J] [J]<1J <1J
C 01Q)rlQ)UD DD
LQ) •> 01<1J~.cI
oQ)~<1J DDrlL:::JEDh Co <1J4-
01[J]rl:::J Uo DD·rl Lr.., •<1JO> DD* L
+' [J]U ·rlQ) .c4-f-4-Q)
DD r..,C OJ
-r-l +'N U
·rl <1Jh h<1J <1J
rl .co U0.
+'DD CC Q)o rlh <1J+' >[J] 0
U(1J
q.,[J] 0 [J]<1J ·rl
.c Q) [J]W :>,
C h rlo DD 0
-r-i Q) h+'DD<1J :>, rlu.c.c U
DD IE -r-i 0 N:::J.c+'
-r-irl <1J Q)rl rl:>'CSJ~-I ·rl o,-j
Q) +' 0D DD 0. Q)
C Q) [IJD'rl UQ) +' [J]DD rl :::Jh :::J [J]<1J [J]
.c Q) :>,U r.., hW> 0
01I
:>, [J]rl C.c 0 DDD'rl C
·rl :::J.c Q) 0
D 0.D -r-i EC h 0<1J 0 U
rlrl .c OJrl U .c(1J +'E OJ[J] .c [J]
+' OJQ) -'L.c C <1Jf- 0 E
+
[J]
1l
+
01rlUOJ[IJ
DC<1J
Co·rl+'<1JU
E:J
-r-lrlrl rl:>,Uh~WIDO
Q) DD.cL+'
EC h<1J 0
.c4-+' oDD+'C'rl DN Q)·rl [J]r.., :>,<1Jrl
rl 0o ho.D
:>,[J].c[JJQ) :>,rl rl
rl[J] <1J'rl ·rl
+'C ho <1J
·rl 0..p<1J :>,Url
CE 0:::J
-r-l [J][J] 'rlQ)C 01DDrl<1JUE a;;
Lr..,OJ OJb.l:hh 0<1J4-rl Q)
hOJ OJ.c .cf-+'
Q)
DDD ·rlC h·rl 0D rlC .co U.0
C4- -r-i
oD
OJ CQ :J:>, 0
f-4-
+'COJrl<1J:>oU
U·rlCoH
+'CQ)rl<1J>oU
enrlUZ
4- 0.o
rl OJ<1JDU ·rl
·rl h[J] 0:>'rl.c .cD... U
Q)+' [J]<1J+'+'[J] <1J
[J]<1J
LJ
D·rlrl
oUJ
co
OJ[JJ·rlUhOJX
W
OJrl.0
<1Jf-
4-o
Q)<1JDrl -r-l:J hE 0hrlo .c
u.. U
+'COJEOJrlW
rlUI
COJb1JohD:>,I
01rlU<1J[IJ
E:::J·rlh<1JII]
CQ)b1Joh+'·rlZ
+'COJrl<1J:>oU
OJD·rlXo
Q)[J]·rlUhOJX
W
CD
Q)[J]·rlUc,Q)X
W
[J]·rl
OJ[J] D
'rl rlX U8 0 :;r:E:::J·rl[J]
DD OJL C
DD<1JE
+Io
[J]E rl C<1J <1J 0OJ :::J'rl
o r..,C +'b1J +"rl UL [J] +' <1J
C OJ<1J 0 h
UC Q)
-r-i Q) >.c 0
b1J +' .0C <1J
·rl Q)+' [J] Q)<1J :J .cQ) <1J +'.c U
OJ DD'D C
h 'rlOJ ,[J]> 01 r..,Q) rl OJ3 U >o DD Q)I L r..,
OJ [J]D <1J·rl DDho [J]rl 'rl.c .cU +'C4-Q) 0DDo OJh UD C:>, OJ.c [J]
W4- ho Q
Q)[J]
-r-i
UhQ)XW
D·rl:JIT·rl-.J
rlU·rlUJ
CoU·rlrl·rlUJ
>H
CoU·rl
'rl[J]
U<1J
~rlU
+
Drl OJU +'I <1Jq- r..,
D r~+ :>,0;-.c
[J]
IT<1J
+'r.., Co OJ
o 4-.~01 D h
I Q) Q)N E Q
h X01 0 Wo 4-
'rlUJ
1l1l
DD[J] C
-r-io +' [J]01 <1J
I OJ rlCD .c U
01 h Irl OJ 0U b1JDD C b1JL 0 L
h+'[J]
Co
U<1J
oen
I
W Nr..,<1J C
o[JJ ·rlOJ +'+' [J](1J OJh :::J
o D IT01 :>,
I .c 0q- +'
4-+ 0 h
Q)OJ 3DD[J]C C
or <1J <1Jrl r..,U W·rl <1J OJUJ ~ U)
o01
I
+
rlUI
.0
o+'OJ W[J] OJ·rl UJr..,
b1J.C D'rl W [J]> U rl·rl :J·rlDD D <1J
o +'L~ h OJC Q Do
-r-i
IT C<1J 0
'--'_or!I
[J] r..,<1J OJ
• DD .co +-'en rl r..,
I U :JI4-
Er.., Q) ho E 04- 0 4-
[J]o+' 0 [J]
[J] +'OJ rl C+' <1J OJ'1J E
-r-t [JJ OJU ·rl rlo W[J] OJ[J] r..,-'L'rl OJ UD .c 0
f-rl[J] [IJ 0C I 01o .)::), I
-r-i Co D
+ 'r! OJ(T) +J...I:--l
.- :::J U'" rl OJ
~Orlo [JJ OJ
01 UJI U~~'rlrl D CD<C 'rl H
L....." U<1J +'
OJ ·rl.c C Cf- <1J :=J
+'[J] OJor ...cX [J] f-
D Q) <1J·rlu<1J
OJD-r-i
~ XOD 0
:>,.c •<1J U 0E :J 01
U [J] I'rl [J] <-l ~U Q) [J]. •
-r-l ·rl Q) 01 rlrlU.pOQ)·rl Q) <1J'rl DD[J] 0. r.., UJ
[J] D:>'[J]s: (1J h
o4-+'o C C
Q) 0Q) [J]'rlDDOJ+'C h :::J
,-... ~ I'D 0.. rl> ~ r.., OJ 0
HI h [J]~O (1JC o~
o ·rlU UJ
-r-trl h'rl 0[J]
(1J
[J]:Jor..,o.c0.[J]o.cQ
IT D(1J OJ
rlrl(1JU
DC(1J
D-r-tU<1J
I 3o 0h .c+' [J]U
[J]r..,:::JUUo
<1J (1J
Nb1Jrl C
• U'rl+' b1J.p[J] L (1JQ) U+' [J] -r-i
:::J Dr.., 0 C:::J r..,·rlo D:>,:>,OJ
.c D4- h C ·rl
• 0 <1J 0 <1J h[J]E U4- 0
-t--J :J rl·rl ..c rlrl ·rl (1J Q Q) +' .c
:::J C U :>, D ·rl U[J] -r-i -r-l .p ·rl 3Q) E 0. r.., C
h :::J :>, <1J 0 Q)rl.p b1J
rl<C OJ.cD 0<1J [J].x: u W r..,.p C'rl :> DC OrlErl:>,OJ [J] .r< :::J 0 .cE Q) +' [J] .r< [J]·rl :J U OJ Ul [J] 4-~4 rl !l] > Q) ·rl 0Q) '1J Q) (1J C Do.:>r..,.cb1J OJX Q) (1J OJ uQ):>,u.oE><1J
+' 'rl '1J h hOJ ·rl +' OJ D .c +' OJ
.c>:>'DOJ +'.p 'rl rl ·rl +' :>, '1J U
+'Oh<1Jrl <1J·(1JhOr..,o..cr..,
[J] b1J D' rl DE+' (1JQ) OJ :>,.c :>,·rl 'rl .c>-c.cu.c[J]3U OJ
rlD<1Jf-
UlL
U1<lJl'l
)0, ~
~ 0OJ 0> o.
