SARCOPLASMK RETICULUM CALCIUM ATPASE GENE … · 2020. 4. 7. · Abstract SARCOPLASMIC RETICULUM...
Transcript of SARCOPLASMK RETICULUM CALCIUM ATPASE GENE … · 2020. 4. 7. · Abstract SARCOPLASMIC RETICULUM...

SARCOPLASMK RETICULUM CALCIUM ATPASE (SRCA) GENE EXPRESSION IN MYOCARDIAL BIOPSIES IN
DILATED CARDIOMYOPATHY AND SUSPECTED MYOCARDITIS:
MOLECULAR/PHYSIOLOGIC CORRELATION
Hui Mei Yang
A thesis submitted in conformity with the requKements for the degree of Master of Science
Graduate Department of The Institute of Medical Science University of Toronto
O Copyright by Hui Mei Yang 1998

National Li'brary u*d of-& BibiiiWque nationde du Canada
Acquisitions and Acquisitions et Bibiiographic Services seMces bibliographiques
395 Wellington Sireet 395. rue Wellington OtEawaON K1AON4 Ottawa ON K1A ON4 Canada Canada
Y a P & volt.rliYlries
Our NP NDbe rliiVnncs
The author has pmted a non- L'auteur a accordé une licence non exclusive licence allowing the exclusive permettant à la National Library of Canada to Bibliothèque natiode du Canada de reproduce, loan, distribute or sell reproduire, prêter, distribuer ou copies of this thesis in microform, vendre des copies de cette thèse sous paper or electronic formats. la forme de r n i c r ~ c h e / ~ de
reproduction sur papier ou sur format électronique.
The author retains ownership of the L'auteur conserve la propriété du copyright in this thesis. Neither the droit d'auteur qui protège cette thèse. thesis nor substantial extracts fiom it Ni la thèse ni des extraits substantiels may be printed or othefwise de celle-ci ne doivent être imprimés reproduced without the author's ou autrement reproduits sans son permission. autorisation.

Abstract
SARCOPLASMIC RETICULUM CALCIUM ATPASE (SRCA) GENE EXPRESSION IN MYOCARDIAL BIOPSIES IN DILATED CARDIOMYOPATHY AND SUSPECTED MYOCARDITIS: MOLECULAR/PHYSIOLOGIC CORRELATION Master of Science, 1998, Hui Mei Yang, The Institute of Medicd Science, University of Toronto.
In cardiac muscle, calcium uptake by the sarcoplasmic reticulum ca2+ -ATPase (SRCA) is
primarily responsible for catdiac relaxation. SRCA rnRNA has been shown to be decreased
in heart failure. To defuie the contribution of gene expression to hemodynamic status in
living patients, a pol yrnerase c hain reaction (PCR) based quantitative technique to assess
SRCA mRNA was developed Right ventricular endomyocardial biopsies were obtained
nom 11 patients with dilated cardiomyopathy and 1 1 patients wîth suspected myocarditis
and total RNA isolated. Intemal control RNA was transcribed in vitro from a synthetic
DNA containing the SRCA sequences and coamplified with target RNA in the reaction. The
mRNA levels of SRCA were correlated with simultaneous hemodynamics. Steady state
mRNA levels of SRCA of the biopsies range from 9.6 x 106 to 1.51 1 x 10' molecules 110
ng total RNA and did not correlate with left heart hemodynamics (LVEDP, Tau, dPldt and
capillary wedge pressure). In conuast, SRCA rnRNA positively correlate with right hem
pressures including pulmonary artery pressure and right ventncular systolic pressure.

Acknowledgments
This work could not have been hished without the help and support ofmany individuals.
1 would like to thank Dr. Tom Parker, my supervisor, for his excellent guidance, time and patience, encouragement, hancial support and for providing a stimulating working environment.
To Dr. Michael Sole and Dr. Peter Li y my thesis committee members, for their tirne and patience, scientik expertise and valuable comments.
To The Center for Cardiovascular Research, The Toronto Hospital and the University of Toronto for financial support.
To Dr. John Parker for generously providing the human cardiac biopsy samples and Dr. Jose Azavedo for delivering human hemodynamic data.
To Tammy Martino for providing human cardiac total RNA; and Lily Wee for her assistance in carrying out Northern blot experiments.
To Dr. J i m Tsopons for his helpful advice in statistics and Eendship; to Chris McMahon and Graham Slaughter for their technical support and friendship; to Linda Kozak for her administrative assistance and fiendship.
To Jack Liew, Dr. MinShun Zhao, Dr. Weei-Yuam Huang, Amy Hao Ly and Kem Thai for their technical advice and fnendship.
To Anne Schofield and Monica Diana for their assistance in delivering cardiac biopsies and pathological iriromiation.
To my father for his encouragement.
Finally, I would like to thank my husband James for his constant support, inspiration and understanding, and to my daughter Victoria and my son Victor for bringing me the joy and happiness of life.

List of Abbreviations
Aldo: Aldosterone
a - M H C : Alpha myosin heavy chak
ANF: Atriai naûiuretic factor
Ang II: Angio temin II
AP: Aorta pressure
BNF: Brain natriuretic factor
f3-MHC: Beta myosin heavy chah
cDNA: Complementary DNA
Cm: Congestive Heart Failme
CI: Cardiac mdex
CO: Cardiac output
CV: Coefficient variation
DNA: Deoxyribonucleic acid
dP/dt: F k t derivative of left ventricular pressure by t h e
EDTA: Ethyienediaminetetraacetic acid
FGF: Fibmbiast growth factor
GAPDH: Glyceradehyde-3-phosphate-dehydrogenase
GTP: Guanosine triphosphate

LVEDF: Left ventncular end diastolic pressure
M-MLV RT: Moloney Mirrine Leukemia Virus Reverse Uanscnptase
mRNA:
MMP 1 :
m:
PAdia:
P Amean:
P M :
PAsys:
PAWP:
PCR:
PKLB:
RA:
RAAS:
RNA:
RNase:
RT:
RV:
RVP:
RVsys:
RYR:
Sm:
Messenger ribormcleic acid
Matrix metalloprotemase 1
New York Heart Association
Pulmomuy artexy diastolic pressure
Pulmanary artery mean pressure
Pulmonaly artenai pressure
Rilmonary artery systolic pressure
Puimonary artery wedge pressure
Polymerase Chain Reaction
Phospholamban
Right atrial pressure
Renin-angio tensm-aldosterone sys tem
RibonucIeic acid
Eüinuclease
Reverse transcription
Right ventricle
Right ventricular pressure
Right ventrïcular systolic pressure
Ryanodine receptor
Sodium dodecyl sulfare

SERCA
SHR:
SL:
SR
SSPE:
TAE:
Tau:
TBE:
TGFB:
Sarcoplssmic reticuhrm dcim ATPase gene
Spontaneously hypertensive rat
sarcolemma
Sarcoplasmic retidum
Sodium chloride / sodium phosphate/ EDTA
Tris-acetate / EDTA
Time constant of left ventricular ceiaxatim
Tns-borate / EDTA
Type B tra~~sfomiing growth factor

Table of Contents
. . ........................................................................................................................... Abstract ........... .. 11
... ........................................................................................................................... Acknowledgments 111
. . ...................................................................................................................... List of Abbreviations iv
.................................................................................................................................. List of Tables .xi
. . ................................................................................................................................. List of Figures xi1
....................................................................................................... Chapter 1 : Introduction 1
.................................................................................................... ..................... 1 . 1 Background .. 1
1 .1.1 Cardiac failure .............................................................................................................. 1
1 -1 -2 Systolic and diastolic dysfunction of the heart .......................... .... ........................... 2
............................................................................... 1 . 1. 3 Adaptivc changes in heart failure 8
1.1.3.1 Cardiac hypertrophy .................................................................................... 8
............................. 1.1.3.2 Myocardial collagen matrix remodelling in heart failure 10
1.1.4 Contractile pro teins and gene expression in h y p e ~ p h i e d and failing heart .......... -12
..... 1.1 -5 Signal transduction, growth factors, pro to-oncogenes and cardiac hypertrophy 17
1.1.6 Subcellular basis of calcium movement and relaxation in myocardium ..................... 21
...................................................................... 1 . 1.7 SR caZ' transport proteins and genes 25
1.1.8 SR c~ '+-ATP~s~ gene expression during cardiac muscle development ..................... 26
............. 1.1.9 SR kc t i on and gene expression in cardiac hypertrophy and heart failure 28

1.1.9.1 Animal models of cardiac hypertrophy ..................................................... 28
1 . l . 9.1 .a Thyroid hormone-induced cardiac hypertrophy ........................ 28
1 . f .9. 1. b . Volume/pressure overload-induced cardiac hypertrophy ......... 30
.................... 1 . i . 9.1 . c. Hypertrophy in spontaneously hypertensive rat 3 2
1.1.9.2 Animal models o f heart fisilure .................................................................. 32
1.1 .9.2. a. Heart faiIure in hereditary cardiomyopathie syrian hamster ...... 32
. . ....................... 1 1.9 .2 b Heart failure by c hronic rapid ventncular pacing 33
1.1 .9.2.c Heart failme induced by dnigs .............................................. ...... 34
Cardiac gene expression during transition from compensated ....................................................................... hypertrophy to heart fdur 34
1.1.9.4 Human hypertrop hic cardiomy opathy and heart failure ........................... 36
Alterations of gene expression in animai models of acute myocardial ................................................................................................................ infarc tion 38
. . . ......................................................... ........................ Lirmtations of available human data ..,.,. -39
............................................................................................................................. Hypotheses 40
. . .................................................................................................. Aims and specific objectives 41
............................................................................................................... Chapter 2: Methods 43
2.1 Introduction of the quantitative approach used in this study .................................................. 43
2.2 H m heart biopsy sarnples ................................................................................................... 45
.................................................................................................. 2.3 Total cellular RNA extraction 45
.................................................................... 2.4 Synthetic Intend Standard DN A preparation -46
................................................................. 2.5 Subcloning of the synthetic DNA into pBluescript 47

. . ..................................................................................................... ....... Vitro transcription ... 49
. . 2.7 Removal of the DNA template foilowing in vitro transcnphol~ ............................................ 50
2.8 The intemal standard ............................................................................................................... -50
2.1 0 PCR amplincati0 il ................................................................................................................. 53
2.1 1 The specificity of the SR ca2+-~~pase primers ................................................................... 55
2.12 Determination of the ratio of sample RNA and intenial control RNA used for cDNA synthesis .............................................................................................................. 56
................................................................................ 2.13 Detennination of the exponential phase 56
2.15 Quantitative analysis of SR c~?' -ATP~s~ mRNA levels ................................
2.1 7 Northem blo t analysis of SR C ~ ~ ' - A T P ~ S ~ in rats heart . . ............................................. .................................................. after myocarchal infarc tion ..... 65
................................................................................................................ Chapter 3: Results 67
3.1 Characterization of group 1 patients with suspected myocarditis .................................... A 7
3.2 Vanability of SR ca2+-~TPase mRNA levels in duplicate biopsy samples .......................... 70
3.3 Correlation between SR ca2+-~TPase mRNA levels and clinical parameters h m ..................................................................... patients with suspected myocarditis (group 1) -70
3.4 Characterization of group 2 patients with dilated cardiomyopathy .................... ... ........... -79
3.5 Correlations between expression levels of mRNA for the SR ca2+-~TEkse and clinical parameters from patients wi th dilated cardiomyopathy ( g~oup 2 patients) .......... ... 82

3.6 Correlations between clinical parameters of group 2 patients and SR C ~ ~ + - A T P ~ S ~ mRNA levels nonnalized with GAPDH .............. .., ........................................................... 87
3 -7 Comlation between SR ca2+-~'Tpase mRNA levels and clinical parameters h m combined group 1 and group 2 patients .............. ... ............................................................ 91
3.8 SR ca2+-ATE'ase expression in infarc ted rat hearts ................................................................ 97
Chapter 4: Discussion ............................................................................. -99
4.1 The development of the RT-PCR quantitative technique . technical aspects ...................... ..99
4.2 Variability of SR ca2'-A~pase mRNA from duplicate biopsies ...................................... 102
4.3 Alterations in SR ~a~+-A'T'~ase gene expression in human heart disease . . ........................................................................................................................ O new mights 103
.................................................................................................... 4.4 Significance of this study 112
.............................................................................................. References 114

List of Tables
Table 1: Oligonucleotides of 5' primers and 3' pnmers for PCR of 3 cardiac genes ..................... 54
Table 2: Characterization of patients with suspected myocarditis (group 1, included in correlation analysis), their hemodynamic data and mRNA levels of
2+ SR Ca -ATPase.. ...................................................................................................... ..68
Table 3: Histologie findings fiom group 1 patients. ............... .. ................................................. ..69
Table 4: SR ca2' -~TPase mRNA levels in 5 duplicates of group 1 patients. .......... .... ...- -.7 1
Table 5: Characterization of patients with dilated cardiomyopathy (group 2), their hernodynamic data and mRNA levels of SR ca2+ ATPase. ........................................... 80
Table 6: Histologie hdings from group 2 patients .............................................................. 8 1
Table 7: SR ca2' -ATPase mRNA showed positive correlation with right sided cardiac pressures fkom patients of suspected rnyocarditis and dilated
.......................................................................................... cardiomyopathy. .... ..... .86

List of Figures
Figure 1: Micromanometer recordmgs of left ventricular pressure and its . . first denvative. dP/dt ................................................................... .... ................................ 5
Figure 2: Diagrammatic representation of ventricular diastolic pressure-volume relations for normal. stiff. and cornpliant ventricles ........................................................ 7
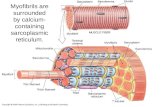
Figure 3: Schernatic presentation of sarcomere structure and the events that produce myocardial excitation-contraction coupling and myocardial relaxation ........................ 13
Figure 4: Partial schematic presentation of ce11 signaling in response to pressure-overload in the myocardium .................................................................. 1 8
Figure 5: Schematic presentation of calcium fluxes in the myocardium ..................................... -24
Figure 6: Synthesis of intemal standard by overlap extension PCR .......................................... A 8
Figure 7: Diagrammatic representation of quantitative PCR using an interna1 control RNA (CRNA) produced from a synthetic DNA .............................................. 52
Figure 8: Autoradiogram of PCR products as a function of cycle number .................................. 58
Figure 9: Plots of PCR products as a bc t i on of the number of amplification cycles ................ 59
Figure 10: Autoradiogram demonstrating quantitative PCR products ......................................... 62
Figure 11 : Quantitative PCR pmduc ts from Molecular Image System ....................................... 63
Figure 12: Quantitative analysis of SR ca2'-ATPase mRNA level in an . . endomyocardial biopsy sarnple ............. .... .......................................................... 64
Figure 13: SR cap -ATPase mRNA levels positively correlate with puimonary artery systolic pressure From patients with suspected myocarditis ........................... 73
Figure 14: SR ca2' -ATPase mRNA levels positively correlate with pulmonary artery diastolic pressure fkom patients with suspected myocarditis .......................... 74
Figure 15: SR ca2' -ATPase mRNA levels positively correlate with pulmonary artery mean pressure from patients with suspected myocarditis ............................... 75
xii

Figure 16: SR ca2& -ATPase mRNA levels positively correlate with right ventricular systolic pressure From patients with suspected myocarditis ..................................... 76
Figure 17: SR ca2+ -ATPase mRNA levels negatively correlate with cardiac output from patients with suspected myocarditis. ............................................................... ..77
Figure 18: SR ca2+ -ATPase mRNA levels negatively correlate with aortic pressure * . fiom patients with suspected myocarditis. ............................................................... ..78
Figurel9: SR ca2'-ATPase mRNA levels positively correlate with nght ventricular systolic pressure from patients with dilateci cardiomyopathy ................................... 83
Figure 20: SR ca2' -ATPase mRNA levels positively correlate with pdmonary artery diastolic pressure fkom patients with dilated cardiomyopathy ........................ 84
Figure 21: SR ca2' -ATPase mRNA levels positively correlate with pulmonary artery mean pressure from patients with dilated cardiomyopathy ............................. 85
Figure 22: SR caZ' -ATPase mRNA levels when nomalized with GAPDH positively correlate with right ventricular systolic pressure from patients with dilated cardiomyopathy ...................................................................... - 3 8
Figure 23: SR ~ a " -ATPase mRNA levels when nomalized with GAPDH positively correlate with pulmonary artery diastolic pressure from patients with dilated cardiomyopathy.. ...................................................................... 89
Figure 24: SR caZ' -ATPase mRNA Levels when nomlized with GAPDH positively correlate with pulmonary artery mean pressure h m
.................................................. patients with dilated cardiomyopathy ................ .. 90
Figure 25: SR caZ'-ATPase mRNA levels positively correlate with right ventricular systolic pressure fiom combined patients of
............................................... suspected myocarditis and dilated cardiomyopathy.. ..92
Figure 26: SR caZ4 -Anase mRNA levels positively correlate with right ventricular diastolic pressure h m combined patients of
............................................... suspected myocarditis and dilated cardiomyopathy ..93
Figure 27: SR ~ a " -ATPase mRNA levels positively correlate with pulmonary artery systolic pressure fiom combined patients of suspected myocarditis
.................................................................................... and dilated cardiomyopathy.. ..94
xiii

Figure 28: SR ca2' -ATPase rnRNA levels positively correlate with pulmomry artery diastolic pressure From combined patients of suspected myocarditis and dilated cardiomyopathy.. .................................................................................... ..95
Figure 29: SR ca2+ -ATPase mRNA levels positively correlate with pulmonary artery mean pressure h m combined patients of suspec ted myocarditis
................................................................................... and dilated cardiomyopathy.. ..96
Figure 30: Northern Blot and ethidium bromide staining of SR ~a~'-ATPase RNA in .................................................................................................... inf'arcted rat heart.. .-98
Figure 31: Schernatic presentation of proposed mode1 of SR ca2' -ATPase mRNA levels in .............. cardiac hypertrophy and failure .. .......................................................... 109
xiv

Chapter 1 : Introduction
1.1 Background
1.1.1 Cardiac failure
As the result of the improved standard of living and quality of life, the Life expectancy has
increased greatly during the past 20 years. Yet cardiovascular disease continues to be the most
serious threat to life and health in the developed world. Heart disease is the leading cause of death
followed by cancer and cerebrovascular diseases in that order. Heart failure is the end stage
consequence of a wide variety of heart diseases, notably hypertensive, coronary, rheumatic, and
congenital heart disease. Two million or more Americans (and by extrapolation 200,000
Canadians) have congestive heart failure (CHF), and the 400,000 new cases that occur yearly
require over 900,000 hospitalization each year (Kannel et al 1 99 1 & Ho et al 1 993). B ecause
cardiovascular disease accounts for nearly one-half of North Amenca's mortality and much of the
continent's morbidity, its cost to the economy is by Far the largest for any diagnostic group, an
estimated $1 10 billion in 1 984 ( Kannel et al 1986). Based on years of cardiovascular data
compiled in Framingharn, Mass, there appears to be a 1 % prevdence of C HF in individuals aged
50 to 59 years. The incidence of CHF increases with advancing age to approximately 10% of
people aged 80 to 89 years (McKee et al 1971). With an increasing geriatnc population, cardiac
failure is becoming a formidable problem. The prognosis of patients presenting with kart failure
I

is generally poor; and in several series 50% of symptomatic patients died within 12 months
(Packer 1987), a mortality in excess of common solid organ tumours. Sudden death, presumably
from ventricular arrhythmia, and progressive failure are the common modes of death. Despite the
availability of a variaty of phannacologic agents encompassing glycosides, diuretics, adrenergic
blokers, angiotensin converting enzyme mhibitors and direct acting vasodilating agents, as well as
surgical approaches such as volume reduction and transplantation, the population of patients
with CHF is continuing to increase ( Kannel et al 1994 & Cahalin 1996) and the overall impact on
rnortality has been modest. Preventive programs require early detection, treaûneuts which do not
potentiate or induce arrhythmia and are not hampered by a kequent lack of symptoms during the
early deveiopment of disease. Thus, preventive management must be employed before the heart
has exhausted its reserve and compensatory mechanisms (Kannel et al 1986). Unfortunately, our
understanding of the fundamental biology contributing to the progression of disease remains
relatively nidimentary, imposing m e r limitations on early therapeutic interventions.
1.1.2 Systolic and diastoüc dysfunction of the kart
Cardiac performance is dependent on both appropriate systolic and diastolic funchon.
With a few exceptions, heart failure is a low cardiac output syndrome characterized by systolic
myocardial dys funchon, diastolic dysfunction or both. Thus heart failure exists when the hart
fails in one or both of its primary fûnctions: 1) during systole, to propel blood into the great
vessels under increased pressure and 2) during diastole, to receive blood into the cardiac ventricles

at low pressure (Grossman 1990).
Systolic dysfunction is detined in tems of insufficiency of cardiac output relative to the
metabolic needs of the body. In the most common forms of cardiac failure (Le. following
myocardial infarction), impairment of systolic function dominates the clinical presentation. The
velocity and extent of ventricular contraction and the rate of pressure developmeat are decreased
in hart failure (Gault et al 1968, Harnby et al 1970 & Field et ai 1973). Systolic function of the
myocardium is a reflection of the interaction of' myocardial preload, afierload, and contractility
(reviewed in Grossrnan 1 986). Preload is the load which stretches myo fibrils during diastole and
determines the end-diastolic sarcomere length. For the left ventricle (LV), this load is o f h
measured as the leR ventricular end-diastolic pressure (LVEDP). Increased preload enhances the
extent and velocity of myocardial shortening. Thus, afterload is also uicreased, and this mcrease
will lessen the increases in extent and velocity of myocardial shortening due to increased diastolic
fiber stretch. Mterload is the force resisting systolic shortenhg of the myofibrils varing
throughout systole as the ventricular systolic pressure nses and blood is ejected fiom the
ventricular chamber. LV systolic stress approximates the force resisting myocardial fiber
shortenhg within the wall of the ventricle (Grossrnan 1986). An inmase in end-systolic wali
stress will result in a decrease in myocardial fiber shortening. For the intact veniricle, an increase
in afterload (end-systolic wall stress) will result in a fa11 in stroke volume and ejection fiaction.
Contractility is the level of activation of cross bridge cyclhg of the heart muscle sarcomere which
accounts for alterations on performance induced by biochemical and hormonal changes.
Since it was fint passed into the human body in 1929, cardiac catheterization has brought

an enormous reservoir of physiologic and anatornic knowledgs of heart disease; it has made it
possible to evaluate both systolic and diastolic hinction of the myocardium (Grossman 1986).
One of the most widely used measures of myocardial contractility is the maximum rate of nse of
LV systolic pressure, dP/dt. It has been shidied in humaa patients with micromanometer
catheters and found that maximum dP/dt in normal LV ranged h m 84 1 to 1696 fTlfnHg/msec
(Figure 1) (Gleason and Braunwald 1962). Exercise, infusion of riorepinephrine, isoproterenol or
atropine caused increase in dP/dt, felt to parallel changes in intrinsic muscle contractility (Gleason
and Braunwald 1962 & Bowditch 187 1). Extensive studies have been done to examineci the
influence of changes in afterload , preload and contractility on maximum dP/dt and have shown
that maximum dP/dt rises with increases in afterload and preload, but the changes are smaller than
10% in the physiologic range (Grossman et al 1 972, Wallace et al 1963, Zimpfer et al 198 1,
Broughton et al 1980 & Barnes et al 1979). Thus, peak dP/dt serves as a relative load-
independent measure of rnyocardial contractility, and by extension, systolic performance.
In several clinical senes, heart failure occurs in the absence of measurable impairnent of
systolic function and results From disordered diastolic filling. Diastolic heart failure is
characterized by increased resistance to diastolic füling of one or both cardiac ventricles. The lefi
ventricular (LV) relaxation rates, assessed by maximum rates of LV pressure deche (-dP/dt), and
the mean velocity of circderential fiber length shortenhg in early diastole are also decreased
(Grossman et al 1979). Physiologically, LV diastolic hinction is summated in the relation
between LV pressure and volume (PV) during diastole ( Grossman 1986). An upward shift in the
diastolic PV relation is regarded as increased LV diastolic chamber s t f iess and a downward shiR