OJ
o
j
OJ
u
~
uo
.>, h
h 0OJ 0> CL
mOJ ~
0. OJE ~
Ul E
uz
'0
...J
'>, h
h 0OJ 0> D.
uu
'0
IT.>, h
h 0OJ 0> 0.
h
OJ ~~ lJ0. OJE ~
.,., 0
GO E
uOJ
~[J"
...J
c-, hh 0OJ 0> D.
o<lJ
Q:
c,<0
~ 0[lOJ
.~ '"3Ul E
uOJ
OJ
'0
o(f)
.>, c,h 0OJ 0> 0.
oUl
'0oco
Ql
>
o
c<lJ
co H
OJ[J]·rlUhOJX
L~J
QJ
Ul
>'0
lJ IT
'O~
04-
U 0
6co h
.,., OJ
U <lJ<t: 3
JOJ
III>U
cOJ
>ou
C
III.>
U
rlb1J U en
I:r: ITrl [Y) Z <1JU:r: + +N U
I+ IT IT
<1J <1J IT +<1J
en 0o 0 IT
DQJUrl CDOJ en I UII] I [Y) :r: u..
N 0I
o :::J 001 01 01 0
:r: :r: :r: 01 0[Y) [Y) I N
:r:+ + +
Ol)C
'0
oOJ
01rlU rlOJ U[IJ [IJ
.0
+
en 0,-I N LLU rl rlZ U U
UH[Y) rl
H+~
E~ :JIT 'rl<1J C
~ -r-l
Een :J.- rl
'" (1J~ <1Jo :J
NITI <1J~ (1Jrl X
D <C OJQ) L.....".c
:::JC
-r-i
+'CoU
oUl NC Io CD·rl+' +[JJOJ:::J
0"en
rl
Url
+' <CCOJE'rl (1Jr..,Q)oXW N
r" ~U>IHq- ~
C+ 0
U~ ·rlITrl(1J ·rl
~ [J]o
OlDI OJ<-l +'. <1J01 h
OD·rl :>,UJ.c
[J]<1J OJ
[JJOJ OJDL:
-r-l f-Xo
N
OJ rl+' 3 <1J<1J D.c .c ·rl+' U 0.r< rl>-.c rl
('t1rl :3: 0DO OJ UQ) .r< -'L +'+' UJ .r< [J] <1J<1J 01 rl -r-lr.., I X [J]
D [J] Q) <1J>- <1J E.crlOJO[JJ
:::J OJ 01 ho E [J] I <1J+' h • Q)
O+' NO.r.., 4- 'rl 0 0.OJ -r-l <1J4- OJ+'UJOJ .c:::J Q)r..,+'.oDD
C ·rl[JJ +' C <1J X.x: 'rl 0 0o ·rl 0o OJ+' OlD.0 > :::J I OJ
0,--j rl N +JW b(! 0 . <1JE [JJ 01 r..,OD ODUJ C C ·rl >-~ <1J 'rl UJ .c
+
+
rlU'rlUJ
D
rlUID[Y) ·rl
U+ <1J[J]OJE
~ U -r-lU·rl +'<1J C OJ
~ 0 E.en.c 0o Q [JJI [J]D... 0 [JJ
N.c 'rlI G.
o01
I[Y)
U+ ·rl OJ
C .co +'.c0. C[J] OJ
rl 0:>U .c 'rlD... D... DD
U
DQ)1] +'[J] rl C CE :::J :::J 0 OJ
E h:::J 0 ·rl rlo :::J ·rl :>, 3 +' <1Jh.cDrl U>
4-QOD+'<1JOrl tn CO -r-! OJ U
OJ:::J .0 r..,D[JJOJOOJ 4-([J rl H en en 0E D ·rl 0. (1J :::J
c.c uow[JJ (1J 3:::J h OJC o.cOho In [J] >- U b1J b1J·rl :::J OJ ·rl·rl OJ.pr..,D .c:>DU O'rl • 3·rl .c h OJDo.ODCOJ [J] rl 'rl ·rlr.., 0 .c r.,o..c U 0
0. rlE +,.ch • C U·rl C OJ4-0rlUC U <1J ·rlo 'rl > CU rl 0 0
-r-i U 'orlo [J]1]
OJ rlrl rlOJ <1J
enenD
OD...I enD...I
01I (1J
rl1] :::J-r-i EU r..,<1J 0
4-
64

u u -'=OJ +-'
+-' [J] X OJ -r-ih
OJ '+-h ·rl ·rl
'+- 3: 3:0
QJ rl h 0 >,ro E 0 OJ 0 +-' -'= 0 ro .0+-' +, .0 rl +-' ro +-' OJ 0 o, >, '"'[J] -r-l DlJ C rl 'rl OJ
U > U QJ ro u I~ +-' C 0 », 0 C -r-l h OJ 0 h :s OJ rlU 0 C +-' 'rl 'rl ro '+- OJ 0 u 0 .r: E 0 o, [J] 00 +-' QJ ro +-' +-' E +-' u
CLL +-' OJ +-' 0 >, ;:,. E
E -'= +-' :J u ro'rl
h 0 C rl <:J(Y] -'= OJ +-' OJ
rl G~ -r-l h OJ +-' C +-' 0 D.O~ " D.O rl D.O 0 OJ U +-' .0C :J 0 0 [J] h II "'~ U " :J OJ 0 [J] u (0 -r-i ro OJ 0 +-' ·rl QJ ·rl u "' E Ln
U 0 0 [J] u-r-] +-' C 0 -'= >, +-' rl >, u E U
0 [J] C QJ C [J] h U ro rl +-' rl QJ :J .0 QJ -'= QJ U lD0 ·rl CD -'= ro 0 0 'rl u U ~.o ·rl [J] c, rl ·rl h Ul r-- 0 (Y]
0 m +-' U 0 rl h C 3: :J :J 0 [J] QJ [J] 0 _-l 0Ul Cf) u 0 h OJ -'= 0 ro [J] 'rl rl 0 +-' G1 [J] .r; -r-l 0 Ln LCJ X:;: OJ +-' OJ 0 U rl -r-i [J] 0 D ·rl X 0 +-' E N N
" [J] U +-' rl <T is rr -'= c .r; :J E OJ > 'rl u o. +-' D.O rl-rl 'r' ro OJ <T ro h U 0 I- >,+-' OJ E QJ .: ·rl <C "' x :'2: 0
[J] .0 OJ E <T -r-i 0 'rl '-,= ro h +-' OJ rl I - E·rl CL E -'= D.O C OJ h +-' >'+-, QJ o, D ro .0 OJ [J] U '+ 0 rl rl X