Figure 1. Microminometer recordhm of kft veatricalrr pressure and ib f i rst derivative, dP/dt.
A A patient with noCm81 Ieft venîxicdar bction. Isoproterd markedy inmeases wntractüity with large increments m positive dP/dt. Atropine produces tachycardia, which results in a treppe effect and a rise in positive dP/dt above controI ( Gleason & Braunwaid 1962).
B. Methoxamine raïses arterid and LV systolic pressure, but does not ïncre85e positive dPldt. In contrast, h e combineci a and f5 adrenergic e f k t s of norepinephrine increase anth LV systoiic pressure and +dP/dt.

indicates decreased stifiess or increased LV diastolic c h b e r cornpliance (Figure 2). One of the
simplest ways of quantimg the time course of LV pressure decline is to masure the maximum
rate of pressure fall, peak -dEVdt. However, because of the load dependency of peak -dP/dt,
other indices including the time constant of LV isovolumic relaxation have been introduced. The
time constant of LV isovolumic relaxation ( T or tau) was fiat calculated by the equation (Weiss
et al 1976) :
p = A t t B
where P is the LV isovolumic pressure decline, t is the t h e after peak negative dPldt and A and
B are constants. This c m also be expressed as:
l n P = A t + B
A plot of In LV pressure versus t h e allows calculation of the slope A, a negative nurnber whose
units are sec -' . The time constant tau or T of isovolumic pressure fa11 is then defined as -UA,
expressed in milliseconcis, and is the t h e that it takes P to decline lle of its value (Grossman
1986). It is worth note that asynchrony of the relaxation process within the ventncular chamber
may result in a prolongation of T, and T is probably not completety independent of loading
conditions. However, the influence of altered loading is relatively small (Grossman 1986).
Therefore, prolonged tau may reflect slow myocardial active relaxation.
Systolic and diastolic dysfunction co-exist and contribute to the clinical presentation of
patients with heart failure, that is impairment of forward cardiac output with elevation of cardiac
filling pressures. Similarly the molecdar basis of impaired systolic and diastolic performance
may be both distinct and inter-related.

NORMAL
COMPLIANT ( t DISTENSIBILITY 1
I
VOLUME *,
Figare 2. Diagrammatic representation of venhieuiar diastoiic pressurtvolume rektions for normai, sbüf, and compliiiit ventricles. The upward or downward displacement changes of the m e are associateci with a change in venmcdar distensibiiity. If the LV diastolic PV plot shiRs upward, the LV chamber has become less distenst'ble; a higher diastolic pressure is required to fl.I or distend the chamber ofits prior volume. Similady, a downward SM in the diastolic PV plot indicates an increase in LV diastolic distensiiility. Modined fiom Grossrnan 1986.

1.13 Adaptive changes in heart fdnre
1.1 3.1 Cardiac hypertrophy
Cardiac hypertrophy refers to augmenteci myocardial mass resulting fiom predominantiy
incresed myocyte volume and is a conservai response to imposition of load on a cardiac chamber,
primady the ventricles. The developrnent of cardiac hypertmphy is a common feature that
normally precedes or accompanies the development of the clinical syndrome of heart failure. The
two most common types of mechanical cardiac stress (overload) are that resulting from an
increased resistance to ventncular emptying of increased afterload ( i-e., aottïc stenosis, systernic
hypertension, etc.), and that resulting fiom an increased preload or increased ventricular filling
(Le., aortic or mitral regurgitation, ventncular septal defkct, myocardiaI infarction, etc.)( Schlant
et al 1986). The basic response of myocardium to an increased afterload (pressure overload) is to
contract more forcefidly but more slowly. By contrast, the ventricle dilates when it is subjected
to an acutely increased preload.
The classic type of cardiac hypertrophy caused by pressure overload is termed concentric
hypertrophy in which there is marked thickening of the lefl ventricular walls (includmg the
ventricular septum), but there is no increase in the size of the lefi ventncular cavity (Schlant et al
1986). It has been suggested that the Uicreased aAerload stimulates myocardial thickening by
replication of sarcomere in parallel (Grosman et al 1983). ui concentric hypertrophy due to
pressure overload there may be special difficulties with the delivery of adequate amounts of

oxygen to the myocardial cells, particuiarly in the endocardium. Some of the factors responsible
for this include the elevated myocardial oxygen requirements and the very high mhamyocardial
pressure, which M e r impairs systolic coronary blood flow ( Vmcent et al 1974, Brazier et al
1975 & Downey et al 1975). An elevated ventricular diastolic pressure, which may be neccessary
to fil1 the hypertrophied ventride, will fiirther impede diastolic coronary blood flow to the
endocardium ( Brazier et al 1974). In addition, the growth of capillaries may be relatively less
than the growth of myocytes, and the dimision distance h m myocardid capillaries to the center
of the hypertrophied myocardial cells may be significantly increased (Honig et al 1974). Overall,
although hypertrophy has the beneficial effect of restoring wali stress toward normal and thereby
improving cardiac performance, the increased muscle mass predisposes the hypertrophied
ventncle to myocardial ischernia Furthemore, a late transition fiom "compensated"
hypertrophy to myocardial failure often occurs although its biochemical and structural basis is
still obscure. This transition to failure is clearly associated with depression of systolic
performance as well as changes in diastolic relaxation and ventricular distensibility (Grossman
1 990).
The ventricular hypertrophy induced by increased left ventrîcular preload (volume
overload) is the development of eccentric hypertrophy in which the venhicular chamber and the
left ventricular wall increase in size proportionately (Schlant et al 1986). It has been suggested
that this type of hypertrophy is produced by a chronic increase in diastolic wall stress and is
associated with the synthesis of additional sarcomeres, predominantly in series (Grossman et al
1975). Since increased preload also inmases systolic wall stress and afterload , some replication

of sarcomeres in parallel also occurs and helps to normalize systolic stress ( Schlant et al 1986).
The marked ventricular dilatation of chrcmic volume loadhg is pmduced by several mechanisais,
including an increase in individual sarcomere length, the synthesis of new sarcomeres in series and
parallel with previous sarcomeres, "slippage" between and within myofibrils and fibers, and the
rearrangement of myocardial fibers along the normal cleavage planes of the ventricle ( Bramwald
et al 1976, Ross et al 197 1, Spotnitz et al 1972, Spotnitz et al 1972, Spotnitz et al 1973, Yoran
et al 1973, Sonnenblick et al 1974, Spotnitz et al 1976, Katz 1965, MacGregor et al 1974,
Grossman et al 1983 & Meerson et al 1972). The signalhg rnechanisms that differentially
contribute to eccentric versus concentric hypertmphy are unknown.
1.1.3.2 Myocardial collagen matrix remodeliing in heart faiiure
While cardiac myocyte growth is a common denominator to left ventncuiar hypertrophy,
the accumulation of fibrillar collagen secondary to fibroblast activation contributes importantly to
myocardial mass and influences performance. Myocardial fibrosis is a diffuse perivascular and
interstitial accumulation of fibrillar collagens within the normal connective tissue structures of the
myocardium. In vivo stuclies have connmied that the growth of myocyte and non-myocyte ceUs
are independent of each other (Briila et al 1990). The hypertrophie remodelling of the
myocardiurn is either a homogeneous or a heterogeneous process, based on whether or not there
is a proportionate or disproportionate growth of the myocardial collagen matrix (Weber
et al 1987). Disproportionate growth of non-myocyte myocardial tissue, particularly the

development of myocardial fibrosis may contribute to progressive diastoiic and /or systolic heart
failure. Thus, trophic factors that promote disproportionate non-myocyte tissue growth or
collagen gene expression will lead to abnormal myocardial structure, representing a feature of
pauiologic hypertrophy with myocardial failure (Brilla et al 1995).
Previous studies have dernonstrated that the trophic factors that mediate cardiac myocyte
and fibroblast growth can be independent of one another. Cardiac myocyte growth appears to be
primady regulated by haernodynarnic stimuli while cardiac fibroblast activation with subsequent
more dependent on humoral regdatory systems, Le. hormones and growth factors (Brilla et al
1995). Several growth factors have been considered as potential growth promoters for cardiac
fibroblasts. Transforming gmwth factor f3 1, platelet-denved growth factor and insulin-like growth
factor 1 are each known to stimulate fibroblast-mediated collagen synthesis (Fine et al 1987 &
Goldstein et al 1989).
The importance of the renin-angiotensin-aldosterone system ( RAAS) in myocardial
collagen ma& remodelling in hart fadure have been studied in several models in the rat ( Brilla
et al 1990). Cardiac fibroblasts express type 1 and LI1 collagen and matrix metalloproteinase 1
(MMPI), the key enzyme for degradation of fibrillar collagem. Collagen synthesis increased
~ i ~ c a n t l y in a dose-dependent rnanner after incubation with either angiotensin II (An@) or
aldosterone (Aldo) compared with untreated control cells in cultured adult cardiac fibmblasts.
This increase in collagen synthesis in AngII-or-Aldo s thulated fibroblasts could be comple tel y
abolished by AngiI-type 1 or mineralocorticoid receptor antagonisis, respectively. In addition,
An@ significantly decreased MMP 1 activity, while Aldo had no eKect on collagen degradation
11

(Brilla et al 1994). These findings suggest that both effectm hormones of the RAAS can d k t l y
lead to collagen accumulation m culhned adult cardiac fibroblasts.
Myocardial diastolic stifiess and contractility are both increased with moderate
myocardial fibrosis; and a rnarked elevation in diastolic stiEmess is associateci with severe
fibrosis, where overali collagen volume hction is increased skfold above controis and accounts
for nearly 25% of the myocardial volume. In the Iate stage of myocardial remodelling, with
progressive myocardial fibrosis due to continued RAAS activation, systolic dysfimction appears
( Weber et al 1990). These structural alterations within the myocardial collagen ma& together
with relevant changes within cardiac myocytes explain in part why a progressive deterioration of
diastolic and uitimately systolic LV function occures that would lead to progressive heart failure
(Brilla et al 1995).
1.1.4 Contractile proteins and gene expression in hypertrophied and faiiing heart
In cardiac as in skeietal muscles, the basic unit of contraction is the sarcomere which is
composed of a diverse set of proteins working together to generate force and contraction. Two
major components of the sarcomere are the thick and thh filaments (Figure 3). Myosin is the
main component of the thick filament of sarcomere. It is a hexameric molecule that composed of
two heavy chains and four light chains. The heavy chin subunits that contam the site for
ATPase activity exist in two isoforms, alpha myosin heavy chain (a-MHC) and beta myosin

F i 3. Schematic presentation of sueomere structure a ~ d the events that produce myoeudiil excitation-conîraction coupling and myocardinl nlaution. Two major components of the sarcomere are the thick and thin filaments. The thick filaments coasist primarily of myosin, while the thui filaments are composed predominantiy of acth, tropomyosin, and the troponin cornplex. Wrili depolarization of the cardiac cell membranes, the Na+ charnels open, followed by the Ca2+ channels. The initial ûanssarcolemmai infi= of Ca2+ triggers the reiease of Ca* from the sarcoplasnic retidum- Ca? 'lm 'uigher concentration then bmds to troponin C which produces codonnationd changes in whole troponin (troponin I- troponin C-troponin T cornpiex) that relieves a troponin I interaction with actin, allowing the uderaaion of actin and myosh to produce contraaion. M: myo* A: achq Tm: tropomyosin; T: troponin T; 1: troponin 1; C: troponin C. Moaed and reproduced f?om Ilconornidis 1996.

heavy chain ($-MHC) (Lompre et al 1990 & Nakal-Ginard et al 1989). The thin filaments of the
sarcomere are composed predominantl y of act in, tropomyosin, and the troponin corn plex. The
thin filament can contain tbree actin isoforrns, a-skeletal actin, a-cardiac actin and a-srnooth
actin ( Lompre et al 1990 & Black et al 1991). Actin-activated myosin ATPase activity generates
force and leads to contraction. These isomyosin and isoactin genes are expressed differently with
ontogeny, aging, and hypertrophy, and this plays a role in the regdation of contraction ( Lompre
et a1 1 990).
Ressure and volume overload produce in the myocyte both qualitative changes,
phenotypic conversions characterized by protein isofom switches and quantitative changes
characterized by modulation of single genes through a mechanogenic transduction the pathways
of which are not fülly elucidated (Geistek-Lowrance et al 1 990, Katz 1990, Schwartz et al 1990
& Tanigawa et al 1990). The qualitative changes involve differential expression of multigene
families of contractile proteins, especially myosin heavy chain and actin.
Al1 situations of pressure overload or of combineci pressure and volume overload activ
the BMHC gene and deactivate the a-MHC one in rodent mudels. Because P-MHC is
predominant in rat fetal ventricies, the induction of BMHC in rat ventricles developed the
concept of reactivation of a fetal program with hemodynarnic overloading. In contrast, the
induction of PMHC by overload in human venûicles is not obvious because human ventricles
contain mainly BMHC under basal conditions. In rodents, the change fiom a-MHC to p-MHC
(or isomyosin V 1, the a-a homodimer, to V3, the homodimer) results in a slower rate of

ATP cycling by myosin, which fully accounts for the slower velocity of contraction of the
hypertrophied fiber. The result is an improved economy of force development ihat has d l y
been considered as adaptative ( Schwartz 1992).
Pressure overload also induces changes in the expression of the a-actin isoforms. In adult
rat, the a-cardiac actin isoform is almost exclusively present. With the onset of pressure-
overload-induced hypertrophy, the a-skeletal isoactin geue in rats is transiently upregulated, and
because it is also active in utero, the a-skeletal isoactin gene represents the second example of a
fetal program reactivation b y hemodynarnic overload (Takahashi et al 1 992, Schwartz et al 1 986
& lzumo et al 1988). It has been show that a-skeletal isoactin expression is associated with
increased contractile function in BALWc m o w hearts (Timothy et al 1994) although it is not
clear how a switch fiom a-cardiac actin to a-skeletal isoacth rnight lead to Functiond alterations
in the myocardium since the two isoactins differ by only four amino acids out of a total of 3 75
(Vandekerckbove and Weber 1979). However, three of the four amho acid diffemces found
between these two protiens occur at the myosin binding site (Sutoh 1982). Thus, it has been
postulated that a-skeletal isoactin can activate the cardiac a-rnyosin heavy chain ATPase
activity to a greater degree than cm a-cardiac actin (Timothy et al 1994), providing an adaptive
augmentation of myocardial contractility.
Interestingly, after imposition of pressure overload in the rat heart, the h e course of
upregulation of BMHC and a-skeietal actin genes is not the same. The amount of BMHC
messenger ribonucleic acid (mRNA) increases in proportion to the extent of hypertrophy and
1s

persists as long as the overload is maintaineci, but a-skeletal actin mRNA returns to control
values (Schwartz et al 1 986 & Inmio et al 1 987 ). Furthmore, during the early stages of cardiac
hypertrophy secondary to pressure overload, skeletal a-actin mRNA is detected earlier than fb
MHC mRNA and skeletal a-actin mRNA is detectable throughout the entire lefi ventncte,
wheras p-MHC mRNA is observed mainly around large coronary arteries and in the inner half of
the lefi ventricular wall ( Schiafio et al 1989). Further extendmg our understanding of alterations
in sarcomere structure, it has also been dernonstrated that smooth muscle a-actin, &
tmpomyosin, and atrial myosin light chams, each found in fetal ventricles, are similady induced
by pressure overload ( Black et al 1991 & h o et al 1988).
In addition to alterations in the sarcomere, the development of compensateci hypertrophy
in rodents has been associated with an increase in the ventricular ievels of the mRNAs encodmg
atrial natnuretic factor (ANF) to levels seen in fetal hearts (Mercadier et al 1989). ANF is one of
the fmt detectable changes in cardiac gene expression in the activation of a pmgram of early gene
expression. Unlike the absence of changes in contractile protein gene expression in human hem
disease, ANF could not be detected in normal ventricle but was abundant in fading human heart
(Feldman et al 199 1). Further studies have shown that in faihg heart, elevated expression level
of ANF negatively correlates with the expression level of SR C~" -ATP~S~ both in humans and in
rats with chronic aortic banding (Arai et al 1993, Takabashi et al 1992 & Feldman et al 1993)
suggesting that elevation of ANF gene expression in the ventricle could be considered as a
molecular marker of human heart failure (Feldmm et al 199 1).

As noted above for transitions in myosin and actin, these genetic alterations have been
viewed as initially adaptive responses to maintain myocardial hct ion and conserve energetics.
However, altematively, such transitions rnay be ultimately maladaptive. As one example, the
expression of BMHC post-infârction in the rat is associated with adverse ventricular
remodelling and impaired hemodynamic performance (Orenstein m al 1995). Perhaps, such
changes in gene expression are neither adaptive nor rnaladaptive and represent conserved
responses of myocardial phenotype to trophic signals in the absence of the capacity for ceil
division.
1.1.5 Signal transduction, growth factors, prot~ncogenes and cardiac hypertrophy
Studies from both in vivo and Ui vitro models have provided insight into the potential
signalhg pathways that might regdate cardiac genes during the development of cardiac
hypertrophy (Figure 4). The mechanisms by which the hemodynamic stress itself leads to
cardiac muscle gene program during myocardial hypertrophy are largely mknown and a cardiac
mechano-receptor temains elusive. However, it has been shown that mechanical stretch alone
induces hypertrophy and the associated pattern of gene expression in cultured neonatal rat
cardiac myocytes (Sadoshima et al 1993). In this in vitro rnodel, stretch causes release of
angiotensin II from cardiac myocytes, indicating that Ang Il may be an initial mediator of the
stretch respmse and triggers the subsequent autocrine/paracrine production of other trophic
factors and intracellular signalling pathway s (Sadoshima et al 1 993). Whether similar mechani-
17

? Catdiac Mechanoteceptor
Proto-oncogenes: eg . H-ras CAMP, PKC, Ca".
inositol phosphates. etc. \ Cell Growth 4-, Transcription Factors \ \
Nuclear Proteoncogenes: eg. c-fos. c-;un. c-myc
U biguitous and cardiac-specif ic / "Faal' Gene Expression Tmphic Factors
BMHC. skeletal and Local: TGFP1. FGFs, All, etc. smooth muscle Systemic: Catechotamines
u-actin, ANF, etc. Al I/Aldosterone
Figure 4. Partid schematic presentation of c d signaIlhg in tesponse to pressure-overioad in the myocardim. Cardiac musde is capable of expressing fetal program of genes in response to pressuce-overload. Systemic neurohumoral stimulation as wen as polypeptide growth -ors piay d e in sustaining the hypertrophic phenotype suggesting an autocrine or paracrine ktor mode1 of the hypertrophic response. Both pressure-overload and growth factor signalling is associated with activation of transcription facon which are encoded by nuclear protwncogenes and important in the reguktion of gene expression and in cardiac growth. Modined fiom Parker 1993.

exist in adult tissue remains controversial. S tretc h-mduced cardiac hypertrophy activates
multiple messemgers such as protein kinase C, tyrosine kinases and mitogen-activated protein
kinases ( Sadoshima and izumo 1993, Yamazaki et al 1993). Activated protein kinase C (PKC) is
capable of activating ANF gene regulation, indicating that PKC is one potential proximal pomts
in the signaling pathway (Chien 1992).
In addition to angiotensin II, other identifiable growth factors are produced by cardiac
non-muscle cells or by the myocytes themselves in response to hymodynamic stress, and that
these factors, through speci fic cell-surface recepton and intracelluiar mgnding cascades, regulate
transcription of gmes of the contractile apparatus, as well as others involved in ce11 growth
(Lembo et al 1995). Fibroblast growth factor (FGF) and transforming growth factor (TGFBl) are
induced by myocardial ischemia, infafction, and load (Parker 1993). Ischemic myocardiurn induce
coilateral vesse1 growth and increase TGFB in cardiac myocytes and endothelial ce11 growth
factor, a precursor of acidic FGF in arteries (Quinckler et al 1989 & Roberts et al 1990). In rat,
both TGFBl and basic FGF expression is suppressed in infarcted cardiac muscle but upregulated
in surrounding suMving myocytes which undergo compensatory hypertrophy (Chiba et al 1989
& Thompson et al 1988). Similarly, basic FGF and TGFB are hduced rapidly in catdiac muscle
cens after aortic banding (Komuro et al 199 l), and insulin-like growth factor1 and endothelin
expression are also induced by overoad (Ito et al 1992). Like h g II, these growth factors induce
hypertrophy and fetal gene expression in cardiac muscle (Parker et al 199 1 ) in keeping with an
autocrine paramine role in hypertrophy.

Cellular oncogenes are a diverse group of n o m l homologues of ûamforming vira1 genes
whose proteins participate in the cellular response to peptide growth factors and whose
mutations can transform cells in culture. The protooncogeues c-cis, crk and raf can encode for
growth factors themselves (Mulvagh et al 1988, Parker et al 199 1 8r Simpson et al 1989),
implying that the cellular oncogene pretein products are important in regulating ceIl growth.
Recent studies have provided direct evidence that the RAS pro-oncogene, a low molecular
weight GTP ( guanosine triphosphate ) binding protein, can activate the expression of the ANF
gene, a market of venûicular cell hypertrophy (Chien 1992). Expression fkom the c-Fos, atrial
natriuretic factor ( M F ) and myosin Iight chain-2 (MLC-2) promoters during phenylephnne-
induced cardiac hypertrophy requires activation of this pathway ( Thorbum et al 1995),
suggesting that G protein is part of the signalling pathway that couples the a adrenergic receptor
to the fetal gene programme. In addition to the G-protein dependent signalhg pathways, it is
apparent that other second messengers also play important roles in this response. Nuclear proto-
oncogenes whose protein products are limited to the nucleus include c-fos, c-myc, c-jun, jun B
( Parker 1993, Bilsen and Chien 1993). They can be induced rapidly by growth factors and other
phamiacologic agonists, as well as by over-expression of ras whose protein is upstream of the
signalling pathway of the cell( Parker et al 1991). C-myc, c-fos, and c-jun is re-expressed m a
variety of experimental models of cardiac hypertrophy (Mulvagh et al 1988 & Starksen et al
1 986). Induction of c-fos is required for the activation of ceil proliferation following stimulation
with growth factors (Riabowol et al 1988). These data suggest that the mduction of proto-
oncogenes is an essential feature of the hypertrophie response. Forced expression of fos and jun
20

can activate the transcription of fetal cardiac genes (Parker et al, in press). However, due to the
large number of nuclear proto-oncogenes involved, their cornplex interactions, and the diEerent
levels of ceIl fùnction that they affect, their precise role in mediahg cardiac hypertrophy in vivo
has not been established (Bilsen and Chien 1993).
1.1.6 Subcellular basis of calcium movement and relaxation in myocardiurn
The calcium ion (ca23 plays a central role in wdiac excitation-contraction coupling. The
process of myocardial relaxation is controlled by cellular mechanisms that restore cytosolic
calcium concentrations at rest to about IO-' movliter ( Ami et ut 1994). The intracellular
concentration of calcium in cardiac muscle is considered to be regdated by different membrane
systems such as sarcolemxna (SL), sarcoplasmic reticulum (SR) and mitochondria. It can be
conceived that defects in one or more these membrane systems will disturb calcium homeostasis
in the myocardial ce11 and produce cardiac dyshction ( Dhalla et al 1978).
Mitochondria are cellular organelles whose main hmction is to generate ATP through
oxidative phophorylation. They have also been shown to accumulate a large quantity of calcium
by both ATP-and respiration-dependent mechanisrns. Although many shidies have shown
impaired changes in calcium transport, calcium uptake activity and oxidative phosphorylation
activity in mitochondria From failing hearts due to different heart diseases, the exact mechanisms
of calcium tramport and the participation of mitochondria in excitation-contraction couplhg
remain to be understood ( Dhalla et al 1978).

Sarcolemma is known to be composed of basement membrane and plasma membrane. The
basement membrane contains glycoprotein and mucopolysaccharides, while the plasma
membrane is composed of phospholipids and various enzyme systems which are involved in the
regdation of ionic permerability and modulation of myocardial contractility. Both basement
membrane and plasma membrane are believed to play a crucial role m the excitation-contraction
couplhg process. Depolarization of the cardiac cell is associateci with calcium influx through
sarcolernma and calcium release h m sarcolemma1 stores and thus result in contraction, whereas,
relaxation is partly a result of calcium efflux through sarcolemma by some energydependent
mechanisms. Bidirectional exchange of calcium with cations such as Na+, C, H' and possibly
M ~ Z ' is also believed to occur at the sarcolanmal level, although the exact mechanisms in this
process are not understood. Thus, any alteration in the composition and structure of sarcolemma,
either in the basement membrane or plasma menbrane, can change caicium infimes and calcium
release, and subsequently produce abnormalities in cardiac contraction and relaxation processes
(Dhalla et al 1 976, Dhalla et al 1977, Langer et al 1 976 & McNutt 1975).
Sarcoplasmic reticulum (SR) is a tubular system which is in close contact with the
contractile apparatus (myofibril), sarcolemma as well as the transverse tubules ( T tubules)
(Dhalla et al 199 1). This membme system is considered to represent a rapidly exchangeable
calcium pool which plays an important role in heart function and metabolism. The structure of
the SR in cardiac muscle is very similar to that described in skeletal muscle. The cardiac SR is
composed of two main components: the junctional SR (terminai cisternae) and the longitudinal
tubules. A contraction-relaxation cycle is initiated when ca2' charnels are opened by

depolarization of s a r c o l m a permittmg ca2' to enter the cytoplasm. This small CC innux
induces the release of a much larger quantity of activated ca2" fkom the intracellular stores in the
SR. The released ca2' interacts with troponin C of the regdatory compiex of the contractile
apparatus to initiate cardiac contraction. Relaxation occurs as ca2+ dissociates h m the
contractile apparatus and sequestered mto the SR by the SR C ~ ~ ' - A T P ~ S ~ pump (Dhalla et al
1982 Br Hasselbach 1964 ) (Figure 5). On the basis of its remarkable ability to accumulate calcium
by energy-dependent mechanisms and to lower the intracelldar concentration of calcium to
initiate the relaxation phase of caniiac muscle, any alteration in the function of SR can be
conceived to affect the cardiac contraction-relaxation cycle.
Alterations in the regdation of mtracellular cap at any of the steps in contraction
relaxation coupling cm cause cardiac contractile dyshction and leads to failtue. The signalling
function of ~ a ' ' demands a very tow ionic concentration of ~ a " inside the myocardial cells
(about 10,000 fold lower than outside) and significant changes a n therefore be achieved easily.
During each depolarization only a very small amount of ~ a " entering the ce11 needs to be
extnided to prevent ~ a ' - overioading of the myocytes (Opie et al 199 1). The bullc of ~ a "
released from the SR must be reuptaken to its original stores (SR) in order to be released during
the next contraction relaxation cycle. Membrane sy stems regulating the intracellular ca2' have
either a low or a high ~ a " afflliity, thus s e h g different purposes in the various phases of the
cardiac cycle (Carafoli et al 1985).

Figure i Schematic presentation of d u m finxu in the nyooudium. The depolarhion of the cardiac ceii membranes induces the opening of the Na' channeis and the Ca* charnels. The initial ~anssarwlernmal i n f h of Ca? triggers the release of Ca" fiom the sarcoplasnnc reticuium Relaxation is initiateci by active uptake of Ca2+ by the SR Ca2 '-ATPase, which is under the control of phospholamban. Most of the fiee calcium that is responsible for contraction is reieased fiom the sarcoplasmic reticulum. During relaxation, Ca* e88w may ocnir botb by Cah-ATPase and by a Na+- c$* exchanga. Mitochondria mi@ acî as a "briffer" against excessive changes in the fkee cytosoiic dcim concentraton SR = sarcoplasmic retidum; MIT0 = mitochondria Modifieci and nproduced h m Opie 1984.

1-13 SR ca2+ transport proteins and gens
The contraction and relaxation of cardiocytes are regulated by inhacellular calcium
concentrations, which, in hini, are controlled primarily by the release and reuptake of ca2' by the
SR ln ment years, the major SR proteins controlling ~ a 2 ' uptake, storage, and release have been
isolated, and sequencing of complementary DNA (cDNA) encoding them has provided the
deduced amino acid sequences (Lytton et al 1 99 1 ). The contraction of cardiac myocytes is
triggered mainly by ~ a ' + release fhxn the SR through calcium release channels, also r e f d to as
the ryanoâine receptor (RyR) (Fleixher et al 1989) and the inosital 1.4,s-triphosphate receptor
(fP3R)( Furuichi et al 1989, Marks et al 1990 & Moschella et al 1993). Three distinct isofonns of
Ca'- release c h e l (RyR) have been described by cDNA cloning (Takeshima et al 1989, Marks
et al 1989, Zotzato et al 1990, Otsu er al 1990 & Coronado et al 1994). The cardiac ryanodine
receptor (RY2) mRNA is unique to hart muscle and is not expressed in fast- or slow-twitch
skeletal muscle ( Zorzato et al 1990 & Arai et al 1992).
Muscle relaxation is initiated by Amdependent aanspoa of ~ a ' - uptake into the SR
Five distinct C a ' - - ~ ~ ~ a s e iso forms encoded by three different genes (SERC A 1, SERCA2, and
SERCA3) have been identified: the adult fast-twitch skeletal muscle isoform (SERCAla)( Brandl
et al 1987). its alteniatively spliced neonatal isoform (SERCAlb)(Bra.mil et al 1987 & Brand et
al 1986), the cardiac/slow-hvitch skeletal muscle isofom (SERCA2a)('acLennan et al &
Zarain-Hetzkg et al 1 990 ), its altematively sp iiced smooth muscldnonmuscle
isoform(SERCA2bXde la Bastie et al 1988 , Lytion et al 1988 & Lytton et aI 1989) and an

isoform expressed in a broad variety of muscle and nonmuscle tissues (SERCA3) (Burk et al
1989 ). Ln cardiac muscle, the SERCA2a isoform is primarily expressed, both in the atrium and
the ventncle (Arai et al 1993). The relaxation mechanisms inclu.de calcium extrusion through the
sarcolemma by sodium-calcium exchange and sarcolemmal calcim pumps, but the most
important one is uptake of cytosolic calcium through the SR ca2 ' -~~pase.
The function of SERCA2a is inhibited by its interaction with a regulatory
phosphoprotein, phospholarnban, but inhibition is relieved by both cyclic AMP (CAMP) and
calmoduiin-dependent phosphorylation of phospholamban (Tada et al 1982). Phospholamban is
encoded by a single gene, and the same protein is expressed in cardiac and slow-twitch skeletal
muscle tissues (Fujii et al 1988).
ca2* inside the SR membrane is stored at a high concentration, which is due to binding
with a number of ca2*-binding proteins in the lumen of the SR: calsequestrin and calreticdin
within the junctional SR and glycoproteins of 53 and 160 kD (1 30 kD in cardiac muscle) within
the longitudinal SR ( FIiegel et al 1989, Campbell et al 198 1, Michalak et al 1980 & Leberer et al
1989). Calsequestrin, a high-capacity, moderate-affity ca2+ binding protein, is the major
determinant of the ca2+ storage capacity of SR. Two distinct isoforms of calsequestrin have been
identified, the skeletal muscle isofonn being expressed in both fast- and slow-twitch fiben and
the cardiac isoform king expressed exclusively in the cardiac muscle ( Fliegel et al 1987, Scott et
al 1988, Arai et al 199 1).
As noted previously, acute as well as chronic forms heart failure involve mechanical
dysfunction during systole andlor diastole. The rapid ~ a " release from and cal' reuptake into

SR are processes that critically detennine normal systolic and diastolic myocardial function.
Calcium uptake by the SR is the main mechanism responsible for cardiac relaxation. The SR ca2'
pump can be considered to be the transport system that presides over the rapid and fme
regulation of intraceilular ca2' linked to the contraction/relaxation cycle and a potential site for
pathologie regulation in cardiac hypertrophy and failure.
1.1.8 SR C P ~ + + - A T P ~ S ~ gene expression during cardiae muscle development
The expression levei of the SR c ~ ~ ' - A T P ~ s ~ during cardiac muscle development had been
studied in animal models by use of gene-specific probes (Arai et al 199 1,1992 & Nagai et al
1989). The SERCA2a is the primary isoform in developing atrial and ventricular muscle (Ami et
al 199 1 & Nagai et al 1989). The level of SERCA2a transcript gradually increases with cardiac
muscle development, but there is no isoform switching during cardiac muscle development The
cardiac muscle also transcribed trace amounts of SERCA2b ( the smooth muscle/nonmuscie
isoform). However, its expression level does not change significantly with development (Arai et
al 1992 & Lytton et al 1989). It has also been observed that at the end of fetal life and in the
early postnatal period , the amount of SERCA2a mRNA increases and remains in a stable high
level during adulthood (Lompre et al 1991). Therefore, during cardiac muscle development,
cardiac growth requires quantitative modulation of the expression level of a single isoform
(SERCMa), but no isoform changes.

1.1.9 SR function and gene expression in cardiac hypertrophy and heart failure
1.1.9.1 Animal models of cardiac hypertrophy
1.1 .9.l.a. Thyroid hormone-induced cardiac hypertrop hy
A number of studies have emphasized the importance of alterations in myocardial
contractility and relaxation that occur during cardiac hypertro p hy and failure. These alterations
have been reported in several animal models which have been developed to study both the
process of cardiac hypertrophy and the underlying mechanisms altering cardiac performance.
Thyroid hormone-induced cardiac hypertmphy is a weil-defined experirnental modei used to
investigate mechanisms altering cardiac function. Thyroid hormone-induced cardiac hypertrophy
is associated with an increased rate of tension development and an enhanced velocity of tension
decline (MaciCinoon & Morgan 1986 , HesenfÙss et al 199 1, Skelton et al L 976, Alpert et al
1986, Conway et al 1976 & Goodkind et al 1974). Although some of these changes in contractile
properties cm be attnbuted to changes in myosin heavy chah expression as manifest by
augmented a-MHC transcription ( Schwartz et al 1983 ), recent studies have indicated that the
ca2' cycling function of the sarcoplasmic reticulum is also altered in this model of hypertrophie
cardiac muscle. It has been reported that the rate of ca2*uptake and the rate of ca2+dependent
ATP hydrolysis by the SR is significantly increased in hypeahyroidism (Suko 1973). Moreover,
intracellular ~ a " transient measurement using calcium-sensitive bioluminescent assays has
28

demonstratecl that hyperihyroid state results in a rapid calcium release and reuptake, apparently
without altenng the peak level of fiee cytoplasmic ca2+ during contraction (MacKinoon et al
1986 & Beekman et al 1988).
Several other studies have shown that thyroid hormone significantly increases the mRNA
levels of ryanodine receptor and SR ~ a ~ ' - ~ ~ ~ a s e , which is in parallel with the ca2+-~TPase
protein level, suggesting that the increase in the ca2'-~'Pase is accompanied with the
upregulated gene expression but without switch From cardiac ca2'-~TPase (SERCA2a) to fast-
hKitch skeletal ~ a ' + - ~ ~ ~ a s e (SERCAl a) (Nagai et al 1989, Rohrer et al 1988 & Arai et al 199 1).
The levels of mRNA encoding the SR C ~ ~ + - A T P ~ S ~ has been shown to be increased, whereas the
phospholamban rnRNA levels to be decreased in the ventricles obtained From hyperthyroid
rabbits (Nagai et al 1989). Another study using primary isolated neonatal rat myocardial cells
incubated with triiodo thyronine (T3) has shown that T3 decreases phospholarnban mRNA levels
to about a half of control in 24 hours, whereas SR c~ '+-ATP~s~ mRNA gradually increases with
time. The same study has also shown that T3 increases Vmax of ~ a " uptake, indicating that
thyroid hormone stimula tes C~" -ATP~S~ but also decreases phos pholarnban (Kirnura et al
1994). interestingly, both hyperthyroid and hypothyroid hearts have no effect in the expression
levels of calsequestrin ( Arai et al 1991), suggesting that in response to thyroid honnone level, the
genes encoding SR ~ a " transport proteins are regulated in a discordant manner. These results
demonstrate that alterations in SR functions are primarily due to the altered expression of genes
encoding SR proteins in this model. It is also important to emphasize that the induction of
hypertrophy by thyroid hormone results in a phenotype distinct h m that induced by load, with

the absence of fetal genetic reprogranmiing.
1.1.9.l.b. Volumdpressure overload-induced cardise hypertrophy
Alterations in SR function and its ca2+-~'T'pase gene expression have also been
extensively studied in volurne/pressure overload-induced cardiac muscle hypemophy. In
pressure-overload hypertrophy in rats induced by abdominal aortic constriction, the function of
SR as assessed by the oxalate-stimulated caZ' uptake is decreased. This decrease is accompanied
by a parallel reduction in the number of functionally active c a 2 + - ~ ~ p a s e molecules, as
determined by the level of ~a-dependent phosphorylated intexmediate (Limas et al 1980 ). In
pressure overload-induced cardiac hypertrophy by pulmonary artery banding, the ATP-
dependent ~ a " uptake and the expression levels of SR ca2' -ATPase, ~a"-release channel
(ryanodine recep tor), phosp holamban and calsequestrin are decreased significantly (Matsui
et al 1 995). In the descending thoracic aorta banding adult guinea pigs, the rates of ca2' uptake
and the affinity of SR C ~ " - A T P ~ S ~ for ca2+ are significantly depressed and these changes are
associated with depressed protein levels of the SR ca2+ -ATPase and phospholamban assessed
by quantitative immunoblotting ( Kiss et al 1995).
However, different degree, acuteness or duration of hemodynamic Ioads can produce
various types of cardiac conditions and gene expression. There is no changes in the concentration
of c ~ ~ ' ' - A T P ~ s ~ mRNA and protein in miid hypertrophy but a significant decrease in severe
hyperiiophy (de la Bastie et al 1990 ). There is a enhanced calcium transport by sarcoplasmic
30

reticulum in mild cardiac hypertrophy induced by pressure overload in rat (Limas et al 1980 ) and
a 20% increased SR ca2' -ATPase activity in mild cardiac hypertrophy induced by volume
overload in turkeys ( Shen et al 1991). Another study ushg the pressure overload-inducecf rat by
abdominal descending aorta banding has shown that the cardiac Ry2 mRNA concentration is
decreased by 50% in severe hypertrophy but not in mild hypertrophy; and both the density of
the high-affiity sites and the Ry2 protein level are decreased by 25% (Rannou et al 1996). Most
recently, in cardiac hypertrophy produced in rats by supraremal abdominal aorta constriction,
C ~ ~ ' - A T P ~ S ~ and Ry2 mRNA levels are increased in rnildly hypertrophied hearts but are
diminished in severely hypertrophied hearts; and ca2+ uptake capacity shows similar changes
along with a positive correlation with ca2+-~TPase mRNA level. In contrast, the level of
calsequestrin mRNA expression is unaltered and that of a-actin is markedly increased ove^ a
range of severity of cardiac hypertrophy ( Arai et al 1996). These hdings suggest that the
expression of SR genes for ca2' uptake and release is up- or down-regulated dependent on the
degree of pressure overload or the magnitude of the cardiac hypertrophie response. These studies
in the voldpressure overload-induced cardiac hypertrophy fiuther suggest that the myocardial
response to load does not involve the uniform down-regdation of sarcoplasmic reticulum ~ a " -
ATPase to the lower levels seen in fetal ventricles but may be bimodal with initial upregdation
by load and dowmegulation at later the-points.

l.l.9.l .c. Hypertrophy in spontaneously hypertensive rat
The spontaneously hypertensive rat (SHR) is another frequently used model in the
study of cardiac hypertrophy. The SHR develops cardiac hypertrophy before the onset of
hypertension suggesting that cardiac hypertrophy in SHR is not entirely due to hemodynamic
overload (Sen et al 1974). A lower resting ca2+ transient and a prolonged tirne to peak ca2"
transient have been reported in the spontaneously hypertensive rat ( Bing et al 199 1) even
though the involvement of the alterations of SR gene in the development of abnomal ca2' cyclmg
stiII remains to be established.
1.1.9.2 Animal models of heart failure
1.1.9.2.a. Heart failure in hereditary cardiornyopathic syrian hamster
The hereditary cardiomyopathic syrian hamster is the most widely used model to study
the alteration of ~ a " cycling in cardiac failure (Bajusz et al 1969). It has been observed that the
velocity and capacity of caZ' uptake are dramatically diminished in hereditary dilated
cardiornyopathic hamster; but the ratios of ca2' uptake velocity to capacity, an estimate of the
functional capability of the SR C ~ ~ ' - A T P ~ S ~ are not changed suggesting a decrease either in the
volume of SR or in the number of SR C ~ ~ ' - A T P ~ S ~ pump sites, with no changes in specific
activity of the C ~ " - A T P ~ S ~ enzyme in cardiomyopathic hamsters (Whitmer et al 1988). Another

study demonstrates that the density of ryanodine receptors was increased in SR h m
cardiomyopathie hamster hearts early in the development of cardiomyopathy, suggesting an
increase in the amount or velocity of ca2' release from SR may contriiute to the development of
ca2' overload in this model of cardiomyopathy (Sepp et al 1994).
1.1.9.2.b Heart failure by chronic rapid ventricular pacing
Heart failure induced by chronic rapid ventricdar pacing is a good mode1 to study the
relation between the mechanical properties and ca2' handling m the failing heart. A defect m ca2'
handling has been shown in this animal model. A study using dogs with congestive heart failure
produced by either rapid ventricular pacing or dilated cardiomopathy demonstrates that activities
are decreased by 36% for the SR ~ a ~ ' - ~ T P a s e pump, 78% for the ca2' release channel
(ryanodine recep tor) and 53% for total ca2+-cycling (Cory et al 1994). S tudy in dogs with heart
failure induced by nght ventricular pacing has shown that at early heart failure, there is decreased
activity of the SR ca2' release channel (O'Brien et al 1994), a 50% decrease in activity of the
myocardial SR caZ' pump and a 75% reduction in SR caZ+ release channel activity (Cory et al
1993). The SR c~'--ATP~s~ activity and SR caZ4 uptake are diminished to half of that of the
control muscle. Importantly, the decrease in SR C~''-ATP~S~ activity is correlated with left
ventricular ejection fraction, an index of degree of myocardial failure (O'Brien et al 1990).