0 0 +-' C ro -'= D 0 rl 0 o, OJ h > ro I 0 " 0 0 N·rl D .r: QJ 4-' ro 'rl +-' >, rl r-, +-' E QJ u N +-' u OJ E E ;;:: ICf) OJ h .0 rl h -'= C 0 Uo h OJ 'M C rl :s 0 +-' X 0
h -'= [J] rl OJ '+- [J] :r: OJ h C rl QJ 0 0 [J] ~ C "' N II "
~
'+- '+- u D.O rl 'rl +-' 0 -'= 0 [lh.o C 'rl ro U 0 :r: 'rl D.O 0 <T I I
0 '+- 0 rl :J 'M +-' ro +-' 'rl ·rl -r-i OJ E c, C 3: -'= L 0 CO 0 0 OJ X0 0 0 0 [J] 3: [J] 'rl +-' [J] D [J] -'= h +-' 0 +-' E " c--' rl
+-' +-' 0 C .0 ·rl OJ 3: m m QJ C ro +-' OJ :J U +-' 0 E ro " c, <TC +-' C -'= QJ D 0 rl N .Y -'= 'M +-' OJ -r-l E h 'I;- X X E CO'rl C -r-i [J] TJ +-' o, C) 'rl rl U -'= Ul OJ C OJ NO 0 II ro0 ·rl 0 u C h E D rl ro ro '" U -r-l u -'= D :r: h '+- <T <T [J] "o, 0 [l CL OJ ro 0 D rn 'rl rl OJ 0 0 OJ +-' 0 CD u.. C CO CO
~ o, I +-' -'= OJ Cf) X ro '+- r., [J] C D -'= -'= 0 0 OJ II "I D.O u ro u o, u 0 +-' 0 .: 0 -r-i +-' OJ +-' N>,+-' -r-i "' " " rlC D.O C OJ OJ 0 rl U h [J] +-' " ·rl U > OJ rl rl +-' E -;;- 0 OJ'rl C -r-i h .r; 0 m u >, OJ 'rl OJ f"--+-' ro '+- ro E u+-' C 0 D II -'= rlt-l 'rl +-' 0 [J] u >, h E +-' ro rl OJ D.O U 0 rl 3: o,rl +-' rl OJ 0 [J] OJ .c U 0 OJ :r: N '+- ro rl C L OJ 'rl ~ 0
<TIDOJ E
OJ rl OJ >,.0 " u ·rl C rl ·rl E CL ·,·1 0 s: 0 h +-' [J] [J] '0 rl C mE QJ E rl OJ
-r-] OJ h 0 +-' rl u 'rl ,·rl ro ~ COO o, ·rl [J]~ E ~ D.Ou h D.O E ro s: 0 > c :r: D ro OJ +-' C +-' OJu N rl '+- E~ C rl OJ C 0 +-' +-' 0 o, C h E 0 ro m D -r-! U 0 ro c OJ
" N 0 0 > ro -r-l .0 [J] C 0 +-' 0 0 N ·rl Nrl D.O X [J] 0 rlrl "' rl h 0 0 .r: D 0 ec [J] :s u ro 0 0 rl .0 h ·rl h c:::; rl <C "' -r-l 0U rl U +-' s: U 0 C QJ 0 'M h OJ 0 ro OJ rl 0 ro E "' OJ -'=·rl U N [J] [J] +-' C [J] [J] -r-i C ~ U D.O ro c > +-' ro rl tlD+-' U I rl OJ 3:Cf) 0.. Cf) [J] 0 ·rl N >, ro c '+- ro h -'= L [J] E 0 u
D Ul 0 +-' h -r-l h -r-l +-' QJ OJ '+- 3: +-' U >, Ln u .c ·rl Ch h h QJ 'r"')D U 0 OJ rl u m U [J] ro s: -'= 0 :J Ul h N 3: h -r-t0 0 0 +-' u -r-i 0 D ro -r-l 0 +-' +-' Ul QJ E 0 U ~ rl 0'+- '+- '+- rn C +-' 0 u E 'rl h 0 OJ h +-' OJ [J] C 0 0 U C 0 C rl I
OJ ro n::: > 0 0 X +-' +-' s: ;:; C tlD +-' OJ 'rl h '+- m ·rl E 'rl .r: rlr-, r-, co L: ro h -r-i 0 :::J m >"rl u C ro U QJ OJ [J] u 0 'i' U u" " " [J] o, L: h OJ C +-' .r: rl 0 0 'M h X N > OJ >, I 0 I
QJ C +-' 0 +-' u C 0 U +-' elL: Ul 0 OJ -r-t 0 C .c h rl rl 0 rl '+- '+-.0 0 rl +-' OJ ·rl >, -r-i OJ 0 U +-' :J [l rl .0 D.O C OJ U U " U 0 0
tlD eo tlD ·rl OJ .c rl .c >,+-' Ul h OJ u OJ ro OJ rl ro m m +-' c::i'rl ·rl 'rl u +-' E +-' +-' .0 ro ro D h c E C > -'= ro E ro + '+- '+- Ul UlLL LL LL rl U c h rl h U >"rl OJ OJ +-' +-' QJ D.O 3: 0 0 Ul Ul
0 ro h ro -'= OJ ro u 0 -'= u +-' 0 Ul .c u c ~ II ro rom 0 OJ 0 +-' +-' 0 -'= +-' OJ [l L: C U +-' >,'+- >, +-' OJ 'rl C " U +-' +-' L Erl ~ ~ ~ -'= h .c h 'M N +-' OJ h ro U OJ h ro OJ ro rl 0 h -p h 3: N ro c c
-rl 'rl -rl [J] [l 0 3: -rl E ro > ro +-' QJ h U rl U D.O ro ro 0 ~ 0 0OJ ~ -rl -rl OJ r:; [l OJ OJ [l OJ OJ :J -'= -'= -rl 'rl ro OJ c h [l OJ 0 0[J] ~ 'rl -rl L: E c [J] OJ OJ rl +-' +-' u u c E D -rl u OJ Ul ef) tlD E E-rl ~ Cf) +-' Cf) 'rl -rl -rl '+- -'= h >, -rl -rl 'M C C -rl ~ C Ul >, h+-' 'rl <C <C <CU U H +-' [l.o H D OJ :s -rl -rl LL ro :=J -'= o. or-! Uh h hOJ ~ ~ ~ OJ ~ ~ QJ ~ ~X ro .0 ~ X ro .0 X ~ .0