1.1.9.2.c Heart fdure induced by drugs
hg-provoked severe kart failure is another mode1 which demonstrates the abnomal
intracellular ca2' accumulation and decreased SR C ~ " - A T P ~ S ~ activity. A significant decrease in
SR ca2'-~Pase activity has been shown in chronically adrninistered adriamycin
cardiomyopathy in dogs ( Olson et al 1974 , Tomplison et (11 1985 & Kusuoka et al 199 1).
Chronic diabetes due to streptozotocin administration has been shown to induce heart
dysfunction characterized by prolonged relaxation tirne as wetl as decreased ca2' transport and
ca2-~TJ?ase activity; but no significant reduction has been found m the relative levels of SR
C ~ " - A T P ~ S ~ mRNA expression and SR c ~ ~ + - A T P ~ s ~ protein (Zarain-Herzberg et al 1994).
These data indicate that abnomial ca2' cycling exists in cardiac hypertrophy and heart failure
induced by drugs and is one of the important causes of cardiac dysfunction.
1.1.93 Cardiac gene expression during transition from compensated hypertrophy to heart failure
After the initiation of hernodynamic stress, the heart undergoes adaptive changes such as
preservation of systolic pressure development and the extent of muscle shortening , a decrease in
the maximum velocities of shortenhg and lenghtening of the muscle and a slowhg of relaxation
(Jouannot et al 1975, Lecarpentier et al 1982, Lecarpentier et al 1987, Lorell et al 1987 & Fifer et
al 1986). Cardiac hypertrophy is widely recognized as an adaptive response that nomializes wall
stress and compensates for an increased load (Gmssman 1980). When the load is chronically

persistent, compensated hypertrophy may progress to heart failure. The mechanism that
contributes to the transition €rom compensated cardiac hypertmphy to heart failure has remained
unknown. There is evidence to suggest that a loss ofkontractile proiein from the myocardium
may contribute to the impaired cardiac function in failhg heart ( Pagani et al 1988). In aging and
hypertension, however, myocyte loss due to myocardial ce11 death and hypertrophy of the
remaining viable myocytes has been proposed to account for irnpaired function and failure
(Anversa et al 1986, Capasso et al 1990 & Engelmann et al 1987).
Alterations in cardiac gene expression during the transition from stable hypertrophy to
heart failure have been studied using spontaneously hypertensive rats with heart failure (Sm-F)
and without heart failure(SHR-NF) (Boluyt et al 1994). This study has documented a significant
loss of a-MHC mRNA from both ventricles of failing hearts, with no significant change in
MHC mRNA levels; a biventricular increase in ANF mRNA levels but no increase in levels of a-
skeletal actin mRNA; a threefold to fivefold increase in fibronectin and collagen mRNAs in both
ventricles but no significant decrease in SR ~a''-~'T'~ase mRNA levels in either ventride during
the transition to failure though SR C ~ " - A T P ~ S ~ mRNA levels has decreased 24% in RV in SHR-
NF compared to control. By contrast, a study using two distinct groups of rats 20 weeks after
aortic banding (20-week nonfailed left veninclular hypertrophy and 20-week failed left
ventriclular hypertrop hy ), has s hown that when compared with the age-ma tc hed control group,
PMHC mRNA increases greater than twofold, ANF mRNA level increases about sixfold in lefl
ventricular myocardium of both gmups (Feldman et al 1993). Of importance in this shidy is the

finding that SR c a 2 - ~ ~ p a s e mRNA levels has decreased by 50% only in the lefi ventricular
rnyocardium of rats with cardiac failure but not in rats of nonfailed le ft ventricular hypertrophy
group. These studies suggested that the expression levels of SR ca2' transport gene are not
related exclusively to imposition of load; and the pattern and regdation of cardiac gene expression
(specifically SR ca2'-~TPase gene) during the transition From compensatory hypertrophy to
heart failure may be model-dependent and the potential for smiilar transitions in human beings
remain to be elucidated.
1.1.9.4 Human hypertrop hic cardiomyopat hy and heatt f d u r e
The SR ~ a " transport Function in failing human hearts has also been studied. ca2' cycling
in cardiac muscle can be assessed by bioluminescent ca2' indicaton a e q u o ~ or hua (Gwathwey
et ni 1987, Beuckelrnam et ai 1992 & Morgan et al 1990 ). Tension-independent heat is
considered to result from the energy consumed for ~ a " transport in cardiac muscle and can be
used to estimate the ~ a " uptake rate. Dilated cardiomyopathie muscle has shown increased
resting [ ca27 i levels, decreased peak [ ca2' ] i levels, slower rise and slower decline of CC
transient and a 50% reduction of ca2' uptake rate, indicating that the calcium release function and
calciirm uptake (sequestenng) function are impaired in human heart failure ( Gwathmey et al
1987, Morgan et al 1990 & Hasenfuss et al 1992 ).
In recent years, the major SR proteins controllhg ca2+ uptake, storage and release have
been defined. Molecular cloning has provideci sequences of the genes encoding SR protehs.
36

Analysis of gene expression level has observed that the messenger RNA(mRNA) for ca2'-
ATPase is rnarkedly decreased by 48% in leR ventricular specimens of patients with heart failure
undergohg heart transplantation (Mercadier et al 1990 ). Another stuây using failing humau
hearts from cardiac transplant recipients with a diagnosis of dilated cardiomyopathy, pnmary
pulmonary hypertension, or ischernic heart disease has demonstrated that the expression level of
mRNA for ca2'-~TPase is inversely correlated with brain natriuretic factor (BNF) and atrial
natriuretic factor (ANF) mRNA level which has been s h o w to increase in moderate heart failure
and to be highest in severe human heart failure (Arai et al 1993). However, other studies have
s h o w that in temminally failing human myocardium resulting fmm dilated cardiomyopathy and
ischemic cardiomyopathy, ca2' uptake activity, c a 2 ' - ~ ~ p a s e activity and the expression levels
of SR ~ a " -ATPase and phospholamban are decreased significantly, whereas both SR ca2' -
ATPase and p hosp holamban protein levels are unchanged in failing compared wi th nonfailing
tissues (Schwinger et al 1 995, Flesch et al 1 996 & Linck et al 1 996). These data may M e r
suggest that an alteration of the SR c~ '+-ATP~s~ activity contributes to an altered intracellular
~ a ' ' handling and myocardial relaxation in human heart failure; and the changes in SR caZ*-
ATPase and phospholamban steady-state protein levels may not contribute to these alterations.
The dissociation between protein and mRNA levels may provide evidence for a post-
transcriptional or post-translational regulation of these proteins.
Other studies have examined the expression Level of phospholamban in human hart
failure. Phospholamban rnRNA level is reduced in kart failure caused by dilated
cardiomyopathy, coronary artery diseaase and primary pulmonary hypertension. Impor*intly,

this reduction is in parallel to that of ~ a ~ ' - ~ ~ ~ a s e and is inversely correlated with the ventricular
ANF mRNA level (Ami et al 1993 & Feldman et al 199 1 ) . Analysis of the levels of cardiac
ryanodine receptor mRNA has shown a decrease in end-stage heart failure c a w d by coronary
artery disease, primary pulmonary hypertension but decreased or unchanged levels of mRNA in
dilated cardiomyopathy ( Arai et al 1993 & Brillantes et al 1992). In addition, the same study by
Arai et al ( Arai et al 1993) has demonstrated that the decrease in the mRNA of ryanodine
receptor is in parallel to that of cd+-~TPase and phospholamban, and the mRNA level of
ryanodùie receptor is invenely correlated with that of ANF M A . It has been shown that the
two cardiac intracellular calcium release channels were regulated in opposite directions in end-
stage hart failure: RyR mRNA levels are decreased by 3 1% whereas IP3R mRNA levels are
increased by 123% (Loewe et al 1995). However, in hart failure caused by dilated
cardiomyopathy, coronary artery disease, or primary pulmonary hypertension ,there is no
signScant change in the level of calsequestrin mRNA (Ami et ai 1993 & Takahashi et al 1992).
These data indicate that except calsequestnn, the expressions of c~''-ATP~s~. phospholamban
and ryanodine receptor are coordinately regulated in human heart failure (Ami et al 1993). Taken
together the bulk of the current literature support a downregulation of SR ca2+ - ATPase activity
and/or gene expression.
1.1.10 Alterations of gene expression in animal models of acute myocardial infarction
Coronary artery ligation in rats has been widely used to examine the cardîac phenotype
38

and remodelling after acute myocardial mfarction. Acute myocardial mfarction is associated with
enhanced expression of insulin-like growth factor 1 and insulin-like growth factor 1 receptor
which may activate genes essential for the reconstitution of tissue mass ( Reiss et al 1994).
Myocardial ùifat-ction is also accompanied by over-expression of angiotensinogen gene,
angiotensin II receptor , c-myc, c-jun and ANF (Lindpaintner et al 1993, Reiss et al 1993 &
Kanda et al 1993). Post-infarction PMHC is re-expressed persistently out to day 35 and
strategies for BMHC downregulation are associated with improvements in myocardial
performance (Orenstein et al 1995). It has recently been reported that skeletal a-actin gene is
re-expressed in rat myocardium at 7 days post-infarction, but subsequently down-regulated at
&y 7 accompanied by the induction of S 100B, a protein normally expressed in brain (Tsoporis
et al 1997, in press ). These data suggest that post-infarct cardiac myocytes undergo phenotypic
changes involving cellular hypertrophy and gene re-programmùig which is comparable to the
hypemophic response of the hart to acute pressurelvolume over-load. In addition the post-
infarct mode1 allows analysis of the temporal sequence of gene expression in association with
progressive ventricular remodelling and the development of systolic and diastolic myocardial
dysfuntion, and the analysis of differences in gene expression between left and nght ventricle.
1.2 Limitations of available human data
Studies to date on buman cardiac hypertrophy and heart Failure involve myocardium
39

procured at post-mortem or from the recipients undergoing cardiac transplantation or hearts
subject to hi& dose of therapeutic medication, reflecting exclusively end-stage or agmal diseased
myocardium. Studies on human with mild cardiac hypertrophy and kart failure and during
transition €mm cardiac compensation to decompensation are currently lacking. Furthennore, the
unavailability of large pieces of cardiac tissure has been the essential limitation to study the
molecular biology of human heart failure in living patients. The ability to study gene expression
in myocardium from intact living human beings, at an earlier stage of heart failure, will provide
better understanding of human heart failure, contributions of altered gene expression to
progression of disease, and possibly provide means to monitor the response to therapeutic
interventions.
1.3 Hypotheses
The quantitation of low abundance of mRNA cm be achieved by quantitative PCR by
using a synthetic RNA as an intemal standard. This method is sensitive and accurate. The
hypotheses of this study are:
(1) A quantitative PCR method wili provide a suitable way to quanti@ the amount of
SR caZ' -ATPase mRNA in a myocardial biopsy sample.
(2) Altered expression of SR c~''-ATP~s~ in living human patients with heart failure
correlates with hemodynamic rneasures of cardiac systolic and diastolic performance.

1.4 Alms and specifk objectives
Cardiac adaptation to hemodynamic stress involves both quantitative (hypertrophy) and
qualitative (pattern of gene expression) changes. Previous studies have provided evidence that
SR ca2''-~~I?ase gene expression levels is altered m cardiac hypertrophy and heart failure both in
experimmtal animal models and in human beings. However, experiments on animal models have
documenteci dissimilar patterns of SR c~*'-ATP~s~ gene expression during transition nom
compensated hypertrophy to decompensated heart failure. It is unclear whether these changes
happen in humans. Sample unavailability has made it unachievable to monitor the changes of gene
expression in different stages of human cardiac hypertrophy and heart failure. Myocardial
infarction in rats induced by coronary artery ligation has been shown to result in phenotypic
transitions, cardiac hypertrophy and utimately congestive heart failure. Alteration in SR ca2'-
ATPase gene expression after myocardial infarction has not been studied The specific objectives
of this study are to:
1. Develop a PCR based strategy to quantitate cardiac specific gene expression, specifically,
the SR C ~ " - A T P ~ S ~ gene expression as assessed by steady-state mRNA levels in
myocardial biopsy samples from patients with suspected myocarditis and dilated
cardiomyopathy .
2. Evaluate possible correlations between SR C~"-ATP~S~ gene expression in living human
being with concorni tant phys iologic rneasurements of myocardial systolic and diastolic
performance.

3. Assess the steady state levels of SR ca2'-~Pase mRNA using Northern blot analyses
post myocardial infarction in rats.

Chapter 2: Methods
2.1 Introduction of the quantitative approach used in this study
A number of methodologies ( Le. dot blots, in situ hybridization, Northem blotting) have
been used for the detection and quantitation of mRNA levels. However, if target mRNAs are
present in relatively low abundance or large quantities of s t h g materiai are unavailable, these
methods might not be ~ ~ c i e n t l y sensitive to reprodicibly ensure mRNA detection and
quantitation. The Polymerase Chain Reactim (PCR) is a powemil molecular biological tool that
allows a small quantity of DNA to be amplified. It is by far the most sensitive known method to
detect gene expression. This technique invoives enzyrnatic amplification of nucleic acid sequences
via repeated cycles of denaturation ( double-stranded DNA separated at a high temperature),
oligonucleotide annealing ( two convergent DNA primers anneal to opposite strands of the target
DNA at low temperatures), and DNA polymerase extension (extension of the annealed primer
along the DNA strands at 72Oc by the heat-stable Taq polymerase in the presence of excess
DNA triphosphates)( Mullis et al 1986 & Saiki et al 1988). However, in rnost instances, the PCR
technique had only provided qualitative results. The availability of quantitative PCR should
provide valuable method to quantiQ the amounts of specific -As in small quantities of
tissue.
The application of RT-PCR (reverse transcriptase-polymerase chah reaction) makes it

possible to analyze srnall numbers of mRNA in a limitai amount of tissue, that is, isolation and
purification of small quantities of RNA, reverse transcription of RNA to complementary DNA
(cDNA) and the PCR for amplification of srnall quantities of cDNA. It has been diffîcult to
quantitate the absolute amount of specific mRNA without an intemal standard of known
concentration. The literature describes a number of approaches to the quantitation of PCR
product ûriginall y, attempts to quantitate PCR amplification of mRNA sequences have invo lved
the use of a relatively invariant mRNA such as Bacth or an unrelated template as an interna1
standard ( Chelly et al 1988 & Rappolee et al 1988). However, this approach provides only
comparative data, in part because of differences in eficiency between the primer pairs for the
standard and the target mRNAs. Altematively, quantitative PCR cm be done by generating an
allelic variant ( e.g., a small deletion or insertion in the gene of interest) such that there is a mal1
difference in the size of the PCR product of this intemal standard and the PCR product of the
native mRNA. Another method that would permit distinction between the PCR products of the
standard and target RNAs is to mate a restriction enzyme site in the target gene ( Wang et al
1989). However, these approaches require that a new standard be constmcted for each target
gene.
In selecting a methodology for the quantitation of cardiac specific mRNAs, efforts have
been made to fùlfill a number of criteria and overcome problerns associated with the methods
descnbed before. The method would be required to control for cDNA synthesis and PCR
reaction, to produce a standard curve and to aliow quantitahg multiple genes of interest. In this
study, an approach fint described by Wang et al ( Wang et al 1989) was selected. A DNA
44

template £km which an RNA molecule with the same primer binding sites as the target RNA
molecule could be transcribed is first consûucted. The target RNA and this intemal standard were
reverse-hanscnbed then coamplified incorporating a radiolabeled nucleotide. Gel slices containhg
the PCR pmducts were subjected to Cerenkov counting or analysis by a computerized phospho-
imager. The amount of target mRNA in the sample was quantitated by extrapolating against a
standard c w e generated with the same intemal siandard.
2.2 Human hart biopsy samples
Endomyocardial biopsies were obtained at the time of diagnostic procedures from right
ventricles of patients with suspected myocarditis (1 1 patients, group 1 ) and dilated
cardiomyopathy (1 1 patients, group 2). Following infonned consent, samples of group 1 patients
were pmcured at The Toronto Hospital, patients' ages ranged from 4 to 74 years ( mean age 41.3
years, fmale = 9, male = 2). Biopsy samples of group 2 patients were obtained kom
Cardiovascular Clinical Research Laboratory, Mount Sinai Hospital, Toronto, Ontario, and
patients' ages ranged h m 3 1 to 76 years (mean age 48.5 years, fernale%, male=S). After removal
h m the heart, biopsy samples were immediately fiozen in liquid nitrogen and stored at -80°C
until used.
2.3 Totai cellular RNA extraction
Total cellular RNA was isolated fkom the fkozen myocardial biopsy sample ushg a
45

modification of the acid guanidiniiimi thiocyaaate/pheno~chlomfomi extraction (Chornczynski &
Sacchi). The hzen tissue was homogenized in 1.3 ml of fresh made RNazol( 4 M guanidiniurn
thiocyanate, 25 rnM sodium citrate, pH7,0.5% sarcosyl, 0.1 M B-mercaptoethanol, 0.2 M
sodium acetate, pH4 and 0.5 volume of phenol) using a tissue grind pestle (sz22 , Kontes
Scientic Glassware, Fisher) until the tissue goes into homogenate. Sequentially, 120~1 of
chlorofomi/isoamyl alcoho1(48:2) were added , mixed thoroughly by vortex for 10 seconds and
cooled on ice for 15 minutes. The supernate was transferred to a kesh tube aRer the mixture was
centrifuged for 15 minutes at 4OC 14 k rpm . Equal volume of ice-cold isopropyl alcohol was
added and mixed well by inversion. RNA was precipitated at -70°C for ovemight. Following
centrifugation for 15 minutes at 4OC at 14 k rpm, the RNA pellet was washed with ice-cold 70%
ethanol( resuspended in 70% ethanol and sedimented ) twice then dried in vacuum pump for 15
minutes. RNA was stored in DEPC water at -70°C. RNA concentration was assessed
spectrophotometrically using a spectrophotometer and microcuvettes (Phamarcia).
2.4 Synthetic Interna1 Standard DNA preparation
Two long oligonucleotides (92 nt each) containhg 20 bp overlapping ends were designed
to contain sequences of interest and synthesized by Vitrogen (London, Ont.). 0.5 pg of each of
the long oligonucleotides were mixeci together for the first PCR (overlap extension PCR)
(Figure 6). The PCR was performed in a DNA thermal cycler 480 ( Perkin Eixner-Cetus) in a

total volume of 50 pl containhg 50 mM KCI, 1.5 mM Mg&, 10 mM Tris-HC1200 p.M each
dNTPs, and 2.5 units of Thermos aquaticus DNA polymerase (Taq polymerase) (Phamacia).
The reaction was denatured at 94" C for 7 minutes, annealed at 50° C for 2 minutes and extended
at 72" C for 3 minutes. The reaction went through seven additional cycles with the denahvation
step reduced to 1 .S minutes. One rnicroliter of this reaction mix was used as template for the
second PCR. In the second PCR, 50 pmol of each of the 2 primers (18 bases each) spanning the
5' and 3' ends of the template fortned from the first PCR were added to the 50 pl PCR Mx as
desaibed above and subjected for 25 cycles of amplification ( denature at 94' C For 1.5 minutes;
anneaIing at 55' C for 2 minutes; extension at 72' C For 3 minutes).
2.5 Subcloning of the synthetic DNA into pBluescript
After the second PCR, the synthetic DNA templates were cloned into pBluescnpt II KS
(+/-) phagernid ( Sambmok et al 1989). The synthetic DNA templates produced in second PCR
were fmt purified using Magic PCR Pres DNA Purification System (Promega). 16 pl of purified
PCR product and 0.2 pg pBluesmpt phagernid was digested with EcoRI and BamHI in 20 ~1
volume with 1 x buffer B(10 m M Tris-HCl, 5 mM Mgch, 100 mM NaCl and 1 m M P
mercaptoethanal ). The digestion reaction was carrïed out in 37 O C for 3.5 hours and in 6S°C for
additional 20 minutes to inactivate the enzyme activity. The digested DNA fbgxnent and
phagernid were purified separately using Magic PCR Preps DNA Purif~cation System (Promega).

PCR 1 3' 5'
PCR 5'
Figure 6: Synthesis of intemal stindard by overiap extension PCR 0 S ~ g each of the two long oligonucleotides (92 nucleotides) with overlapping ends (mdicated by broken lines) were mixeci together in standard PCR (100 pl vohime). The reaction was denaturd at 94'C for 7 min, mealeci at 5S°C for 2 min and extended at 72°C for 3 min. The reactions went through seven additional cycles with the denaturation s e p reduced to 1.5 min. One microiiter of this reaaion mk was used as template for second PCR In the second Pm two primers (20 nucleotides each) spamhg the 5' and 3' mds of the template formed m PCR 1 were added to the standard PCR mix and subjected for 25 cycies of ampiifidon

The ligation reaction was canied out in 20 pl volume containmg 1 x ligation buffer (0.05 M Tris-
HCl pH 7.6, 10 mM MgC12, 10 mM dithiothreitol and 50 pg bovine semm albumin), 2 units T4
ligase, 6 pl DNA fragment and 2 pl pbluescript phagemid in 16°C for ovenllght. The ligation mix
(10p.i) was transformed into 200 pl competent cells and was plated onto 1.5% agar plate
containing 40 pg/ml Ampicillin, 50 pl of X-gal(2% stock solution) and 25 pl IPTG (O. 1 M stock
solution). Single white colony was picked and grown in 3 ml LB containing 40 pg/ml Ampicillin
for ovemight. Phagemid DNA was prepared fiom the ovemight culture using Wizard Minipreps
DNA Purification System (Promega). The phagemid DNA was sequenced by automated cycle
sequencing reaction. The sequences of sites for binding of p r i m a for the SR C ~ ~ ' - A T P ~ S ~ were
confirrned to be 100% correct.
2.6 In Vitro transcription
The pBluescript containing the intemal control synthetic DNA was first linearized to
produce transcripts derived front the insert sequence only. 4.8 pl of phagemid DNA was
digested with 20 units of BssHn. (New England Biolabs) in 20 pl reaction rnix containing 100
mM NaCl, 10 mM Bis Tris Propane-HCl, 10 mM MgCl* and 1 mM DIT). The reaction was
carried out in 50°C for one hour then in 65OC for 10 minutes. The BssHU Eragment (about 300
bases) was pwified h m a low-melting agarose gel using Magic PCR Preps DNA Purification
Systern (Promega). The In Vitro transcription was perfomed in 1 O0 pl volume containing 10 rnM

DIT, 1 x transcriptiom buffer (40 rnM Tris-HCI, 6 mM MgC12, 2 2 Spermicide and 10 mM
NaCl), 100 units RNasin ribonuclease inhibitor ( Promega), 2.5 mM each ATP, GTP, CTP and
UTP, linearïzed template DNA and 50 unites T7 RNA poiymerase ( GIBCO BU). The mixhire
was incubated at 37OC for one how and additionai 30 minutes after additional 20 units T7 RNA
poiymerase was added.
2.7 Removai of the DNA template foilowing in vitro transcription
Mter performing the in vitro transcription reaction, 40 units RQ 1 RNase - fkee DNase
(Promega) were added and incubated at 37OC for 30 minutes followed by phenoVchloroform
extraction. 50 pl each of phenol and chlorofom was mixed weli by votexing with the reaction
mixture. Following one minute of centrifugation at 14K rpm at room temperature, the supernates
were mixed with equal volume of chloroform. After the mixture was spun at 14K rpm for 1
minute, RNA pellet was washed with ice-cold 70% ethanol once and resuspended in 30 pl DEPC
treated H20 and stored at -70°C. The concentration of the inteml control RNA was assessed
spectrophotometrically.
2.8 The interna1 standard
The end result of PCR is an exponential increase in the total number of DNA hgments

that include the sequences between the PCR primers. If the efficiency of amplification method is
100%, the total amouni of target DNA formed followmg amplification can be represented at a
theoretical abundance of Zn, where n is the number of PCR cycles perfomed. However, in
praaice, the efficiency of amplification can deviate h m ideal. SmaU ciifferences in efficiency
could lead to large difference in the yield of PCR product. The rnethod selected in this shidy
overcomes the problern of possible variations in efficiency in quantitative PCR by hcluding an
intemal control RNA in the PCR reaction.
The internal control synthetic DNA was made by overiap extension PCR which was
previously described (Kanangat et al 1992)(Figure 6). î h e 164 oligonucleotides produceci by
overlap PCR were cloned into pBIuescript phagemid. The structure of the pBluescript phagemid
containhg the internal control are shown in figure 7. The internai control contains the 5' primers
of 3 genes of interest (SR c~''-ATP~s~, phospholamban and TGFB) followed by the
complementary sequences of their 3' pnmen. The size difference between the PCR products
fmrn the two RNAs permit easy separation by gel electrophoresis. Unique restriction enzyme
recognition sites (RsaI, BgUI and MspI) were located after the set of 5' primes and the set of 3'
primers to provide the provision of additional pairs of primer inserted as needed. The unique
BamHI and EcoRi sites are used to clone the synthetic DNA into pBluescript. The polyA tail at
the 3' end is used to facilitate reverse transcription using oligo(dT) (Figure 7).

cRN# 176 bp
+ ' cDNA - / -
total RNA PCR 107 bp
t myocardial biopsy
Figure 7: Diagrammatic representation of quantitative PCR using an internai contml RNA (CRNA) produced from a synthetic DNA. The synthetic DNA contains 5' p h e r s of3 target genes (SR Cah-ATPase, TGFP and phospholamban) foUowed by the complimentary sequences of their 3' primers. Restriction enzyme linkers are placed after the set of 5' prima and the set of 3' primers to aiiow insertion of additionai pairs of primer as needed. nie multiple primer region flanked by poly A sequences was subcloned into pBluescript downstream of T7 poiymerase promoter. The interna1 standard utilizes the same primer sequences as the target mRNA by design but yields a PCR product of different sire which perds easy separation of the dCNA product fiom the target mRNA product by gel electrophoresis.

2.9 Reverse-transe ri ption
A 20 pl reverse transcription reaction mixture containing 50 mM Tris-HCl (pH 8.3), 75
mM KCI, 3 mM MgC12, 0.5 mM each MTP, dTTP, dATP and dCTP, 20 units RNasin
ribonuclease inhibitor (Promega), 0.2 pg hexamer @âN6)(Pharmacia), 10 m M DTT, 200 units
Moloney Murine Leukaemia Virus Reverse transcriptase (M-MLV RT) (GIBCO BRL), 0.25pg
human heart total RNA and lng intemal control RNA was incubated at 37OC for one hour and
additional 5 minutes at 95°C then stored at -20°C.
2.10 PCR amplification
The oligonucleotides of 5' and 3' primers of SR ca2'-~TPase, phospholamban and TGFB
were chosen f?om the published report ( Lytton et a2 1988, Fujii et al 199 1, Quan et al IWO)
(Table 1). In this study, only the levels of the SR ~a' '-~'I '~ase gene were quantitated. The 5' and
3' primer of SR C ~ " - A T P ~ S ~ spanned a mial1 intron (8 1 bp) for easy identification of
amplification from contaminating genomic DNA if there is any. The same primer sequences are
used but the sues of PCR products from human kart RNA and intemal control RNA are
different based on the design of the interna1 control template. These primers were also chosen to
have 100% homology with the same gene of rat hart accomiodating the initial experiments on rat
heart tissues.

Tablel. Oligonucleotides of 5' primers and 3' primers for PCR of 3 cardiac genes
- -
GAGACGCTCAAGmGTG 1 76 107 - -
SRCA2a
PHLB I
TGFP ..
r I 1
. --
AGAACATCTGGCTCGTG
CAATACCTCACTCGCTC
CGAGGTGACCTGGGCACCATCCA TCAC
ATCATCGTOATGCTTCTC
GTGGGTCGCAAGCCCAAGCTGGA GCAG
144
405
72 I
100

DNA amplification was perfomed Ui 50 pl volume as desaibed before except that 37.5
pmol of each of the 5' and 3' primers of SR c a 2 + - ~ ~ p a s e and 0.5 pl of "P-CIC-~CTP were added
to the reaction. The PCR profile involved denahvation at 94OC for 1.5 minutes, primer annealmg
at 50°C for I .5 minutes, and extension at 72°C for 1.5 minutes. This reaction was carried out for
28 cycles.
2.1 f The specifieity of the SR C ~ ~ + - A T P P S ~ primers
The control RNA obtained by in vitro transcription using T7 RNA polymerase from the
synthetic gene was demonstrated to be of free of significant template DNA by doing PCR from
the control RNA without pnor doing reverse transcription. After 30 cycles of amplification of
the control RNA, no PCR produc ts can be visualized after ethidim bromide stainning. The
specificity of the SR C a ' + - ~ ~ ~ a s e primers was verified by perfomhg PCR using the primen
and first-strand cDNA fiom control RNA and from hurnan heart RNA. As designed and
expected, the SR C ~ ' \ A T P ~ S ~ primen gave single sharp band of 107 bp PCR product for
intemal control RNA and single sharp band of 176 bp product for sample RNA (Figure 10 &
Figure 1 1) , and no nonspecific PCR product could be seen in all samples. The nature of each
band was confirmed by sequencing.

2.12 Determination of the ratio of sample RNA and internal control RNA used for eDNA sy nthesis
It is very important to note that in quantitative PCR using a internal control RNA as a
standard, the starting amounts of the control RNA can influence the amplification effciency of
the sample RNA and vice verm. In this study, thmefore, initial experiments had been doue on
adult rat heart tissues to investigate the proper ratio of intenial control RNA and sampie RNA
should be used in the first-strand cDNA synthesis in order to allow both RNAs to be amplified
within the exponential range. In the case of rat heart tissue, 1 ng of internal control RNA and
about 0.25-0.3 pg of tissue RNA were found io be the suitable ratio. For group 1 and group 2
patients, 1 ng of internai control RNA, 0.25 pg and 0.3 pg of tissue RNA, respectively, were
used to do the quantitation.
2.13 Determination of the exponential phase of ampüficatioo
Having established the specificity of the SR C ~ ~ + - A T P ~ S ~ pnmen, and the proper ratio
of starting RNAs, the next step was to detennine the optimum number of PCR cycles required to
keep the amplification in the exponential phase and also to amplify only the specific product. To
do this, one tenth of the fint-strand cDNA from one of the human samples was amplified for 12,
14, 16, 18,20,22,24,26, 28 and 30 cycles. The PCR products were eletrophoresed on a 6%
TBE polyacrylamide gel. The appropriate bands representing amplification products from
differemt cycles were excised From the dried gel. Radioactivity nom the bands was plotted a g a k t

the nwnber of PCR cycles. At the p r e d e t d e d ratio of human heart RNA and control RNA,
the rates of amplification were exponential for both the intemal control and sample RNA when
the PCR reaction was performed for 24 to 30 cycles (Figures 8 & 9). It is ideal to keep the PCR
cycles to the minimum number in order to avoid nonspecific amplification, therefore, 28 cycles
was chosen for a11 quantitation experiments.
2.14 Electrophoresis
7.5 p1 each of PCR reaction mixture and 1 -5 pi of 6 x sample buffer (Novex) were
eletrophoresed in 6% polyacrylamide TBE gel (Novex) m 1 x Tris-borate/ EDTA buffer
according to the instruction (Novex). For the initial experiments doue on rat kart tissues, the
mini gel was dried for 1 hour then exposed on a Kodak film; and the s p d c bands were cut out
from the dried gel according to the autoradiogram and their radioactivities were determmed by
Cerenkov counting. Otherwise, the gel was transferred ont0 a cellophase small membrane (Novex)
and dried in a vacuum pump (Virtis, New York), then analyzed in a computerized
phosphoimager.

Figure 8. Autoradiogram of PCR products as a function of cycle number. 0.25 pg of human hem total RNA and 1 ng intemal control RNA were reverse-transcribed. One tenth of the first-strand cDNA was amplified for 12, 14, 16, 18,20,22,24,26,28,and 30 cycles. Afier the indicated number of cycles, samples were removed and analyzed by electrophoresis. Lanes 1 - 10 represent amplification cycles 12, 14, 16, ... 30, respectively. Base pair (bp) sizes of sample (176) and control(107) are indicated. Radioactivity of SR ~ a ~ + - ~ ' I ' ~ a s e PCR products for intemal control RNA and sample RNA increase with increasing cycle number.

- cpm (c ) 18n - cpm(s) 18n I
Cycle number
F i 9. Plots of PCR prodncts as a funetion of the number of amplif~cation cycles. PCR products shown in Figure 8 were cut out fiom the gel and radio- was determined by Cereakov couting. The variable concentdons ofthe intemal standard RNA (molec~Iles) and the sample total RNA (ng) were plotted against the number of amplification cycles. When PCR was perfomed for 22 to 30 cycles, the dopes of the m e s for the human heart cDNA and the internai control cI>NA were similar, therefore, the ampiifïcation &ciency couid be assuneci to be the sarne for both cDNAs w i t h the exponemial ranges.

2.15 Quantitative analysis of SR c ~ ' ~ - A T P ~ s ~ mRNA leveb
The SR c~'*-ATP~s~ mRNA levels h m 18 endomyocardial biopsies h m 1 1 patients
with suspected myocarditis ( group 1) and 1 1 endomyocardial biopsies fkom 1 1 patients with
dilated cardiomyopathy ( group 2) were quantitated using the interna1 control RNA described
above. Because of the dimculty ofacquiring human cardiac biopsies, initial experinients had been
doue using adult rat heart tissues before pursuing studies on hurnan biopsy samples following
procedures 2.3 to 2.12 to ensure this developed quantitative PCR technique is accurate and
reliable. Before the computerized phospho-image system was available in the laboratory,
intensity rneasurements of the PCR products were done on the Cerenkov counting machine
( initial experiments on rat hem tissues and cardiac biopsies from group 1 patients). For
quantitative analysis. one tenth of the kt-strand cDNA fkom each of the samples was serially
1 :2 diluted for seven cimes then subjected for 28 cycles of ampiification with the presence of SR
~a''-~TE'ase primers and '*~-a-dcTP. After electrophoresis, appropnate PCR products were
subjected to Cerenkov counting or analysis in a computerized Moiecular image System ( Bio-
Rad)( Figure 10 & Figure 1 1). A standard cuve was constmcted with varying concentrations of
intemal control RNA used in the analysis and the corresponding counting of their PCR products
( Figure 12). Since in the exponential phase of the PCR reaction, the efficiency of PCR for the
two RNAs is the same, the amount of target mRNA is quantitated by extrapolating against the
standard curve. As in figure 12, the molecule number of mRNA (Yc) in 10 ng total RNA (Ys= 1 O)
nom sample can be calculated by solving the equations as below:
60

Yc=Ac+BcX Ys=As+BsX
when Ys = 10 (ng), X = (1 O - As) , thus, Yc (molecules) = Ac + Bc i l O - As)
Bs Bs
and was expressed as molecules mRNA per l h g of total human heart RNA (Figure 12). The SR
ca2'-~Vase mRNA levels from two groups of patient are show in Table 2 and Table 4.
2.16 Statistical analysis
Correlation analysis were made in each group of patients between SR C~''-ATP~S~
mRNA levels and their haemodynamic data. Correlations were also made when the two groups of
patients were combined. Al1 correlations were tested by lmear regression analysis using Pearson
Correlation method. The test was considered significrmt when p value4.05. Variability of the
mean of SR c~''-ATP~s~ mRNA levcls in duplicate biopsy samples ( two separate biopsies
taken kom the same patient at the same procedure ) was tested using coefficient of variation
(CV), which is defied by CV = SD/M x 100%, where SD is the standard deviation, M is the
mean value fkom the two duplicate biopsies.

Figue 10. Autoradiogram demoIlSfraüng serial dihrtEons of quantitative PCR products. Trines 1-7 repfesent FCR pmdms h m serial 1:2 dilutions of one te& of a cDNA mixture reverse tmnscribed h m 0.25 vg of human heart total RNA plus 1 ng of interna1 control RNA after amplifiaiion for 28 cycles. The dried gel containing @taîive PCR products was exposed onto a Kodak film for 15 hours. The 176b products h m sample RNA and the 107-bp pucts h m intemal c0iltroI are i n d i d

Figure 11. Serial Dilution of Quantitative PCR products analysed by Molecular Image System. Lanes 1-8 represent PCR products from serial 1:2 dilutions of one tenth of a cDNA mixture reverse transcribed from 0.25 pg of human heart total RNA plus 1 ng of interna1 control RNA after amplification for 28 cycles. The dried gel contaking quantitative PCR products were analysed in a Molecular Image System (Bio-Rad). The 176-bp products fiom sample RNA and the 107-bp products ftom intemal control are indicated.

, 100
10 ' total
- RNA (4)
, 1
O. 1
Figure 13. Quantitative a d y & of SR CsM-ATP= M A ievd in rn endomyocaxdiai biom wmpk 0.25 pg of human heart total RNA and 1 ng of- ~0ntr01 RNA were revezse-tm~~&ed to cDNAs. One tath of the cDNA niamne was 1 3 serially ûiiuted for seven times and ampüfied for 28 @es. The intendnies of each band of the PCR procfucts were Aclterrnined by Molenilar Image System and plotteci against the concaltcationa of hternal control RNA (standard m e ) and brmian heart total RNA used in the adysis. Since the rates ofamplification were e x p o n d for both intanal corn01 RNA and human heart RNA, and the two dopes were in parallei, the moleaile number of mRNA in 10 ng of total RNA can be caladated by solving the two equations as bebw:
in ttiis patient, there are 2.135 x 1 O' m o l d e s of SR Ca%%TPase mRNA in 10 ng human h m total RNA
64

2.17 Northern blot andysis of SR c a 2 + - ~ ~ p a s e in rats h a r t after myocardinl infarction
Sprague-Dawley rats were subjected to proximal left coronary artery ligation. At 1,2,7,
14,21,28 and 35 days after the operation, rats were sacrificed and RNA was extracted from peri-
infarct myocardium using acid guanidinium thiocyanate-phenol-chlorofom method as described
be fore (Orenstein et al 1995). For each sample the total RNA (1 5 pg) was denatured in
fomialdehyde, nm in 1.2% agarose-formaldehyde gel and tramferred ovemight onto a positively
charged nylon membrane (Gene Screen plus; dupont, Wilmington, DE). The transfer was done by
an absorption process using I O x SSPE (1.5 M NaCl, 0.1M NaH2P04 x H20, 0.01 M EDTA) as
a tramferring buffer. The filters were incubated in prehybridization buffer (5 x SSPE, 50%
formamide, 5 x Denhardt's solution, 1% SDS, 100g/rnl denatured salrnon sperm DNA ) for 4
hours at 42'C. The filters were then hybndized ovemight at 42OC in fresh prehybridization
butTer containing the denatured 32~-labelled rat SR ~a~'-~TPase-specific probe. The filter was
sequentially washed for 30 min with 2 x SSPE at room temperature, and then 1 x SSPE at 52OC,
followed by 0.1 x SSPE and 0.1% SDS at 52T until the radioactive background was negligible.
The filter was then autoradiographed at -70°C with intensifjkig screens.
To confirm equality of loading of the extracted RNA from the heart samples, the same
blots were M e r washed with 0.1 x SSPE and 0.1% SDS at the boiling temperature for 15 min
and rehybridized with rat GAPDH and B a c t h probes, which are housekeeping genes to act as

controls.
The SR ~ a " - ~ ~ ~ a s e specific probe of 176-bp was derived by PCR gene amplification of
rat SR c a 2 + - ~ ~ p a s e target using the same primers as in quantitative PCR in human. Reverse-
transcription-PCR amplification was the same as described in 2.10 & 2.1 1 except that rat heart
total RNA was used as target RNA. The amplified SR c~*'-ATP~s~ product was excised and
M e r purified with Sephaglas (Phannacia Fine Chernical) and labelled with a--"pdATP.

Chapter 3: Results
3.1 Characterization of group 1 patients with suspected myocarditis
18 right ventncular endomyocardial biopsies obtamed km 1 1 patients were investigated
in group 1 experiments. These patients were a11 hospitalized with acute CHF and were suspected
of having acute viral myocarditis. Al1 the biopsies were procureci at the same time when cardiac
pressures were meanireci using fluid filled catheter-manometer. Duplicate biopsies ( two separate
biopsy fragments ) were taken from seven patients. The cardiac output of these patients ranged
fiom 2 to 6.7 L h i n and cardiac index ranged from 2.12 to 3.88 L/mm/m2. A broad range of PA
pressure, RV pressure, RA pressure and LV pressure can be seen in this patient population. The
clinical characteristics of 1 lpatients ( group 1) submitted to endomyocardial biopsy and
quantitative PCR are summarized in Table 2.
In group 1 patients, retrospective clinical data were rniuing in two patients ( patient #9 &
patient # 1 1). In addition, RNA from patients #8 and # 10 was not suficient to adequately
quantitate mRNA levels of SR c~''-ATP~s~. Therefore, these four patients (#8 to #Il) were
excluded fkom the correlation analysis.
In this group of patients, endomyocardial biopsies were submitted for pathologic
analysis. One patient's pathologic report was unavailable for review and archivai slides were not
located. Two to six pieces of cardiac tissue were studied from each of the 10 patients. No
evidence of myocarditis was presented in any of the biopsies. Four of the patients provided no
67

Table 2. Characterization of patients with suspected myocarditis ( group l), hemodynamic data and mRNA levels of SR Ca2' -ATPase
PAP RVP RA fmmHgl (mmHg) (mmHg)
LVEDP 1 mRNA (x 1 0' rnolocule/ 1 0ng total RNA) Patient Age(yr) CO Sex (Wmin) ( m m 9 Biopsy 1 Biopsy 2 Mean
CO, cardiac output; CI, cardiac index; PAP, pulmonary arterial pressure (systolic/diastolic/mean); RVP, right ventricular pressure (systolic/diastolic); RA, right atrial pressure; LVEDP, lefi ventricular end diastolic pressure; ND, not detectable; * patients excluded fiom correlation anrlysis.


positive abnomatities. The other six patients showed minimal to mild interstitial fibrosis, mild
cardiac fibre hypertrophy and mild fat infiltration. The histologic hdings Grom the biopsies are
compiled in Table 3.
3.2 Variability of SR ~a ' ' -~ ' I '~ase mRNA levels in dupücate biopsy samples
In group 1 patients, 7 of the 1 1 patients have two biopsy samples taken at the same time
of diagnostic procedure. To prevent variations in pmcessing h m affecting the experimental
resuits, al1 the sarnples from this group of patients were processed at the same time. However,
RNA from two biopsies ( #6 & #9) was not sufficient to quantitate mRNA SR ca2' ATPase.
Therefore, thses two patients were excluded kom the variability analysis. Sampling variability of
SR ca2' ATPase mRNA levels in two separate biopsies has been analysed using Coefficient of
Variation method and is shown in Table 4. The sampling variability between two separate
biopsies in these £ive patients is kom 2% to 83%. The mean variability of gene expression
between these separate biopsies is 38%.
3.3 Correlation between SR c~ '* -ATP~s~ mRNA levels and clinicd parameters from patients with suspected myocarditis (group 1)
Al1 patients h m group 1 were biopsied for suspected myocarditis. The hemodynamic
parameters were obtained using fluid filled catheters and this component of the study was done
retrospectively. The relationship between the expression levels of mRNA for the SR ca2'-

Table 4. SR CaB-ATPase mRNA levels and samphg variability in 5 dupikates of group 1 patients
(x 1 O' molecdes/l O ng total RNA)
Patient Biopsy 1 Biopsy 2 % variability 1 16.55 18-83 13 2 1 .O9 1.682 42 3 3 A65 5.765 49 4 2.164 2.216 2 5 3.032 7.514 - 83
mean 38
SR Ca2+-ATP~S~ rnRNA levels were detennined in two separate biopsies obtained in 7 patients. However, RNA fiom two biopsies fkom two patients was not sutncient to quantitate SR Ca2+- ATPase mRNA level. Thus, these two patients were ornitted from the variability study . Sampling variability nom two separate biopsies in one patient range fiom 2% to 83%. The mean variability of gene expression between two biopsies among these five patients is 38%.

ATPase and hemodynamic data was examined using Linear regression foilowed by Pearson
coefficient correlation method. The patient nurnber analyzed for each parameter were not the
same since in this group of patients some hemodynamic parameters were missing. SR ca2+-
ATPase mRNA levels positively correlated with right sided cardiac pressures including
pulmonary artery pressure ( systole, r=û.895, p=û.007, n=7; diastole, r=O.883, p=0.02 1, n=7;
mean, r4.883, p4.009, n=7) ( Figure 13. Figure 14 & Figure 15), nght ventncular systolic
pressure (r=O.82 1, ~ 4 . 0 2 5 , n=7) ( Figure 16) ( Table 7) and a trend towards a positive
correlation with right ventricular diastolic pressure (r=0.68 1, p4.094, n=7) and right atrial
pressure, r=0.664, p=0.106, n=7). These fmdings suggest that there is a direct relation between
SR c a 2 + - ~ P a s e gene expression levels and nght heart pressures in this patient population and
in particular, in patients with relatively mild cardiac fmction impairment.
The correlation between SR C ~ " - A T P ~ S ~ mRNA levels and leA sided cardiac parameters
were also analyzed. SR c ~ ' - - A T P ~ s ~ mRNA levels showed negative correlation with cardiac
output ( ~ 4 . 7 6 6 , p=0.046, n=-7) ( Figure 17), aortic pressure (r-0.768, p=O.O45, n=7) ( Figure
18) and a trend towards a negative correlation with left ventricular end diastolic pressure
(LVEDP) (F-0.730, y0.103, n=6). These results denote that in this patient population, there is
a weak inverse relation between nght ventricular SR C~"-ATP~S~ gene expression and LV output
and aortic pressures. This may reflect the contribution of seventy of left sided hemodynamic
embarrassrnent to elevated PA pressures which strongly and positively correlate with gene
expression.