W ~ ~ W ~ ~WI ~
-'= OJ [J]
+-' .0 :s u-rl 0 OJ:s u rl E
rl rl hu U. 0 0 0 .cOJ OJ 0 '+- '+- OJX E 3: rl U'rl h Ul OJ +-' -rlE 0 +-' ro .0 +-' -'= ~4- H "' .. 0 :sh [J] >, rl OJ .0 'rl0 +-' 'rl rl U h OJ ro0 -rl rl rl ro h u[l >, c ro <C QJ -rl h +-'ro [J] +-' 0 U Ul > X 0 Ul
> ro 'rl -rl -rl '+- C -rl QJ 0 0 -rlh +-' +-' 0 0 OJ C [l 0
h OJ :::J ro OJ Ul U -rl E ro EOJ u [l rl h tlD 0 ro QJ h h >+-' -rl E 0 0 0 OJ h 0 0 >,ro h -rl U OJ N h rl '+- h .0
:s 0 rl L: D -'= OJrl C tlD ro +-' >, C U 0 +-' [J]
[J] L: ro U X rl ro +-' ro -rlD.O tlD tlD tlD ro U rl u "' "' OJ [J] :s Ul
Ul U OJ rl rl .Y OJ [J] h >,Ln 0 0 0 0\° -'= E CD :r: -'= 0 u U Ln +-' -rl .0 OJ -rl r;;; rlN N CD <T (Y] U 0 CD +-' 0 rl rl N U
...J :J U ro 0
0(Y] 0 'rl OJ 3: <C <C :::J
[ill+-' X :::J h
0 0 0 UJ C U + [J] 0 t-l u OJ rl U D'rl ·rl +-' +-' '+- '+- U 0 C ro -rl >,E X rl ro 0 0 X :J h Ul 0 .c 0 [J] -'=
[J] :J 0 0 -'=w~1
u [l u -rl +-' u OJ0 rl [J] +-' tlDl 0 rl +-' 'rl -rl h +-'+-' ro E "' OJ h '+- OJ [J] :s Ul OJ C
N ro 0 0 h "' [l 0 0 -rl :J OJ .0 >, OJ
'" h OJ -rl N rl • f"-- 0 >,.0 >, h 0.0 >ro .c c rl C "' U (Y] (Y] N BIN '+- [J] E ro +-' OJ[l+-, 'rl <C OJ rl rl (Y] (Y] 0 [J] :s 0 :s -'= u h
+-' [l E E u <C ro 0 U ro +-' c OJ [lu ro OJ 0 -rl rl [J] E X ~i -rl 0 [J]
~I ",'"' [J] rl U <C '+- [J] C u :s -rl >, 0u (j) >, ro OJ 0 [J] [J] [J] ro rl 01 -rl OJ +-' rl +-'
E .L rl [l QJ OJ OJ E ro ;Iz OJ -rl [J] [J] 0+-' 0 C [J] [J] U U U U > [J] h +-' 0 h >,
0 OJ [l h -M tlD [J] 0 0 0 -rl -rl C h U .0 u hef) .0 ¥ c u ro OJ ro u u u ro +-' b£ 'rl ro ro E >, roOJ 0 U -rl >, +-' C 0 -'= E 0 0 0 0 OJ ro U OJ 0 -'= [J]h +-' C ",N .~I -'= c 0 N +-' tlD h h h +-' h 0" c E h U 01
ro +-' 0 'rl :r: OJ [l [l [l U 0 OJ QJ [J] [J] OJC C C U U +-' (Y] E N .c CD OJ +-' h -rl E OJ -rl UOJ g! OJ OJ rl U 0 rl +-' rl rl rl -'= '+- :J -'= OJE .0 [J] 0 u ro + h U rl <C <C <C +-' u 0 +-' +-' -rl +-' +-' C-rl -:=i 0 ~I OJ 0 rl OJ '+- OJ <C rl U U C UU U +-' '" h :s 0 h rl +-' 4- 't- '+- OJ +-' :J :J -rl C 0 [J]QJ E OJ [l 0 ~ ro ro 4- 0 0 0 -rl C D u E 'rl u -rl[l 0 [l c 3: OJ +-' + rl 0 D >, QJ 0 0 :J 0
Cf) -M [J] OJ +-' OJ OJ -'= "' ro :J tlD CD tlD rl E h h rl [J] h h," c E u h C +-' +-' rl U U b£ OJ 0\° -rl [l [l ro c [l 0-g >, -rl 0 0 -rl U U ~ rl "' 0 Ln -rl h ·rl +-'OJ +-' U u +-' h 0 >, rl OJ roLn N N >, OJ OJ OJ OJ ro OJ ro
N .0 0 D OJ 0 N [J] 0 D .0 <C -'= rl UN D E E E E E UC;;; E [l h 'rl rl 0 N +-' <C 0 0\° X 0 0 0 OJ 0 U
+-' ro OJ en [l +-' 0 .c: h u ,0 QJ (f) Cf) (f) hCf) ·rlC 1-- C E u [l OJ CD +-' [J]QJ '+- '+- '+- '+- u OJ E c [J] E [J] OJE LC 0 0 0 0 E >, OJ OJ h 'rl h 0 -r-!,.-------."'--'" ,.---., u-rl +-' OJ 'rl C -'= -'= 0 [J] -rl h .cro.oU uh [J] [J] [J] [J] -rl h <C +-' l- '+- :=J LL '+- I- ~ ~ ~ <COJ 0 en [J] [J] UJ >, OJ[l en ro ro ro ro [lX OJ L L L L X ~ reiw cr W <T
65

Q)r-i
ClJUUJ
enC
.r!r-i
::JClJ0..