Pulmonary artery systolic pressure(rnd3g)
Figure 13. SR CaB-ATPase mRNA levels positively correlate with pdmonary artery systolic pressure from patients with suspected myocarditis.

(MokcuWlO ag total RNA)

rnnnnnnnn L
Figure 15. SR CaBaATPase mRNA leveis positively correlate with pulmonary artery mean pressure from patients with suspected myocarditis.

Right ventricalv systoiic pressure (mmHg)
Figure 16. SR CaN-ATPase mRNA leveis positivety correlate with right ventricuiar systolic pressure from patients with suspected myocarditis.

CardYc output (litnlminute)
Figure 17. SR CaN-ATPase mRNA levels negatively correlate with cardiac output fmm patients with suspected myocarditk

Aortic pressure ( m e )
Figure 18. SR CaN-ATPase mRNA levels negatively correlate with aortic pressure from patients with suspected myocarditis.

3.4 Characterization of group 2 patients with dilateci eardiomyopatby
Al1 1 1 patients from group 2 were clinically diagnosed with dilateci cardiomyopathy and
no patient had CO-existent coronary artery disease. This group of patients were al1 out-patients
will less CHF symptoms and were seletively chosen for endomyocardial biopsy. Cardiac
pressures and other parametee of myocardial performance were measured prospectively at the
time when biopsy sample was taken using micromanometers in order to reduce the mass and
inertia of the pressure measurement system, decrease artifacts associated with overdamping and
catheter whip, and improve the frequency response characteristics which is necessary to
accurately measure the rate of ventricular pressure rise (+dP/dt) and other indices of cardiac
performance (Grossman 1986). The majority (73 %) of patients were in the II-III class according
to the New York Heart Association classification. Two patients were in class 1 (1 8%) and one
patient was in class IV (9%). Ejection £?action were from 23 % to normal. There were broad
ranges of cardiac output From 3 -3 to 6.7 L/mk and cardiac index from 1 -9 to 3 -3 L./min/m2. The
c harac teris tics of these patients, their hemodynamic paremeters and the SR C$=ATP~S~ mRNA
levels are shown in Table 5.
Biopsies from this group of patients were submitted for pathologic analysis. One
patient's pathologic report was unavdabte for review and archival slides were not located. Thé
histological data from group 2 patients are summarized in Table 6. Arnong the ten patients, four
to five pieces of cardiac tissue h m each patient were submitted for histological studies. Four
patients showed non-speci fic changes. Three patients were diagnosed with myocarditis whiie the

Table 5. Characterization of patients with dlated cardiomyopathy, their hernodynamic data and mRNA levels of SR Caw-ATPase
Patient 1 Ag=@) 1 Tau 1 .@dT 1 R* No. and Sex (mec) max fmmH@
Tau, tirne constant of lefl venricular relaxation; dPldT, first derivative of lefi ventricular pressure by the ; RA, nght ventricular pressure; RV, nght veniricular pressure (systolid diastolidmean); PA, pulmonary artery pressure (systolicl diastolid mean); PAWP, pulmonary mery wdge pressure; AP, aorta pressure (systolicl diastolid mm); LVEDP, leR veniricular end diastolic pressure; LV by RNA, ejection fnction; CO, cardiac output; CI, cardiac inclex; mRNA, messager ribonucleic acid ( moleculel 10 ng total RNA).

Table 6. Histologie data €rom patients with dilated cardiomyopathy (group 2).
Patient Evidence of myocacditis No No Yes - Yes No Yes No No No No
- No No Yes No Mild No Scattered
Inters titiai fibmsis No No Prominent - No No Yes Yes No No r n d
Fat infiltration No No No - No No Patchy No No No Patchy

other three showed changes consistent with dilated cardiomyopathy (muscle fiber hypertrophy, muscle fiber degeneration, interstitial fibrosis, endocardial fibrosis and fat infiltration).
3.5 Correlations between expression levels of mRNA for the SR ca2'-~~pase and cliaical parameters from patients with ditated cardiomyopathy ( group 2 patients)
Patients from group 2 were clinically diagnosed with dilated cardiomyopathy. SR ca2+-
ATPase mRNA levels showed significant positive correlation with most of the parameters of
augmented load on the right heart ( right ventricular systolic pressure, d . 6 1 5, p=0.044, n=l 1 ;
pulmonary artery diastolic pressure, r=0.656, p=0.029, n= Il; pulmonary artery mean pressure,
r=0.626, g~0.040, n= l 1 ) ( Figure 19, Figure 20 & Figure 2 1 )( Table 7). There is no correlation
between SR C ~ " - A T P ~ S ~ mRNA levels and right atrial pressure, nght ventncular diastolic
pressure, pulmonary artery systolic pressure and pulmonary artery wedge pressure. These
results are consistent with the results from group 1 patients indicating that the expression level of
SR C ~ - - A T P ~ S ~ positively correlaies with imposed Load in this patient population.
The SR c ~ ' - - A T P ~ s ~ mRNA did not show any correlation with parameters of lefi heart
function ( dP/dT max, F-0.343, NS, n=l 1; Tau, ~ 0 . 4 5 9 , NS, II=$; systolic AP, F-0.292, NS,
1142; diastolic AP , I= -0.073, NS, n= 12; mean AP, ~ - 0 . 2 16, NS, n=12), although it did show a
tendency towards a positive correlation with LVEDP (r=0.557,p=0.060, n= 12). The relationship
between SR C ~ " - A T P ~ S ~ mRNA and left-sided pressures is not apparent in this patient
population, and combined with the weak correlation in group 1 suggests that endomyocardial
biopsy ( RV) rnay provide little insight into left-sided cardiac dysfunction. In particular, no
correlation could be found between gene expression and measures of diastolic performance.

Figure 19. SR CaN-ATPase mRNA levels positively correlate with right ventricuiar systolic pressure from patients with dilated cardiomyopathy.

Figure 20. SR Car-ATPase mRNA leveis positive1y correlate with pulmonary artery diastok pressure fmm patients with dilated ardiomyopathy.

Figure 21. SR CaN-ATPase mRNA Lw& positively correlate with pulmonary artery mean pressure from patients with dilated cardiomyopathy.

Tabk 7. SR Ca*-ATPase mRNA showed positive condation with nght sided eudiac pressares from patients o f snspected m y o d i t h and dilrted diomyopathy
"
r v d r r e o aoll
r vrhie(MC) ~ = 7
r vdue(DCM+MC) p l 8
P m ' s
0.491
0.8% **
0594 *+
PAdia
0.656 +
0.833 * O S 7 *
PAmeaa
0.626 - 0.883 +*
0.630 **
RVqs
0.615 +
0.821 - OS94 +*

3.6 Correlatiuns between cünicnl parameters of group 2 patients and SR ca2+-~TPase mRNA IeveIs normalized with GAPDH
In the patient group with suspected dilated cardiomyopathy, SR ca2'-~'I'pase mRNA
levels were also expressed as mRNA molecule per unit of GAPDH (Glyceradehyde-3-
phosphate-dehydrogenase). This was done to control for variability in cellularity between biopsy
samples. The first strand cDNA used to do the quantitative PCR was also amplified using
GAPDH primers. The ethidium bromide stained PCR products were quantitated using a gel-doc
system ( Bio-Rad). SR C & A T P ~ S ~ mRNA Ievels were normalized according to the formula as
below:
mRNA per unit GAPDH = Molecule per 10 ne. total RNA GAPDH / GAPDHrnean
After normalized with GAPDH, SR C ~ ~ ' - A T P ~ S ~ mRNA levels persisted in showing positive
correlation with parameters from the right heart ( right ventncular systolic pressure, r=0.633,
~ 0 . 0 3 7, n= 1 1 ; pulmonary artery diastolic pressure, ~0 .700 , p=0.0 17, n= 1 1 ; pulmonary artery
mean pressure, ~ 0 . 7 3 4 , p 4 . 0 10, n= 1 1 ) ( Figure 22, Figure 23 & Figure 24). These results are
consistent with those when SR C ~ " - A T P ~ S ~ mRNA levels were expressed as mRNA moiecule
per 10 ng total RNA and support the initial correlations.

(MolecukllO ng total RNA)

Puhonary arterg diastotic pressure (mmHg)
Figure 23. SR Ca*-ATPase mRNA levels when normaiized with GAPDH positively correlate with puimonary artery diastolic pressure from patients with diiated cardiomyopathy.

Patmonay arterg mun pressure (mmHg)
Figure 24. SR CaN-ATPase mRNA levels when normalized with GAPDH pitively correlate with pulmoaary artery mean pressure from patients with diLted cardiomyopathy.

3.7 Correiation between SR ca2+-~TPase mRNA leveis and c l i n i d parrimeters from combined group 1 and groip 2 patients
Despite ciifferences in the approach to assess hernodynamics, the correlation between SR
ca2+-~TPase mRNA levels and clinical hemodynamic data when these two groups were
combined was tested. As expected, SR ca2'-~TPase &A levels showed positive correlation
with nght ventridar pressures ( systolic, d .637 , pi0.005, n=18; diastolic, 4 . 5 2 2 , p4I.026.
n=18) and puimonary artery pressure ( systolic, 4.594, ~ 4 . 0 0 9 , n=i8; diastoiic, M.567,
p 4 . O 14, n= 18; mean, 4.630, p=0.005, n= 18) ( Figure 25, Figure 26, Figure 27, Figure 28 &
Figure 29)flable 7). The combbed &ta again reveals no correlation with lefi-sided
hemodynamics.

Figure 25. SR CaN-ATPase mRNA levels positively correlate with right ventncuiar systolic pressure from combined patients of srispected rnyocarditis and dilated cardiomyopathy.

SOOOOOOO
Figure 26. SR Cas-~TPase mRNA levels positively correlate with right venticular diastoiic pressure from combined patients of suspected myocarditis and diiated cardiomyopathy.

Pnimonay artery systolic pressure (mmHg)
Figure 27. SR CaH-ATPase mRNA leveis positively correlate with pulmonary artery systoüc pressure from combined patients of suspected myocarditis and dilated cardiomyopathy.

(Molecu W t O mg total RNA)

Figure 29. SR CaN-ATPase mRNA levels positive& correlate with puimonary artery mean pressure from combined patients of suspectesi myocarditis and dilited cardiomyopathy.

3.8 SR CP~'-ATP~S~ expression in infarcted rat hearts
In order to provide a controlied experimental context for the hdings that SR ca2+-
ATPase mRNA levels showed positive correlation with hemodynamic load in the nght ventricle
in human, Northm blot analysis was performed using tissue from ùifarcted left ventricle in rats
in 1,2,7, 1 4,2 1,28 and 3 5 day s af'ter coronary ligation using cDNA encoding SR ca2'-~'Pase.
The tissue analysed was Eom surviving myocardium and included ventricular septum
(comparable to the site of hurnan RV endomyocardial biopsies). There was an increase in the
expression levels of SR ca2 ' -~~pase in the second day post-infarction when compared with
that of the €mt &y, which then gradually decreased to below basal levels at later time points
associated with veniricdar rernodelling and heart failure ( Figure 30). Mer nomalized with 28s
RNA, SR C ~ " - A T P ~ S ~ gene expression persisted in showing an increase 2 &ys post infarction.
By contras4 such a biphasic response was noi seen in sham controls (non-infart rat hearts).
Thus post-inf'arction in a rat mode1 the initial response of suMving myocytes to imposed load is
the augmented expression of SR ca2' -ATPase gene expression pnor to subsequent
downregulation.

Figure 30. Northern BIot and ethidium bromide staining of SR ca'+-~'I'~ase RNA in inf'arcted rat heart. ( A) Northern Blot of serial changes in mRNA expression of SR Ca2+- ATPase in infarcted rat heart. Representative RNA samples from the infarct heart tissue serially on days 1,2,7, 14,21,28, and 35 following coronary ligation. There was an increase in SR ca2+-~Wase gene expression on day 2 after coronary ligation then a gradua1 decrease throughout the observation period of up to 35 days. ( B) Ethidium bromide staining of RNA eletrophoresed in a 1.2% agarose gel containing formaldehyde demonstrating relative 28s RNA expression.

Chapter 4: Discussion
4.1 The development of the RT-PCR quantitative technique - technical aspects
The polymerase chah reaction (PCR) is a recently developed procedure for the in vitro
amplification of DNA sequences that has gained widespread acceptame in many areas of
moleciilar biology and is makmg an impact on medical diagnostics. The extreme sensitivity of
PCR brings with it a unique set of problems when attempting to use it as a quantitative method.
It has been demonstrated that mRNA levels can be quaatified using the PCR and a synthetic
RNA as an internal standard ( Wang et al 1989). The quantitative PCR method used in this study
was similar to that described by Wang et al in which a synthetic RNA molecule is used as an
internal control to allow the amplification reaction to be made quantitative. By presenting a
contml RNA in the reverse-transcription and amplification reactions, this technique provides a
reliable method for quantification of mRNAs of interest, which obviates inherent assay-to-assay
variations in sample preparation, reverse-transcription, and gene amplification.
It is necessary to emphasise that the intemal control described here could be used to
measure 3 different human cardiac genes in paraIlel since the synthetic interna1 standard contains
primer sequences and their cornplementary sequences of three target genes. An endomyocardial
biopsy sarnple usually weighs 3-5 mg which can yield about 2 pg of total RNA. In ihis study,
0.3 pg of total RNA is added to the reverse-transcription reaction; and only 1/10 of the first

strand cDNA mixture is to be amplified by PCR. The fact that this quantitative method can
provide sufficient sarnple for multiple PCR miction demonstrates a major advantage of this
technique, considering that much more RNA is required for Northem-blot analysis of multiple
genes. An added advantage of this PCR quantitative technique is that the constmct as designed
bas provision to accommodate other primers of target genes and can be readily rnodifîed to
analyse diverse genes of interest £tom any tissue.
Efficiencies of first strand cDNA synthesis and PCR amplification can influence the
accurate quantitation of mRNA levels by the technique applied in this study. Differences in
nucleotide sequences, length of poly(A) tails, and the distance between the PCR primen and the
poly(A) tail are among the facton that may affect the efficiency of cDNA synthesis of the target
mRNA and the intemal control RNA. However, these effects have been overcome by using
random prime hexamen instead of oligo dT primer.
There are numerous variables that could affect the efficiency of the PCR amplification.
Some of the facton that can be easily controlled are WTPs, MgC12, polymerase and the PCR
cycle profile. Another factor which has to be taken into account is the difference in length of the
target sequences and the intemal control sequences. In this study, however, the difference in
length of the target sequences (176 bp) and intemal control sequences(l07 bp) would not
significantly affect the amplification efficiency cons ide~g the fact that the Taq DNA
polymerase can incorporate >60 nucleotides per second. Primer efficiency is the most important
parameter among the various parameters that could affect the PCR amplification efficiency.
Previous observation (Wang et al 1989) indicated very clearly that it is critical to use the same

primers for amplification of the target mRNA and the interna1 standard in any attempt to
quantitate mRNA expression by PCR. By reverse transcription and amplification of the target
mRNA and intemal control RNA in the same tube, variable effects due to primer differences,
tube differences, differences in sample preparation, conditions of the reverse transcription, or the
PCR amplification are intemally controlled and will affect the yield of PCR product equally for
the target mRNA and the standard CRNA.
It has been demonstrated that the amount of an amplified DNA fiagrnent in a given
sample has a tremendous influence on the amplification efficieucy. When a high template
concentration is used or occurs as a result of the PCR amplifications, phenornena such as the
subsmte saturation of enzyme, product inhibition of enzyme, incomplete strand separation, and
product strand reannealing c m be limiting factors for efficient amplification. It is worth noting
that in quantitative analysis of mRNA levels with a standard control RNA, the concentrations of
the control DNA can affect the amplification efficiency of the sample cDNA and vice versa. In
this study, the quantities of intemal control RNA and sample RNA used in the first strand
cDNA synthesis were adjusted for each sample so that the ratios of sample ( 0.25-0.3 pg) and
control(1 ng) RNA would alIow for equal amplification efficiency (calculated between 8045%).
One of the limitations in quantimg mRNAs in small samples of tissue such as biopsy
samples is the difficulty in determining the quantity of total RNA precisely. In this study, the
concentration of RNA is acquired spectrophotometrically. The availability of sensitive
spectrophotometric instruments and microcuvettes has made it possible to obtain concentration
of total RNA accurately. Furthemore, the quantities of RNA were verified by repeating the
10 1

measurements at a different t h e . In this study, the total RNA yielded h m one endomyocardial
biopsy sample of human heart ranged h m 1.58pg to 6.24 pg (average yield, 2.85 pg) consistent
with a previous report (Feldman et al 1991). It is important to emphasize that this previous
report used "bopsy-sized" samples of myocardium from explanted end-stage hearts and the data
in this present study reflect the Fust use of this approach on achial biopsies with correlation of
gene expression with hemodynamics in intact living patients.
4.2 Variabüity of SR ~ a * + - ~ ~ F @ a s e mRNA from duplicate biopsies
Endomyocardial catheter biopsy had becorne a promising tool for the evaluation of the
morphologie-functional relationship and the follow-up of the morphologie course of cardiac
disease since its introduction in 1962. The variation of morphologic fmdings in different biopsy
specimens fkom a well defined part OF the heart had been well studied. A study using 112
biopsies nom 25 patients with hypertrophie cardiomyopathy suggest that five endomyocardial
biopsies are desirable and give the most information at an acceptable strain, considering the
sarnpling variability of fiber diameter (Coefficient of Variation, CV = 5%), volume density of
interstitiun ( CV = 9%), fibrous tissue ( CV =17%), endocardial diickness ( CV = 79%) and
muscle fiber disarray ( CV = 100% ) differed. Moreover, in the same study, the percentage of the
unusable biopsies was 28.6% ( Schwartzkopff et al 1987). However, variability and
reproducibility of assessing cardiac gene expression levels from cardiac biopsies have not been
studied by any investigators to date. Therefore, the finding h m this study that CO-efficient of
102

variability of SR ca2 ' -~~pase gene expression between two biopsies in one heart is 38%
compares with a mean sampling variability for rnorphological snidies is 42%. To mcrease the
precision of the estimation of cardiac gene expression From endomyocardial biopsies, a greater
number of biopsies would be required. This may not be possible in experirnental studies for both
technical and ethical reasons and as such our estimate of variability must be kept in mind when
interpreting any subsequent descriptions of the use of this approach.
4.3 Alterations in SR ca2+-~TPase gene expression in human henrt disease - new
insights
Previous studies using animal models and human failing heart sarnples showed that SR
c ~ ~ * - A T P ~ s ~ gene expression levels were altered in both cardiac hypertrophy and heart failure.
Thymid homone-induced cardiac hypertrophy is associated with increased SR C ~ " - A T P ~ S ~
gene expression (Nagai et al 1989 & Kimwa et al 1994) while in pressure overload-induced
cardiac hypertrophy, SR c~' '-ATP~s~ gene expression had been shown to be significantly
decreased (Matsui et al 1995). However, other studies showed no change or enhanced SR ca2+-
ATPase gene expression levels in mild cardiac hypertrophy with a decrease o d y in severely
hypertrophied heart (de la Bastie et al 1990, Limas et al 1980, Shen et al 199 1 & Arai et al 1996),
indicating that cardiac hypertrophy induced by pressure/volume overload is not invariably
associated with decreased expression of SR C ~ " - A T P ~ S ~ gene.
In anmial models of heart failure, SR c~'''-ATP~s~ activity was decreased 36% to 50% m

failing heart induced by chronic rapid veniricular pacing ( Coty et al 1993, Cory et al 1994,
O'Brien et al 1994 ). A significant decrease in SR ca2+-~TPase activity was also shown in cimg-
induced heart failure (Olson et al 1974, Tomplison et al 1985 & Kusuoda et al 1991). However, a
study camed out by Feldman et al in 1993 demonstrated that SR C ~ ~ * - A T P ~ S ~ mRNA levels
were decreased by 50% only in Failing ventricular myocardium of rats but not in rats with cardiac
hypertrophy without heart failm. These data Eûrther suggest that in animal models, dom-
regdation of SR c a - ~ ~ ~ a s e gene may contribute to the development of heart failure (or at least
serve as a marker for failure).
In human cardiac hypertrophy and heart failure, calcium release and calcium uptake
hmction are impaired (Gwathrney et al 1987, Morgan et al 1990 & Hasenfuss et al 1992). The
expression levels of SR C ~ " - A T P ~ S ~ were decreased up to 48% in myocardiwn from patients
with end-stage heart failure ( Mercadier et al 1990) and were inversely correlated with ventricular
ANF ( atrial natriuretic factor) mRNA ( Arai et al 1993 ). These fmdings suggest that SR ca2'-
ATPase gene expression is down-regulated in end stage heart fasure in human.
Unlike previous studies, the present study was undertaken to investigate the correlation
between SR C~"-ATP~S~ &A levels and hemodynamic rneasures of systolic and diastolic
perfomance in patients with clinical heart failure and suspected myocarditis or dilated
cardiomyopathy prior to the development of temiinal disease resulting in death or myocardial
transplantation. A prUnary finding was a stmng positive correlation between measures of
imposed load on the right ventricle (eg. PA systolic and PA mean pressures) and RV SR ca2*-
ATPase mRNA levels both in patients with suspected myocarditis and io patients with dilated