Q)s:.j..J
ea
UJ3a
r-ir-i
a4-
UJClJ
Q)~ClJ
UJQ)ueQ)~ ill (Y) 0 I'.Q)4- N4-.r!"0
(Y)
N
Q)UJ
-r-i
U~Q)
~ ~
N N
(Y) (Y)
Lf'l o
~a4-
blJe-r-t
"0eaD
u.r!ea
.r!
.,.oLf'l >.
u~ CQ) ill •> r-i ea ClJ 0> u.j..J O·r!UJ U r-i::J .r!
•....., 4- UJa
a ~.j..J Q) OJ
Q).j..J
UJ ~ 4-"0 blJ ClJC OJa "0 ~0. Q)UJ Q) .cQ) blJ.j..J~ ~ ~~ ClJ ::J0r-i4-
UClJ UJ
N Q)O.j..JUJ-r-i U ClJen Q) Q)o.~~ x uo Q) e4- "r"i
"0Q) r-i ~U ::J Q)CO.j..J
Q) 3 U~ ClJQ) Q) ~
4- s CU4- £.r!.j..) U"0 ClJ
£ .j..J>..j..J e
.j..J Q)
-r-i a r-i> UJ ClJ-r-i >.j..J -0ClJ ~ UblJQ)Q).j..J4-
C U aa ClJr... r... OJ
.j..J CU OJU £ r...Q) U DCr-i OJOJ U "0
.r!Q) e OJ£0£I- .r! I-
.j..J
CQ)
r-i
CU>aU
a.j..J
U.r!eo
-r-t
Ea~ .
4-"0
aUJ or!Q) ~blJQ)e 0.CU£ OJO£
.j..J
UJQ) UJ"0 UJ-r-i ax ~a U
ClJ
"0 ~o OJ•..-1 D~ EQ) ::J0.. eC U
•..-I.r!E
blJOC.j..J
.r! ClJ"0e blJo eD •..-1
UJ4- ClJo OJ•..OJ 0o.c>. ...-1
.j..J
£OJ.j..J£ ...-1I- 3
D
osn
oa..
"o~o 0..g: (G >- ~ >
o(/)
UJ
~::JUJQ)
et::eQ)
E-r-lUQ)0.(f) (Y)
OJ(Y) r-i
D.j..J ClJe I-Q)E UJ•..-1 .j..J
~ r-iQ) ::J0. UJX Q)W et::
~I~E
o
>~o JJ~o c-,~~cI0. 0-
>. (J) N Lf'l
~ ci>•..-1
.j..J
OJ Lf'l Lf'l Lf'lOJ)OJ (Y)ea~.j..J (II IfI
U 0 0 (II IfIQ) N 0 (II 0 0 (IIr-i ClJ blJ r-i •..-1 N 0W Z :E <:( (/) 0.. (/)
Lf'l Lf'l Lf'l Lf'l
t-o
(IIr-i
U
IfI.j..J0
o N• ..-1 r-i"0<:(Q)~ ~0.0
4-OJ3 blJ
eUJ •..-1Q)"OU ec aQ)D~Q) U4- -r-i4- C.r! 0"0 •..-1
>.>..j..J r-i-r-l .j..J
> C-r-i ClJ.j..J eClJ •..-1blJEOJ aeLlo Q)~ ~.j..J0.
UOJLlr-i eQ) ClJ
OJOUJ bJJQ):E£.j..J -
(II
EOo (II~ ClJLLZ
.-<N
~0.EClJUJ
C•..-1
r-i
U
4-aUJUJI1JE
~0.EClJUJ
4-aUJUJClJE
Q).-<0.EClJ bJJUJ
(Y)
~ ~i? ci3OJ.C en.j..J
r-,C CD
• ..-1 "-0...
4-aUJUJClJ:E
~0.EClJUJ
OJ.-<as:3:
ills:.j..J
C• ..-1
0..en
4-'OJ a
.j..J
eo ::JaEClJ
r--.-<aE
'"Io
~UJEa
.j..J
ClJ
rlU
4-a
IT .j..J .j..J1lJ C C
:J ::Ja 0
N DO E EN <:( <:( <:(
OJUJ
·rlU~Q)
~ ~
UJ·rl
"0Q)UJ::J
DO<:(
4-a
.j..J
C
~EClJ
ea• ..-1
.j..J
::J.-<~ ~18 ~
"' 0 o,.-< 4- N.,- EU 0 f1JDO X UJ
<:( '"Eo
UJ
lJlN
IT Cf1J -r-t
+ 4-a
ITlITl EE UU
oo .r-- Lf'l Lf'l
.-< N NaE x
ITlI
o
x
o
.-<oE
ITlIo
xo
N
Q).-<as:3:
C·rl
.-<U
4-a
N
~o,Ef1JUJ
~oL3:
C-r-i
ca
·rl
OJLl·rl~a
.-<s:U
4-o01UJf1JL
..•I.-<aE
DO
Lf'l(Y)
x.-<aE
NI
o
en
Q).-<D.EClJV1
Q).-<a
.t:3:OJ£.j..J
C·rl
blJ DO
o 'OJN "-'OJ CD
o
OJ.-<o,EOJUJ
GJ.-<o£3:
OJ.c.j..J
C·rl
(/)
4-o
.j..J
C:JoE
<:(
..•I (II
blJr-i Io 0~ ECD DO X
ci 0
.-<aE
N(Y) N
QjCoOJ~o4-OJ~9:!~ S--is.
~.-<::J ::J-is.UJ
'-<4-
iii 0Ll~e aOJ E
C£a u·rl f1J
OJ OJLl£·rl .j..J
~ .r!,S 3:£"0U ill
4- .;:;aDUJ E
.j..J ae U:J UJ
~ ·rl
f1J GJ
riD
I1l ·Cg~
£OJ u
~'6ill>... OJQJ .-<.c 0I- E
f1Jri::JE>...a4-
~I1l
.-<::JUill
.-<oEOJ.c
.j..J
UJ~GJD
N E::J
Lf'l C(Y)
.-<I1l
UJ :J·rl IT
OJGJLl C·rl ·rl~o •....ri :J.c uU u
oill.c 01
.j..J Eo4- .j..J
o f1J
UJ riUJ UClJE "0
e~ 1lJ 3
en .-<o UE QJ (/)
UGJ C.c ·rlI- (/)
CD
[,.J~.-<N
• NUI (j)(Y)
"':JQ)::JC
-r-l.j..J
eou
GJUJ
·rlU•....Q)x
W
xcoCD
(Y)
'-;;- •• .-<UJ U::J•...• 4-o 0.c0. r-i
UJ 0a E.c0. N
I ri
'6 0 ~~
rioEoo
OJ"0·rl~ori.coQ).c
.j..J
4-oI1J
.-<:JE•....o4-
l8OJ
.-<0..E
·rlUJ
GJ.cI-
Q)
N'--
II
ITlE"0
ITlI
E"0
rioEoo
ci(/)
'+-oUJenI1lL
D
UJUJI1lE•....I1lrloE
I1lUJ riUJ :Jf1J EE •....
o•...• 4-f1Jrl •...•
o f1JE .-<:JuOJ
.-<o~ E
•...• OJo .c4- I-
..•I
blJr-ia'OJ E
00
~oE
X (II 0I -c--'
'OJ 0co v-' x
lfl
~
f.-.oLL
OJ xcoe CDo s: 'OJ •
.j..J co (Y)
£ ·rl.j..J 3:.r!3: UJ L
GJ .j..J
"0 C ·rlOJ·rl 3:C D·rl E UJD 0 Q)E u Ca ·rlU 0.. D
EOJ 4- 0C 0 U·rl•...• rl 0..o 0rl E 4-.c au '"I ri4- 0 0o E.j..J xC:J coo CDE
<:( (Y)
66

0) l!1UJ'rl
~ ~0) .0X tUW f-
l[1
N
0)UJ'rlU~0)x
W
0)r-t0.E·rlUJ
4-oUJ+JUJ
'rlUJCou
4-0) 0s:+J C
a~ »'rl0) .0 +J+J ·rim U UJ3 0) a3 0.caE'ri •....• a
.....•uU 0 0)0) 4- U>.....•aUJUJ'riU
ITtU
ITtU
(II
o(II
I
~III
o+
oIII
IN
ITmIII
oIII
IN
CD
»"I
"'. u
I
<D
I
<D~ 0
"'" z
I
-c
> •~.'" o.
~ °
<n "'"
CDN
0)UJ
-r-i
U~0)xW
I C<D.o
.0-0~I
!
J
IIIo(f)
UJ0)•..::J+JU::J~+JUJ
IIIo
C III0) Is:3 +
(f)
co
'rl+J(f)
QJ:JG
+JCQJE·rl>-..QJ0.Xw
.'"
Q)
!
U'ri
0) 0) OJ ClJ lJ lJ a'ri ·ri -r+ 'riX X Xo a 0 +J~ -r+ COJ E U tU0.::J 'ri
'ri ~ !lOE C ::J::J -r-i .c:s ~ .8-a r-t ::JUJ tU UJ
+J CC a'ri 'rio +J0. ::J I1 •.••.• 0en 0 mC UJ Z·ri N+J QJ•....•COJ -r-lE .....•
m+J -"'-UJ •....•0) tU3a.....•
OJ •~ UJtU 0)
r-t
"'::Jo UIII OJ.....•.....•
<I:: 0 OJE .c+J
oC IIItU I
NUJOJ +>·rien ~
o0lJ:L
+o
III:r: 0CD 0 III
III I:r:
o'" .....•o u(f) I
III N:r:
(f)
N
.0 U D QJ
D OJC •....•
!1J 0.E
H'ri •H (f) OJ
c, 0 -e+
H +J ~o
UJ ,r-to.OJ.c::J D Uo -r-t~ X DL'J 0 C
mC~
-r-i > ~H ::J
CI)~.cOJ C 0.>-.. 0 •....•::J U ::J OJ+J -r-l CI) OJ.cU •....• .c +J::J'ri ' +J~ CI)+J(f)
ITm
0-tU
CI)::J•.. ~
o 0U4-.c'rl 0.C OJ CI)o •.• 0
-r-i ::J.c+J 0.
+J UC ::J >-..tU ~ 0
-r-i +J 4-!lOCI)
CI)eo +J OJC C •.•'rl OJ ::JOJri+J.0 ro U> :JE 0 ~o U +J>-.. CI)4-
coCs: -r-i
!lOC::J -r-io tU~ E.c OJ
+J •.•
CI) OJOJ.cD +J-r-iX4-o 0
.Y (f)
U OJODri -r-i.0 XI 0
UJ
>-..~ 004-4-
UU -r-i'rl DCI) 'rlro U.0 tU
E 0O+J>-..4- OJ
D(f) 'rlOJ X0lJ0Cro E.c ::JU -r-i
C0) -r+•.•E:J :J+J r-<tU tUC •..QJ 0Cl)4-m.0 UI -r-l
D •.• CI)-r-i OJ +JU +J CtU 0 0)
.c E0) 0. OJ.c E riI- m 0)
o+J
CI)U 0)'rl 0lJC Co ro
-r-t .cU
+JC OJ!1J ~'rl :::J0lJ+J
tUE Co•.• OJ4- CI)
tU(f).o0) I!lODC -r-lro U.c roU >-..(f) ·rlOJ OJD.c-r+ +JXo 0
CI)OJ
.cD+J 0
-r--i4- >-..o OJ
0.onC OJ·rl .cD +JCo CI).0 CI)
oD >-.. UC U -r-iro ro D
-r-iOJ >-.. U•.• ro tU::Jri
+J :::J 0U U +J:J OJ>-.. ri U4-l 0 'rlUJ E CI)
<UOJ OJ .0.c r-t+J 0. EE 0CI)'rl >-..c:( CI) 4-
UE ·ri:::J, U
.c 'rl CI) -r-i+J C OJ U t\(J'rl'rl CI) m C3 E ro 0 'rl
:J.o UJri+JCri ritUU
-r-t <. D CD U roC -r-i OJ
+J tUCI)o.~-r-i -r-i »4- CI) UJ +J,
OJ U OJ Uo U'rl D tU'rlD C U'rl D
OJ ro x UJ 'rlUJ'" 0 ro UOJ QJ .c ro
D4-+J~ 0lJ-r-i 4- 0 > C riX -r-l .0 H -r-i rio D ~ > m
i: C mcYJ»+JO.cOJ
+J -r+ U OJ •.•D'r! 3 'r! .0 ro (f)
o > r-t C'rl'r! (f) 'r! (f) C 0•.• +J+J(f)·ri O'r!OJrou riU+J
0... 0lJ ro ro'rl:::JOJ OJ '-"'-riri
4- C >-.. OJ r-t -r-i 0o 0 0. m CI) CI)•.• ,»UJ4-l "'+J.cD UCUOJ +JC'rlOOJ+JD'rlOD
'r! ri ro C 3 »'rl+J OJ 3 0 OJ UU .oCl).ororo E C +JOJ 0 'rl OJ U UJ OJ•.••.• +J m 0) >
4- OJ m OJ D -r-iU '--<'rl H 'r! b.(OJD.oD x>OJ:::JOJ+JOO"'+J.--<E::J +JOJUO •.• 'O+JCI)'r! CI) 0) C •.•.oUC+J"'QJOJo OJ -r-i C OJ r-t +J
•.• -r-i +J ro roOJo..c ro>3.c eo (f) 3 0+JOJ::J4-l u.c
O. 0 'r! C +J, ».c -r-i OJ 'r!
•....• +J+JbD £3ro riCOJI-•.•D ro -r+ ri »OJC +J.o riCO,U::J·+JOJ .0 OJ OJ r-t OJ U0lJ DriOUOJ
OJ 'rl 4- UJ 'rl •.•C£XOJCX'rlH 4-l 0 •.•.r! 0 D
c
""
oc.