cardiomyopathy. One interpretation of this finding is that imposition of load on the right heart
results in augmentation of SR gene expression in proportion to the severity of that load at least at
the stage of disease studied. This would be in agreement with data that m fact mild experimental
cardiac hypertrophy is associated with augmented SR c a " - ~ ~ ~ a s e mRNA levels a n d h activity
(Limas et al 1980, Shen et al 1991 & Arai et al 1996). Histologie findings fiom both group 1 and
group 2 patients did demonstrate predorninantly mild myocyte hypertrophy and absence of
severe cardiac muscle damage or fibrosis in keeping with an early stage of RV pathology.
However, this conclusion is limited by the absence of data on the normal levels of expression of
SR ~ f l - ~ T P a s e mRNA in the right ventricle in the absence of disease. Normal RV could be
obtained at autopsy or from hearts not used for implantation. Mternatively. RV biopsy samples
could be taken from patients who have undergone heart transplantation. However, such tissue
may not be comparable to that obtained by biopsy in intact humans or may not be possible
because of ethical reason. Thus, it can not be definitively stated that the gene is upregulated From
normal levels by load in the RV but only that it is positively correlated It is unlikely that
patients with normal PA pressures have lower levels of SR ca2--~TPase expression as a
reflection of uicreased disease severity as more severe myocardial disease is associated with
increases in PA pressures not decreases. Similarly, a weak negative comlation between the SR
~ a " - ~ ~ ~ a s e mRNA and cardiac output was seen in gmup 1 patients in the face of a positive
correlation with right sided load
The result of this study is disparate from earlier studies in hiling to demonstrate a
uniform downregulation of SR ca2'-~TPase steady state message levels in patients with heart

failure. In addition, there was weak or no correlation between SR C ~ ~ + - A T P ~ S ~ gene expression
and detailed measures of left ventricular systolic or diastolic performance. It is unlikely that the
differences between the result of this study and those from previous literatures can be attributed
to differences in the techniques used to assess mRNA levels. The quantitative PCR approach has
been well validated by previous studies (Wang et al 1989, Feldman et al 1991 & Feldman et al
1993). In addition, initial experiments have k e n done in adult rat heart to make sure that the
internal control RNA was free of any contarninated DNA by PCR without doing cDNA
synthesis. All the biopsy samples h m each group were processed at the sarne time in order to
reduce the variations between samples. Effort have been made to insure the amplification for both
the internal control RNA and the sample RNA were within the linear range of the assay, and the
data were obtained from the exponential phase of the amplification. Furthemore, the identity of
the amplification products were confmed by sequence analysis. Therefore, it is more likely that
the results of this study differ from previous results because of invinsic differences in these
patient populations.
In previous studies, human cardiac tissues used to study SR c~*'-ATP~s~ mRNA levels
were taken h m patients undergohg cardiac transplantation, reflecting exclusively end-stage
failing hearts. In this study, however, cardiac biopsy samples were procured from living patients.
The majority of patients from group 2 are in New York Heart Association (NYHA) functional
classes II and III ( 73%, moderate symptoms), 2 patients are in class 1 (1896, mild symptoms) ,
and only 1 patient (9%) is in class N (severest), indicating that this group of patients are for the
most part not s u f f e ~ g h m end-stage heart failure and clearly distinct kom patient populations

undergoing cardiac transplantation. A second possible explanation for the resuit of this study
may be the existence of dispatities in gene expression during deveiopment of cardiac hypertrophy
and heart failure between right ventricle and left ventricle which is not assessed in this study. A
study conducted by Kolar et al in 1992 using two experimental models of cardiac hypertrophy
(chronic thyroxin or isoprenaline treatrnent of adult rats) showed that in the thyroxin treated
group, the functional reserve of the left ventricie rose very noticeably, whereas the nght
ventricular functional reserve did not differ from that in the controis. Tnis shidy also showed that
there was an incomplete regression of ventncular hypertrophy and persistent structural and
functional impairment in the left ventrîcle that did not happa in the right ventricle in the
isoprenaline treated animals. Another study done by Boluyt et al in 1994 showed that in SHFt.,
there was no significant decrease in LV SR C ~ ' - A T P ~ S ~ mRNA as a result of either
hypertension or during the transition from cardiac hypertrophy to heart failure, although there
was a 24% decrease in the right ventricle of SHR-NF, with no M e r decrease during the
transition to failure. This finding is contrary to the result of diminished expression of the SR ca2*
-ATPase gene after aortic constriction in the rat heart. These studies suggest that investigation of
gene expression in cardiac hypertrophy and the transition to heart failure in animals may be
model-dependent, and that hearts undergoing a comparable degree of experimental hypertrophy
and failure may have different hctional and structural properties as well as different patterns of
gene expression; and significant ciifferences can be found between the nght and left ventricular
response. Given the hypothesis that there may be disparities in gene expression between right
ventricle and lei? ventricle during cardiac compensation and heart failure, assessrnent of gene

expression levels in endomyocardial biopsy (right ventricle) may reflect mainly the alterations of
gene expression in the nght hem. Autopsy data on differences in right versus left ventricular
expression of the SR ca2+-~TPase gene are not available and no data on expression in LV
biopsies are provided in this study, again for the technical and ethical reasons of the higher risks
associateci with LV sampling.
The result showing that SR C ~ ~ ' - A T P ~ S ~ gene expression is increased 2 days after
coronary artery Ligation in rats supports the findings of positive correlations between SR ca2'-
ATPase gene expression levels and right hart pressures in human reflecting induction of the gene
by initial augmentation of load. The infarcted rat heart model also allows analysis of the
progression of heart disease to failure by remodelling of the leA ventricle. The downregulation of
SR c ~ ~ ' - A T P ~ s ~ gene expression at later stages post-infarction parallels changes in skeletal a -
actin gene expression (Tsoporis et al 1997) and likely reflects the development of decompensated
myocardial failure. The finding of animal data from this study is supportive of the human data
and may provide a new insight into the eifects of acute myocardial inf'arction in the expression of
~ a " transport proteins and therefore the development of cardiac diastolic dysfunction in a model
with ready clinical application.
While cytoskeletal actin mRNA has been widely used as a "control" for normalking
mRNA levels of interest, steady-state rnRNA level of p-actin is decreased in failing human hearts
(Feldman et al 1991). Thus, SR C ~ ~ + - A T P ~ S ~ mRNA has been noxmalized with GAPDH, a
constitutively expressed glycolytic enzyme wildly used as a house-keeping gene. BMHC has

Normal Mild Moderate Severe Failure Hy pertrophy
Figure 31. Schematlc presentation of proposed model of SR Ca2+ -ATPase mRNA levels in cardlac hypertrophy and failure Normal adult cardiac muscle has a relatively siable expression level of SR Ca2+ -ATPase which is portion 1 of the curve. After the hem is subjected to pressure/volume overload. the myocardium compensaies by cardiac hypertrophy, elevating calcium transport by sarcoplasmic reticulum ( Limas et al 1980) and increasing the expression bvels of SR C ~ " - A T P ~ S ~ which is portion 2 of the curve. If overload persists, the myocardium will undergo decompensation accompanied by abnormal calcium handling and reduction in the calcium transport as well as the expression of SR ~ a ' + - ~ ~ ~ t t s e irnitating portion 3 of the curve. In the end-stage of heart failure, the calcium transport capacity is further decreased and so as to the expression levels of SR ~a~+-ATPase reflecting portion 4 of the curve.

been used as control related to myocyte specific RNA (Mercadier et al 1990). However,
contradictory results regarding PMHC gene expression in human heart failure have been reported
(Feldman et al 199 1 & Ami et al 1993). Therefore, this study does not control for the volume of
cardiac myocytes in a single biopsy. However, 0u.r histologie data shows that these biopsies have
minimum non-myocyte tissue, such as fibrosis.
It has been suggested that alterations in steady-state levels of mRNA paraIIel changes in
the levels of their protein products ( Limas ei al 1980, de la Bastie et al 1990 & Arai et al 199 1 ) .
One of the limitations with quantitating gene expression at the mRNA level is that it is not
necessary correspondent between mRNA and protein levels. However, analysis of gene
expression may provide insight into the basis for functiooal protein modification. In addition,
there is substantial data showing dissociation between protein and rnRNA levels in contributing
to funetional impaiment, providing evidence for a post-transcriptional or post-translational
regulation.
The result of this study showing a positive correlation between SR C ~ " - A T P ~ S ~ mRNA
levels and right h a r t pressures is consistent with a recent study which demonstrated that SR
~ a ' ' - ~ T ~ a s e mRNA levels were upregulated in mild cardiac hypertrophy but downregulated in
severe cardiac hypertrophy induced by pressure overload ( Ami et al 1996), and can possibly be
explained by a proposed model of compensatory mechanism of' SR C ~ " - A T P ~ S ~ gene expression
when the heart is subjected to overload, develops progressive hypertrophy, and oniy
subsequently decompensates and fails ( Figure 3 1).
The c w e in figure 3 1 shows varied expression levels of SR C ~ " - A T P ~ S ~ gene during
110

different stages of cardiac hyperhophy and failure. Nomial adult cardiac muscle has a relatively
stable expression level of SR C ~ ~ + - A T P ~ S ~ which is portion 1 of the curve. m e r the heart is
subjected to ovedoad, the myocardium compensates by cardiac hypertrophy, elevating calcium
transport by SR ( Limas et al 1980) and increasing expression levels of SR c a 2 + - ~ P a s e (Arai et
al 1996) which is portion 2 of the curve. If overload penists, the myocardium will undergo
decompensation accompanied by abnormal calcium handling and reduction in the calcium
transport as well as the expression of SR C~''-ATP~S~ imitahg portion 3 of the curve. In the
end-stage of heart failure, the calcium transport capacity is M e r decreased and so as to the
expression levels of SR C ~ ~ ' - A T P ~ S ~ reflecting portion 4 of the curve. Assumingly, h e m , at
l e s t the right heart, analysed in this study both from group 1 and group 2 are in well
compensated stage which is portion 2 of the curve, and there is an increased expression levels of
SR ~ a " - ~ ~ ~ a s e when the right heart pressures go up. Comparably, previous studies used end-
stage failing heart tissues reflecting portion 3 or even portion 4 of the curve, showing that the
expression level of SR ca"-~TPase is downregulated in this stage of human myocardial disease.
Myocardial hypertrophy is widely recognised as an positive adaptive response that
normalizes wall stress and compensates for an increased load. Not only the amount of contractile
elements increase, but the h a r t itself undergoes complete remodelling at the organ, cellular and
nibcellular level. When the load is chronically elevated for an extended period of time,
compensated hypertrophy may progress to heart failure by unclear mechanisms. It rnakes
teleologic sense that hypertrophied myocardiurn which is subjected to elevated resting calcium
concentrations would initially augment transport mechanisms into the sarcoplasmic reticulurn.

Whether the progression to failure is mediated, in part, by downregulation of SR ca2'-~TPase
gene expression, allowing further pathologie calcium overload, or altematively downregulation is
only a marker of the development of failure awaits mechanistic testmg. Similarly the mechanisms
responsible For initial mduction of gene expression and subsequent trans-repression of the same
gene in myocytes require Çurther study .
4.4 Significance of this study
The technique of quantitative PCR developed in this study to detect cardiac-specific gene
expression levels in human endomyocardial biopsies provides a novel approach to understand the
molecular genetics of human cardiac hypertrophy and hart failure. Variabil ity of cardiac gene
expression beh~een duplicate endomyocardial biopsies has not been studied previously, and
provides insight into [imitations in interpretation of such data. Conclusions concerning the
opha1 number of biopsies required to precisely quantitate gene expression is lirnited by the
small number of patient observed in this shidy. In patients with suspected myocarditis and
dilated cardiomyopathy, SR C ~ " - A T P ~ S ~ gene expression levels positively comlate with right
heart pressures. That the SR C ~ " - A T P ~ S ~ is up-regulated early afier ùifarction in infarcted rat
heart is a new finding. These observations h m human and animal studies may suggest potential
rnechanism(s) of a compensatory responses of the heart to load which differs f?om the findings in
end-stage failing hearts. Irnpomtly, development of the quantitative PCR technique may
provide ways to better understand mechanism(s) underlying cardiac hypertrophy and failure, to
112

monitor changes in respmse to therapeutic medication in Living human bemgs at the level of gene
expression, and to extend the molecular investigation of cardiac disease to the bedside.

References
A f A N, Dhalla NS. Differential changes in leR and nght ventricular SR calcium transport in congestive heart failure. Am J Physiol. 1 992:262 :H868-H874.
Alpert Mt, Mulieri LA. Detenninants of energy utilization in the activated myocardium. Fed fioc. l986;45 :2597-2600.
Anversu P, Hiler B, Bicci R, Guideri G, Olivetti G. Myocyte ceil loss and myocyte hypertrophy in the aging rat heart. J Am Coll Cardial. 1 986;8 : 144 1 - 1448.
Arai M. Alpert NR, Periasamy M. Cloning and characterization of the gene encoding rabbit cardiac calsequestrin. Gene. 1991;109:275-279.
Arai M, Alpert N R MacLennan DJ, Barton P, Periasamy M. Alterations in sarcoplasmic reticulum gene expression in human heart Mure: a possible mechanisrn for alterations in systolic and diastolic propeties of the failhg myocardium. Circ Rex 1993;72:463-469.
Arai M, Otsu K, Mademan DH, Alpert NR, Periasamy M. Effect of thyroid hormone on the expression of mRNA encoding sarcoplasmic reticulum proteins. Circ Res. 199 1 ; 69:266-276.
Ami M, Otsu K, MacLennan DH, Penasamy M. Regulation of sarcoplasrnic reticulum gene expression during cardiac and skeletal muscle development. Am J Physiol. 1992;262:(CelZ Physiol 3 l):C6 14--C620.
Arai M, S d T and Nagai R Sarcoplasmic reticulurn genes are upregulated in mild cardiac hypertrophy but downregulated in severe cardiac hypertmphy induced by pressure overload. J Mol Cell Cardiol. l996;28: 1583- 1590.
Avruch J, Zhang XF and Kyriakis M. Raf meets Ras: completing the framework of a signal transduction pathway . Trenk Biocliem. Sci. 1 994; 1 9:279-2 82.
Bajusz E, Baker JR, Nixon CW, Homberger F. Spontaneous hereditary myocardial degeneration and congestive heart failure in a strain of Syrian hamster. Ann NY Acad Sci. 1969; 156: 1 05- 129.
Bames GE, Horwitz LD, Bishop VS. Reiiability of the maximum denvatives of left ventricular pressure and interna1 diameter as indices of the inotropic state of the depressed myocardium. Cardiovusc Res. 1979; 13:652.
Beekman RE, van Hardeveld V, Simonides WS. Effect of thyroid state on cytosolic kee calcium in resting and electrically stimulated cardiac myocytes. Biochirn Biophys Acta. 1988;969: 18-27.

Beuckelmann DI, Nabauer M, Erdrnann E. intracellular calcium handling in isolated ventrïcular myocytes h m patients wi th terminal heart failure . CircuIation. 1 992;8S : 1 046- 1 05 5.
Bing OHL, Brooks WW, Conrad CH, Sen S. Perreault Cl, Morgan JP. Intracellular calcium transients in myocardium from spontaneously hypertensive rats during the transition to heart failure. Circ Res. I991;68:1390-1400.
Black FM, Packer SE, Parker TG, Michael LH, Roberts R, Schwartz Ri & Schneider MD. The vascular smooth muscle alpha-actin gene is reactivated during cardiac hypemophy provoked by load JCZin Inves. 1991;88:(5):1581-1588.
Bloch W. A biochemical perspective of the polymerase chah reaction. Biochemistq. 199 l;30:2736-2747.
Boluyt MO, O'Neil L, Meredith AL, Bing OHL, Brooks WW, Conrad CH, Crow MT, Lakatta EG. Alterations in cardiac gene expression during the transition from stable hypertrophy to heart failure: marked upregulation of genes encoding extracellular matru< components. Circ Res. l994;75:23-32.
Bowditch HP. Uber die Eigenthumlichkeiten der Reizarbeit, wilche die Muskelfasem des Herzens zergen. Ber Verh der kongiglich sachsischen ges Wissenschaften ni Leipqig. 187 1 ; 23:652.
Brandl CJ, deLeon S, Martin DR, MacLennan DJ. Adult foms of C ~ " - A T P ~ S ~ of sarcoplasmic reticulum: expression in developing skeletal muscle. J Bi01 Chem. 1987; 262L3768-3774.
Brandl VJ, Green NM, Korczak B, MacLeman DJ. Two C~''ATP~S~ genes: homologies and mechanical implications of deduced amino acid sequences. Cell. 1986;44:597-607.
Brilla CG & Maisch B. Regulation of the structural remodelling of the myocardium: nom hypertrophy to heart failure. (Review). Eur Heurt J. 1994; 15(suppl D):45-52.
Brilla CG, Pick EL, Tan LB, Janicki JS, Weber KT. Remodelling of the rat right and lefi ventricle in experimental hypertension. Circ Res. 1 WO;67: 13 55- 1364.
Bdla CG, Rupp H and Maisch B. The renin-angiotensin-aldosterone system and myocardial collagen matrix remodelling in congestive heart failure. Eur Heart J. 1995; 16(supplO): 107- 109.
Brilla CG, Zhou G, Matsubara L, Weber KT. Collagen metabolism in cultured adult rat cardiac fibroblasts: response to angio tensin II and aldosterone. J Mol Ce11 Cardiol. 1 994;26: 809-820.
Brillantes A-M, Allen P, TakahashiT, Inimo S, Marks AR. Differences in cardiac calcium release
115

chamiel (ryanodine receptor) expression m myocardiurn fkom patients with end-stage heart failure caused by ischemic vernis dilated cardiomyopathy. Circ Ra. 1992;7 1 : 18-26.
Broughton A, Komer PI. Steady-state effects of preload and aAerload on isovolumic indices of contractility in autonomically blocked dogs. Cadiovasc Res. 1980; l4:245.
Buccino RA, Harris E, Spann JF Jr, et al. Response of myocardial comective tissue to development of experimtntal hypertrophy. Am J Physiol. l969;2 16:425.
Burch G, Giles T. Senile cardiornyopathy. J Chronic Dis. 197 1 ;24: 1.
Burk SE, Lytton J, MacLennan DH, Shull GE. cDNA cloning, funciional expression, and mRNA tissue disbiiution of a third organellar ~ a ' + pump. J Biol Chem. l989;264: 1586 1 - 1 5868.
Bumett JC. Kao PC, Hu DC, Heser DW, Heublein D, Granger .JP, Opgenorth TJ, Reeder GS. Atrial natriuretic peptide elevation in congestive heart failure in humans. Science 1986;U 1 : 1 145- 1147.
Campbell KP, MacLennan DH. Purification and characterization of the 53,000 dalton glycoprotein from the sarcoplasmic reticulum. J Biol Chem. 198 1 ;256:4626-4632.
Capasso JM, Palackol T, Olivetti G, Anversu P. Lefi ventricular failure induced by long-tm hypertension in rats. Circ Res. l990;66: 1400- 14 12.
Carafoli E. The homeostasis of calcium in heart cells. J nt02 Cell Cardiol. 1985; 1 7:203-2 12.
Carafoli E & Bing RJ. Myocardial failure. J Appl Cardiol. l988;3:3- 18.
Carrier L, Boheler KR, Chassagne C, dela Bastie D, Wisnewsky C, Lakatta EG, Schwartz K. Expression of the sarcomeric actin isogenes in the rat heart with developrnent and senescence. Circ Res. l992;70:999- 1005.
Chelly J, Kaplan JC, Maire P, Gautron S, Kahn A. Nature (London) 1988;333:858-860.
Chien R. Signaling mechanisms for the activation of an embryonic gene program during the hypertrophy of cardiac ventricular muscle. Basic Res Cardiol. 1992;87:49-58.
Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloro fom extraction. Anal Biochern. 1987; 1 62: 156- 1 59.
Conway G, Healitt RA, Fowler NO, Gabel M, Green S. The effect of hyperthyroidism on the sarcoplasmic reticulum and myosin ATPase of dog heart J Mol Cell Curdiol. 1976;8:39-5 1 .
116

Coronado R, Momssette J, Sukhareva M, Vaughan DM. Structure and function of ryanodine receptors. Am J Physiol. l994;266:C 1485-C 1504.
Cory CR, Shen H, O'Brien P.J. Compensatory asymmetry in down-regulahon and inhibition of the rnyocardial ca2' cycle in congestive heart failure produced in dogs by idiopathic dilated cardiomyopathy and rapid ventncular pacing. J Mol Cel Cardiol. 1994;26(2): 173- 1 M.
Cory CR, McCutcheon U, O'Grady M, Pang AW, Geiger JD, O'Brien PI. Compensatory dowuregulation of myocardial ca2+ chan.net in SR From dogs with heart failure. Am J Physiol. l993;264 (3 Pt 2):H926-937.
Davis W. The mitogen-activated protein kinase signal transduction pathway. J Bi01 Chem. l993;268: 14553014556.
de la Bastie D, Levitsky D. Rappaprot L, Mercadier W, Marotte F, Wisnewsky C, Brovkovich V, Schwartz K. Lompre A-M. Function of the sarcoplasmic reticulum and expression of its ca2+- ATPase gene in pressure overload-induced cardiac hyperirophy in the rat. Circ Res. 1 990;66:554-564.
de la Bastie D, Wisnewsky C, Schwqrtz K, Lompre A-M. (ca2' M~*' )dependent ATPase mRNA h m smooth muscle sarcoplasmic reticulurn differs h that in cardiac and fast skeletal muscles. FEBS Lett. l988;229:45-48.
Dhaila NS, Das PK, Sharma GP. Subcellular basis of cardiac contractile failure. JMol Cell Cardiol. 1978; 10:363-385.
Dhalla NS, Dixon IMC, Beanich RE. Biochemical basis of heart hnction and contractile fahre. JAppl Cmdiol. 1991;6:7-30.
Dhalla NS, McNamara DBmand MB. Heart sarcolemma as a dynamic excitable membrane. In The sarcolemma: Recent Advances in Studies on Cardiac Structure and metabolism. Vol. 9, pp. 1-19. Paul-Emile Roy and N.S DhaIla, Eds. Baltimore: University Park Press (1976).
D M a NS, Pierce GN, Panagia V, Singal PK, Bermish RE. Calcium movernents in relation to heart function. Basic Res Cardiol. 1982;77: 1 17-1 39-
Dhalla NS, Ziegelhoffer A, Harrow JAC. Regulatory role of membrane systems in heart function. Canad J Physiol Phar. 1977; 55:1211-1234.
Endo W. Calcium release h m the sarcoplasmic reticulum. Physiol Rev. l997;57:7 1-1 08.