<0
U
o~ .•w ••.......•x c
o orz
.0.0•• U
.•.•.0•• 3>
o '" 0
~ E
" ..•• '>-.. ~......• 0 0.
'"'"> '" muI-+--+--+--+-+--I ~;
o'" ....•.~ o,
-o 0j ;;1--+--+--+---+---!----1 ~~
0.
~jI----t-Jl-+_"'_t-"'_I--"'-----;I----1
0 ii
o '"u > c-c
ooc,
..o
j-o
o •.<D U1 >
oc,
o• 0m til
c"o
III illo 0
III III IIIro •....• 0Z <I:: (f)
UC+Jm C CI)
0) roIIIr-t £ III III
-r-i -e+ 'r! 0 ro 0 0-r-i 'ri III> III III (II
·ri ro a 0 m tUZ U (f) Z Z
.'>-
,,'"10 eo til 0
U'O .oOl<, c, Ql '"'
U ~
U 10 0 s: .•..•'------'-U1---L_U_~--L__'""_c--L'-'---__L.:<D=__.J f-- 0
OJri.0roI-
.0
C'r!
0-ro
»riOJ+J OJrori•.• .0OJ ::JD •....•o 0E CI)
D »C •....•ro+J
£•.• 0lJOJ'r!+J r-tro CI)3 »D Hri OJo >U
c.~ ~-r-i N ,-..
0-m
IIIo
IIII
+
0-tU
IoroZN
£»U :r:
•.••.•• r-i 0+J £
!lO i: 3on
III -r-i 'o .....•OJCI) D
-r-l
UJ X+J 0U HroD
o QJ» 0III !-. £ III
I IN
+
oIII
:r:N
+
OJ OJD£-r-i +JXo E•..E 0:::J4-
-r-l(f) 0
III OJ 4-lo CIII bO +J
I CD 0N :L s:
~III
oIII
roZ
0-<U
67
CI)+
~ 0lJ 0o III III~ 0
0... [f)
+
4-lC •.•
OJ ro robO'r! •....•C 0lJ :Jro U£ m OJU ri
.c 0UJ 0lJ EOJ ::JD 0 +J-r-l H Cx s: OJO+J •....•
mOJ H >£ H 0
U
+0lJ
.....•u
~ +III
I
+
0- I Iro 0 0
I ea r-to :L c:(tUZ
o 0III (II
o I I(II N cYJ
I
+
.0
0lJ ~
III III
I IN cYJ
+
+ +

I U)
c >,OJ N OJ0 ~, D IJ::
-r-i OJ ·rl. blJ-I-'-I-' U) >-. Lm OD C
rl rl >, D mOJ UJ:: CJ::>-'-1-' (jJ -I-'
C-I-' >-.rl OJ 0 OJ I >-.(jJrl CJ:: -r-i OJC (lJ -I-' ui -1 -I-'0 > U) 0 mblJO OJ OJ C OJm U 04-rl OJ r....
-r-i D 0 :::J OJ enD U 3
rl en OJ OJ -I-' -I-'OJ (lJ I bJrl OJ 0
J:: r.... rl C 0 D C-I-'-I-'<C (jJ E
:::J >-. D OJOJ OJ U) -I-' C r....-I-' C m m C m mm OJ OJ~-I ...c >-. Erl en OJ
-1-'-1-' OJ r.... m I U)U) OJ:: 0 > rl OJ 0.:::JD 34- 0 <CJ:: :::Jrl U -I-' 0rl OJ C D r....-r-l r.... U) 0 OJ C - b()
m OJ U rl m r....>, r.... ·rl 0. OJ OJrl en :::J rl E N> Er.... I -I-'·rl -r-i I OJ m(jJiD U U) U'J OJ 3: U)
OJ :::J CO 0rl D >-'DJ:: I OJU C -I-' C -I-' C J::
(lJ U) m ·rl OJ -I-'C 3: OJ0 ~ >-. C 3 OJ CUI (jJ 0 UJ -I-' D. -r-l
-r-i -r-l rl r.... OJ OJ >,rlCf) :::J 0 U) D-I-' U)-r-l UD m I OJlfl OJ en lflDD
• rl OJ OJ C -r-l
D L~ 0 r.... rl ·rl 0 r....C -I-' E 0 m -I-'DDm C E
r.... -r-i >,OJ OJ r.... -I-' r....DJ::
C Erl OJ :::J m C0 OJ o.J:: OJ rl m Cr....rl E-I-' c 'rl OJ0 OJ ·rl >-. E >-. OJ
D UJ :::J OJ ·rl OJ 3:OJ l.L r.... lfl-l-'+'
4-J::J:: (jJ' U OJo+'+' OJ (jJD
-r-l • J:: E >-.lfl C 3: en U 0 m UJ
a: OJ OJ I -r-t lflJ:: OJN D OJ lfliDJ:: U -r-i
-r-i 3: OJ 3: OJ +'OJ >-.+' U OJ r.... U 'rllfl D OJ Crl4- (jJ -r-i r....
-r-i >,D (jJD 0 UJ mU J:: +' E OJ (jJrlr.... o.lfl OJ +' r.... D'rlOJ OJ'rl D U) UJ OJ EX J:: J:: :::J OJ 0 J:: C ·rl
W f- UJ UJr.... E f- -r-i UJ
C D OJ 4-
D >-. -r-l OJ > 0C 0 N rn OJm 4- D + 'rl J:: UJ J::
D 0. 0. D J:: OJ I r.... D +'N +' G:
E I ~ 'rl E +' UJ m UJ 0 0J:: ~ -c 0 0 >,4- rl :::J UJ J:: UJ
UJ on rn rl 0 0 J:: rl +' (jJ
D ·rl 0 UJ o.f- (jJ OJ E0 r.... r..... OJ r.... E·rl ~ c, +' U 0 OJ >, C>-. 0
~ UJ U C +'r.... OJ +'
OJ +' c, c ~ ru C OJ OJ 0. 0 C J:: C <r0... m .>'D Qru ~ 0 rl UJ OJ 4- 0 +' OJ Ln
+' IE ~ >
I o uE ~
0. > 'rl OJ D U OJ -r-ir.... c-'
C 4- ~ Ul E 0 Ul E0.
(jJ U r.... UJ OJ cico 3 m u u-r-l OJ I C m OJ +(jJ OJ 4-
rl I OJ OJ J:: ·rl 4-
UJ .c J:: C +' Z +' 'r-i +'+' E ~ ~ D 3: -I-' 0 >-. D lflC 0 C +' lfl OJ OJ ·rlOJ >-. ui ~ c c ((j OJ b.O 0 -r-i J:: 0. >,rl
E 4- ~ u ~ f :;;'~Qru D C >-. +' 0 DOJ I
E ~ > >I
0 E ~ > >+((j
0 ·rl 0. +' r.... UJ
rl U 0 ITl Ul E0.
U ci Ul E U C E H >, 0. C .Y
OJ ·rl Z m r.... UJ D OJ 0D ·rl (lJ U > 0
OJ ·rl UJ OJ 4- +' -r-i -r-i 1..l
J:: U CJ:: C +' +' C C b.O
+' m -r-i +' 0 U U OJ 0 OJ
~ c ...., ~ m U m 0- OJ +' -r-l OJ E4- 0 m
C ~m m +' +' m 0. X
r.... 0 UJQ)"'"0 -I-'
I ird > > Iif> Q) u:;~
> C (jJ U J:: 'I X OJ rl m Cf) OJu 0 Z U U Ul 0 W C
0.
U 0 -r-i U OJ m ·rllfl rl U C UJ ·rl I
r.... U .: ·rl rlOJ ((j OJ (jJ J:: 0 +' OJ -r-l -r-l 0.
D >-. I b.OD 3: J:: tn 0. OJ D UJ 0.-r-i +' m 0 + en lfl >,+' m OJ (jJ>-. :::J Z >-. 0 tn ·rl OJ +' U
r.... UD OJ ~ m ~ rn c ~ D UJ C E rl m C rl» C
~E 'rl » rl 0 b.O OJ >-. U m rl
J::I c
E ~ > > I c, if> m ...., :! u~ • J:: m ·rl OJ m > (jJ ·rl +' 'rl
J:: ci Ul E0.
U '" 0
0. o 'rl mUJ N C m J:: C UJ -I-'
ITl Z U Z.D "'~ ITlOJ eo C OJ OJ I I 0 0 J:: U 0 -r-i UJ
J:: :::J 0 U r....I +' ·rl D+' 0 ·rl :::J ro C lfl +' +'
r.... D ,+- m D OJ C 4- r.... C4- J:: I 0 >, 0 J:: C U OJ 0 m OJ0 +'
~~ >,Q) I r.... OJ +' m C rl OJ ED D o.J:: OJ .n m lfl rl :::J
OJ U Im
l- Im 'rl ~
'rl > ~ D f- U r.... C +' > OJ U on>-. 'rl a] 0 >: ITl
no ci m 0.C UJ C OJ 0 UJ 0 :::J :::J r....
ITl >: a] "'~ L U ITl
D:::J If) ((j OJ OJ 0 b.O·rl D U rl C m-I-' m U cD UJ N
r.... :::J ((j r....OJ m D ':' C OJ rn UJ ,+- > OJ >-.:::J C .0 ·rl m +' r.... rl ·rl 0 +' :::JC E ~ -1 -I-' m 0. + -1 OJ +' C 0
-r-l OJ 0 lfl +' lfl UJ OJ C ·rl+' UJ r.... lfl
.~
ru U· UJ D U) OJ -r-i rl OJ OJ OJ +'C ((j 4- D I
m~ C
0.I ": m 'rl 0.
0. C C :::J J:: 0- rl J:: >-. r.... E :::J0 D 0 ~ 'rl 0. m 0. 0. 0.
·rl UJ C +' m C m +' b.O OJ 0 DU ITl ""rl ""rl
I UJ 'rl <1J OJ 'I 0 E OJ 4- r....
D OJ r.... +-' OJ +-'D ·rl UJ J:: D 4- 4-I·rl en OJ ~ C lfl rl C I b.O 'rl
CD U C 0. I'- 0 OJ 0 <1J I OJ :::J J:: D C IN ((j ((j N ~ >
~> U s: E I .c 0 en 0
J:: J:: -I-' U) +' J:: 'rl -I-' -r-i 4-OJ OJ U +-' OJ I'- m m I OJ C OJ +-' J:: m -I-' 0U) J:: 0 U) D
no no'rl 4-J:: 0 J:: .>, rl J:: ((j
-r-l f- 0l D ·rl C I- no -1 0-1-' -r-l f- D <1J ((j -I-' rl U)U U m OJ D c, c !! ~ c :::J :::J>-. >-. rl s: D OJ U 'rlOJ OJ c;o D ru
0.+-' rl D
X ~ X ((j 1; c C
0.
~ :s 0 m <1JW W f- a] a]
Z c r....
68

CONTRIBUTORS
Project Director
Colin Robertson, Inspector for Science, ILEA
Writing team
The materials were written and revised by practising teachers seconded to theProject for limited periods:
Lambros AtteshlisLesley BulmanMike FoleyAnn FriendLawrence HalsteadTerence Kelly
Prod uction Team
Tony LanghamVanda ChanJohn SangwinPeter FaldonDawn DevereuxConstance Godfrey:Stella Jefferies
Frank McManusLeonard RoselaarFran RoweAlec ThompsonSteve Waxman
i/c production and cover designGraphicsAVA TechnicianAVA TechnicianOffice and typingTypingTyping and layout
Videotapes
Brian Babb, Producer, Educational Television Centre, ILEA
Reader
John Stephens, Department of Natural Sciences, South London College
Evaluator
John Gilbert, Institute for Educational Technology, University of Surrey
ILPAC trial schools
The following schools and colleges took part in the trials of the IndependentLearning Project for Advanced Chemistry. The Inner London Education Authoritywishes to thank the teachers in these schools and their students for their help.
Abbey Wood SchoolAcland Burghley SchoolBacon's C.E. SchoolBrooke House SchoolDunraven SchoolElliott SchoolEltham Hill SchoolEnsham SchoolForest Hill SchoolHighbury Grove SchoolHull College of Further EducationHydeburn School.
John Roan SchoolLadbroke SchoolLondon Nautical SchoolMorpeth SchoolNorth Westminster Community SchoolQuintin Kynaston SchoolSt. Mark's C.E. SchoolSydenham SchoolThomas Calton SchoolWalsingham SchoolWoodberry Down SchoolWoolverstone Hall

© ILEA o 7195 4051 8