EngeImann GL, Vitullo JC, Gemty RG. Morphometric analysis of cardiac hypertrophy during development, maturation, and senescence in spontaneously hypertensive rats. Cire Res. 1 987;60:487494.
Fabiato A. Calcium-induced release of calcium h m the cardiac sarcoplasmic reticulum. Am J Physiol. 1 983;24S(Cell Phvsiol 14):C 1 C 14.
Feldman AM, Ray PE, Silan CM, Merce JA, Minobe W. Bristow MR. Selective gene expression in failing human heart: quantification of steady-state levels of messenger RNA in endomyocardial biopsies using the polymerase chain reaction. Circulation. 1 99 1 $3 : 1 866- 1 8 72.
Fifer MA, Bourdillon PD, Lorell BH. Altered lefi ventricular diastolic properties during pacing- induced angina in patients with aortic stenosis. Circulation. 1986; 74:675-683.
Fine A, Goldstein RH. The effect of transfortning growth factor-6 on ce11 proliferation and collagen fornation by lung fibroblasts. J Biol Chem. l987;262:3897-3902.
Flesch M, Schwinger RH, schnabel P, Schiffer F, van Gelder 1, Bavendiek U, Sudkamp M, Kuhn- Regnier F, Bohm M. Sarcoplasmic reticulum ~ a ' - - ~ ~ ~ a s e and phospholamban mRNA and protein levels in end-stage heart failure due to ischemic or dilated cardiomyopathy. J Mol Med. 1996;74(6):32 1-332.
Fliegel L, Burns K, Opas M, Michalak M. The high-affïnity calcium binding protein of sarcoplasmic reticulum: tissue distribution, and homology with calregulin. Biochim Biophys Acta. 1989;982: 1-8.
Fliegel L, Leberer E, Green NM, MacLennan DH. The fast-twitch muscle calsequestrin isofom predominates in rabbit slow-twitch soleus muscle. FEBS Zen 1989;242:297-300.
Fliegel L, Ohnishi M, Carpenter MR, Khanna VK, Reithmeir RAF, Mademan DH. Amino acid sequence of fast-twitch skeletal muscle calsequestrin deduced kom cDNA and peptide sequencing. Proc Nat1 Acad Sci USA. 1 987;84: 1 167- 1 17 1.
Furuichi TS, Yoshikawa A, Miyawaki K, Maeda WN, Mikoshiba K. Pnmary structure and fûnctional expression of the inositol 1,4,5-trisphophate-binding protein P400. Nature (Zond). 1989;342:32-3 8.
Geistefer-Lowrance AAT, Kass S. Tanigawa T, Vosberg HP, McKanne W, Seidman CE, Seidman JG (1990). A molecular basis for familial hypertrophie cardiomyopathy: a P-cardiac myosin heavy chai. gene missense mutation. Cell62:999- 1006.
Gibbs CL, Wendt IR, Kotsanas G, Young IR. Mechanical, energetic, and biochemical changes in I l8

long-terni volume overload of rabbit heart. Am J Physiol. 1992;262:H8 19-H827.
Gibbs RA. DNA amplification by the polymerase chah reaction. Anal Chenr. 199O;62: 1202- 1214,
Gilliland G, Perrin S, Blanchard K, and Bunn F. Analysis of cytokine mRNA and DNA: Detection and quantitation by cornpetitive polymerase chain reaction. Proc Notl Acad Sci USA. 1990;87:2725-2729.
Gleason WL, Braunwald E. Studies on the fint derivative of the ventncular pressure pulse in man. J Clin Invest. 1962; 4 1 $0-9 1.
Go LO, MoschelIa MC, Watras J, Handa KK, Fyfe BS, Marks AR. Differential regdation of two types of intraceliular calcium release charnels during end-stage heart failure. J C h Invest. 1995;95:888-894.
Golsstein RH, Poliks CF, Pilch PF, Smith BD, Fine A. Stimulation of collagen formation by insulin and insulin-like growth factor 1 in cultures of human Iung fibroblasts. Endocrinology. 1989; 124:964-970.
Goodkind MJ, Damback GR, Thrum PT, Luchi M. Effect of thyroxin on ventricular myocardial conûac tility and ATPase activity in guinea pig. Am J P hysiol. 1974;226:66-72.
Green NK, Gammage MD, Franklyn JA, Sheppard MC. Regdation by thyroid status of c-mye, c- fos. and H-ras mRNAs in the rat myocardium. J Endocrinol. 1 99 1 ; 1 30:239-244.
Grossman W. Diastolic dyshuiction and congestive heart failure. Circulation. 1990;8 l(supp1 I I I ) : III- 1-111-7.
Grossman W, et al: Alterations in preload and myocardial mechanics. Circ Res. 1972; 3 1 :83.
Grossman W, McLaurin LP. Diastolic properties of the left ventricle. Ann Intern Med 1976; 84:3 16.
Grossman W. Cardiac hypertrophy: usehl adaptation or pathological process? Ant JMed. 1980; 49576-584.
Gwathmey JK, Copelas L, Makinnon R, Shoen FJ, Feldman MD, Grosman W, Morgan P. Abnomial intracellular calcium handling in myocardium fiom patients with end-stage heart failure. Circ Res. l987;6 1 :70-76.
Hasenfuss G, Mulieri LA, Blanchard EM, Holubarsch C, Leavitt BI, Ittleman F, Alpert NR.
119

Energetics of isometric force development in control and volume-overload human myocardium: cornparison with animal species. Circ Res. 1 99 1 ;68:836-846.
Hasenfuss G, Mulieri LA, Leavitt BJ, Allen PD, Haeberle JR, Alpert NR. Alteration of contractile function and excitation-contraction coupling in dilated cardiomyopathy. Circ Res. I992;7O: 1225-1 232.
Hasselbach N. Relaxing factor and the relaxation of muscle. Prog Biophys Mol Biol. 1 964; 14: 1 67- 222.
Hewett TE, Grupp IL, Grupp G, Robbins S. Alpha-skeletal actin is associated with increased contractility in the mouse heart. Circ Res. l994;74(4)740-746.
Iwaki K, Sukhatme VP, Shubeita HE, Chien KR. a and adrenergic stimulation induce distinct patterns of immediate early gene expression in neonatal rat myocardial cells: fos/jun expression is associated with sarcomere assembly ; Egr- 1 induction is primady an a 1 mediated response. J Biol Chem. 1990; 265:13809-138177.
Ho KKL, Anderson KM, Kannel WB, et al. S u ~ v a l aRer the onset of congestive heart failure in Framingham Heart Study subjects. Circulation. 1 993;88: 107- 1 1 5.
Izumo S, Lompre AM, Matsuoaka R, et al. Myosin heavy chah messenger RNA and protein isoform transitions during cardiac hypertrophy . J Clin Invert. 1 987;79:970-977.
Izumo S, Nadal-Ginard B, Mahdavi V. Proto-oncogene induction and reprogramming of cardiac gene expression produced by pressure overload. Proc Nail Acad Sci. USA. 1 988;85:339-343.
Jouannot P, Hatt PY. Rat myocardial mechanics during pressure induced hypertrophy development and reversal. AM J Physiol. 1 975; 229:H355-H364.
Kanangat S, Solomon A, Rouse B.T. Use of quantitative polymerase cham reaction to quantitate cytokine messenger RNA molecules. Mol Imrnunol. l992;29(No. 1 0): 1 229- 1 23 6.
Kanda T, Nakano M, Arai M, Yamamoto A, S h i T, Murata K. Myocardial atrial natriuretic factor gene expression afier coronary Iigation in rats. Exp Clin Endocrinol. 1993; 10 l(3): 1 73- 177.
Kamel WB, Ho KKL. Thom T. Changing epidemiological leahires of cardiac failure. Br H m J. 1994;72:S3-S9.
Kannel WB, Pinsky J. Trends in cardiac failure: incidence and cause over three decades in the Framingham Study. J Am Coll Cardiol. 199 1 ; 1 7:87A. Abstract.

Katz A M. Cardiomyopathy of overload: A major deteminant of prognosis in congestive hart failure. N Engl J Med. 2 990; 3 22: 1 00- 1 1 0.
Katz AM. The cardiomyopathy of ovedoad: an unnatuni1 growth response. Eur Heurt J. 1995; 16 (Suppl0):llO-114.
Kimura Y, Otsu K, Nishida K, Kuniya T, Tada M. Thyroid hormone enhances ca2' pumping activity of the cardiac sarcoplasmic reticulum by increasing ca2'- ATPase and decreasing phospholamban expression. J Mol Ce1 Cardiol. 1 994;26(9): 1 1 45- 1 1 54.
Kirchberger MA, Tada M, Katz AM. Adenosine 3',5'-monophosphate-dependent protein kinase-catalyzed phosphorylation reaction and its relationship to calcium transport in cardiac sarcoplasrnic reticulum. J Biol Chem. l974;249:6 166-6 1 73.
Kiss E, Bal1 NA, Kranias EG, Walsh RA. Differential changes in cardiac phospholamban and sarcoplasmic reticular ca2+- ATPase protein levels. Effects on ca2' transport and mechanics in compensated pressure-overioad hypertrophy and congestive heart failure. Circ Res. i 995; 77(4):759-764.
Knowlton KU, Baracchini E, Ross RS, et al. Co-regulation of the aaial natriuretic factor and cardiac myosin light chah-2 genes during a-adrenegic s timuiation of neonatal rat vennicualr cells. J Biol Chem- 199 1 ; 266:7759-7768.
Knowlton KU, Michael M, Shubeita J. et al. The alA-receptor subtype mediates biochemical, molecular, and rnorphologic features of cultured rnyocardial ce11 hypertrophy. J Biol Chem (in press).
Komuro 1, Kurabayashi M, Shibazaki Y, Takaku F, Yazaki Y. Molecular cloning and characterization of a ca2' NIg'*-dependent adenosine triphosphatase From rat cardiac sarcoplasmic reticulum: regulation of its expression by pressure overload and developmental stage. J Clin Inver. l989;83: 1 102-1 108.
Komuro 1, Kurabayashi M, Takaku F, Yazaki Y. Expression of cellular oncogenes in the myocardiu. during the developmental stage and pressure-overloaded hypertrophy of the rat heart. Cire Res. 1988;62: 1075-1 079.
Kusuoka H, Futaki S, Koretsune Y, Kitabatake A, Suga H, Kimada T, houe M. Alterations of intracellular calcium homeostasis and myocardial energetics in acute afiamycin-induced heart Mure. J Cardiovasc Pharmacol. 1 99 1 ; 1 8:437-444.
Lamers JHJ, Stinis JT. Defective calcium pump in the sarcoplasmic reticulum of the hyperirophied rabbit heart. Life Sci. 1979;24:23 13-2320.
12 1

Langer GA. Events at the cardiac sarcolemma: localization and movement of contractile- dependent calcium. Fedn Prec. 1 976;3 5 : 1 274- 1 278.
Lecarpentier Y, Martin JL, Gastineau P. Hatt PY. Load dependence of marnmalian heart relaxation during cardiac hypertrophy and failure. Am J P hysiol. 1 982; 242 : H8S 5-H86 1.
Lecarpentier Y, Riou B, Clergue M. Lambert F, Chemla D. The contraction-relaxation coupling during pressure-induced cardiac hypertrophy. Eur Heurt J. 1990; 1 1 (suppl G):46-53.
Lecarpeutier Y, Waldenstrom A, Clergue M, C h d a D, Oliviero P, Martin JL, Swynghedauw B. Major alterations in relaxation during cardiac hypertrophy induced by aortic stenosis in guinea pig. Circ Res. l987;6 1 : 1 07- 1 16.
Lembo G, Hunter JJ & Chien KR. Singnaling pathways for cardiac growth and hypertrophy: Recent advances and prospects for growth factor therapy. Ana NY Acad Sci. 1995;752: 1 15-1 27.
Levi* D, de la Bastie D, Schwartz K, Lompre A-M. caZ'- ATPase and function of sarcoplasmic reticulum during cardiac hypertrophy . Am J Physiol. 1 99 1 ; 26 1 (suppl) :23-26.
Limas CJ. Calcium transport ATPase of cardiac sarcoplasmic reticulum in expenmental hyperthyroidism. Am J Physiol lW8;235:H745-H7S 1 .
Limas CJ, Spier SS, Kahlon J. Enhanced calcium transport by sarcoplasmic reticulum in mild cardiac hypertrophy . J Mol1 Cell Cardiol. 1980; 1 2: 1 1 03- 1 1 1 6.
Linck B, Boknik P, Eschenhagen T, Muller FU, Neumann J, Nose M, Jones LR, Schmitz W, Scholz H. Messenger RNA expression and immunological quantification of phospholarnban and SR ca2+- ATPase in failing and nonfaiiing human hem. Cordiovas Res. 1 996;3 1 (4):625-632.
Lindpaintner K, Lu W, Neidermaier N, Schieffer B, Just H, Ganten D, Drexler H. Selective activation of cardiac angiotensinogen gene expression in pst-infarc tion ventricular remodelling in the rat. J Mol Cell Cardiol. 1993;25(2): 133- 143.
Lompre AM, Lambert F, Lakatta EG, Schwartz K. Expression of sarcoplasmic reticulum ca2'- ATPase and calsequesûin genes in rat heart during ontogenic development and aging. Circ Res. 1991;69:1380-1388.
Lompre AM, Mercadier JJ, Schwartz K. Changes in gene expression during cardiac growth. inter Rev C'OZ. 1990; 124: 137-186.
Lorell BH, Grossman W. Cardiac hypertrophy : the consequences for diastole. J Am col1 Cardiol.
122

Lytton J & MacLennan DH. Sarcoplasmic reticulum. In: Fozzard HA, haber E, Jennings RB, Katz AM, Morgan HE, eds. TIte HeuH and CurdiovascuIar System. 2nd ed. New York, NY: Raven Press Publishers; 1992: 1203- 1222.
Lytton J, Zarain-Hertzberg A, Periasamy M, MacLennan DH. Molecular cloning of the mammaIian smooth muscle sarco(endo)plasmic reticulum c a 2 + - ~ ~ p a s e . J Bi01 Chem. 1 989;264:7059-7065.
Maciel LMZ, Polikat R, Rohrer D, Popovich BK, Dillmann WH. Age-induced decreases in the messenger RNA coding for the sarcoplasmic reticulum ca2+-~'T'~a.se of the rat hem. Circ Res. 1990; 67~230-234.
MacKinnon Et, Morgan P. Influence of the thyroid state on the calcium transient in ventricular muscle. mgers Arch. 1986;407: 142- 144.
Marks AR, Tempst P, Chadwick C, Riviere L, Fleischer S, Nadal-Ginard B. Smooth muscle and brain inositol 1,4,5-trisphophate recepton are stmcturally and functionally similar. J Biol Chem. 1990;265: 1-4.
Marks AR, Tempst P, Hwang KS, Taubman MB, Inui M, Chadwick C, Fleischer S, Nadal- Ginard B. Molecular cloning and characterization of the ryanodine receptor/junctional chamel complex cDNA h m skeletal muscle sarcoplasmic reticulum. Proc Natl Acad Sci USA. 1989;86:8683-8687.
Matsui H, MacLennan DH, Alpert NR, Penasamy M. Sarcoplasmic reticulum gene expression in pressure overload-induced cardiac hypertmphy in rabbit. Ani J Physiol. 1 995;268(1 P t 1):C252- 258.
Mayer Y, Czosnek J, Zeelon PE, Nudel U. Expression of the genes coding for the skeletal muscle and cardiac ac tin in the heart . Nucl Acids Res. 1 984; 1 2: 1 087- 1 1 00.
McKee PA, Castelli WP, McNamara PM, et al. The natural history of congestive heart failur: the Framingham Study. NEngl JMed. 1971:285:1441-1446.McNutt XS. Ultrastructure of the myocardial sarcolermna. Circ Res. 1975; 37: 1- 13.
Mercadier J-J, Lompre AM, Duc P, Bohler KR, Frays J-B, Wisnewsky C, Allen PD, Komajda M, Schwartz K. Altered sarcoplasmic reticulum ~ a " - ATPase gene expression in the human ventricle during end-stage heart failure. J C h Invat. 1 99O;85 :3 05-3 09.
Mercadier J-J, Samuel JL, Michel J-B, Zongazo M-A dela Bastie K, Lompre A-M Wisnewsky
123

C, Rappaport L, Levy B, Schwartz K. Atrial natnuretic factor gene expression in rat ventricle during experimental hypertension. Anr Jphysiol. 1 989;257:H979-H987.
Michalak M, Campbell KP, MacLennan DH. Localization of the hi& affmity calcium binding protein and an intnnsic glycoprotein in sarcoplasmic reticulum membranes. J Biol Chem. l98O;2S5: 13 17- 1326.
Moe GW, Angus C, Howard RI, Parker TG & Armstrong PW. Evaluation of indices of leR ventricular contractility and relaxation mvolving canine experimental heart failure. Curdiovmc Res. 1992;26:362-366.
Morgan JP, Emy RE, Allen PD, Grossman W, Gwathmey JK. Abnomal intracellular calcium handling, a major cause of systolic and diastolic dysfunction in ventricular myocardium from patients with heart failure. Circulation. l99O;8 1 (suppl III): 111-2 1-111-32.
Morioka S, Honda M, Ishikaqa S, Ishinage Y, Yano S, Tanaka K, Monyama K. Changes in contractile and non-contractile proteins, intracellular ca2' and ultrastructures during the development of right ventricular hypertrophy and failure in rats. Jpn Circ J. 1992156:469-474.
Moschella M and Marks A. Inositol 1,4,5-triphosphate receptor expression in cardiac myocytes. J Cell Biol. 1993; 120: 1 137- 1 146.
Movsesian MA, Bristow MEt, Kra11 J. Calcium uptake by cardiac sarcoplasmic reticulum fiom patients with ideopathic dilated cardiomyopathy. C h Res. 1987;65 : 1 14 1 - 1 144.
Murray MT, White K. Munro JN. Conservation of femtin subunit structure: Implications for the regulation of femtin gene expression. Proc Nad Acad Sci USA. 1987;84:7438-7442.
Nadal-Ginard B, Mahdavi V. Molecular basis of cardiac performance:Plasticity of the myocardium generated through pro tien iso fom switches. J Clin Inve.sf. 1 989; 84: 1 693 - 1 700.
Nagai R, Zarain-Herzberg A, Brand1 DJ, Fujii J, Ta& M, MacLennan DH, Alpert NR, Periasamy M. Regulation of myocardial C ~ " - A T P ~ S ~ and phospholamban mRNA expression in response to pressure overload and thyroid hormone. Proc Nat1 Acad Sci U S A. 1989;86:2966- 2970.
Neyses L & Pelzer T. The biological cascade leading to cardiac hypertrophy. Eur Heurt 1995; 16 [Suppl N):8- 1 1.
Nuss HB, Houser SR. Voltage dependence of contraction and calcium current in severely hypertrophied f e h e ventricular myocytes. J Mol Ce11 Cardiol. 199 1 ;23:7 17-726.

O'Brien PJ, Moe GW, Nowack LM, Grima EA, Armstrong PW. Sarcoplasmic reticulum ~ a - " release channel and ATPase-synthesis activities are early myocardiat markers of heart failure produced by rapid ventricular pacing in dogs. Con J Phosiol Phamcol . 1 994;72(9):999- 1 006.
Olson HM, Young DM, Pneur DJ, LeRoy AF, Reagan RL. Electrolyte and morphological alterations of myocardium in ahiamycin-treated rabbits. Am J Pathol. 1 974;77:439-454.
Opie LH. Intracellular ca22+ fluxes and sarcoplasmic reticulum. In : The heart. Physiology und metabolism (Opie L H . Ed.) Raven Press. New York, 199 1 : 127- 146.
Orenstein TL,, Parker TG, Butany N , Goodman IM, Dawood F, Wen WH, Wee L, Martino T, McLaughlin PR & Liu P. Favorable lefl ventricular remodeIlhg following large myocardial infarction by exercise training: eflect on ventncular morphology and gene expression. J Clin lnvest. 1995;96:858-866.
Otsu K., Willard HF, Khama VK, Zorzato F, GreenN, MacLennan DH. Molecular cloning of cDNA encoding the ca2'-release channel (ryanodine receptor) of rabbit cardiac muscle sarcoplasmic reticulum. J Biol Chem. 1990;265: 13472- 13483.
Pagant ED, Alousi AA, Grant AM, Older TM, Dziuban SW Jr, Allen PD. Changes in myofibrillar content and M$' -ATPase activity in ventncular tissues nom patients with heart failure caused by coronary artery disease, cardiomyopathy, or mitrai valve insuficiency. Circ Ra. 1988;63:380-385.
Parker TG. Molecular biology of cardiac growth and hypertrophy. Herr. 1993; 18:245-255 (Nr.4).
Parker TG, Chow KL, Schwartz RJ & Schneider MD. Dif'ferential regulation of skeletal a-actin transcription in cardiac muscle by two fibroblast growth factors. Proc Nat Acad Sci (Wà.sh). lWO;87:7066.
Parker TG, Chow KL, Schwartz RI & Schneider MD. Positive and negative control of the skeletal a-actin promoter in cardiac muscle. J Bi01 Chem. l992;267:3343.
Parker TG, Schneider MD. Growth factors, proto-oncogenes, and plasticity of' the cardiac genome. Ann Rev Physiol 1 99 1 ;53 : 1 79.
Parker TG, Schneider MD. Peptide growth factors can provoke " fetal" contractile protein gene expression in rat cardiac myocytes. J Clin Invest. 1990;85:507.
Parker TG & Vijan S. Regdation of human Fmyosin heavy chain transcription by tcansforming

growth factor 1 and nuclear proto-oncogenes (abstract). Circulation. I992;86: 1400.
Raff GL, Glantz SA. Volume loading slows left ventricular isovolumic relaxation rate. Circ Res. 1981; 48:813.
Rannou F, Dambnn G, Marty 1, Carre F, Prouve P, Lornpre AM, Charlemagne D. Expression of the cardiac ryanodine receptor in the compensated phase of hypertrophy in rat heart. Cardiovasc Res. I 995;32(2):238-255.
Rappolee DA, Mark D, Banda MJ Werb 2. Science 1988;241:708-7 12.
Reiss K, Capasso JM, Huano HE, Meggs LG, Li P, Aversa P. h g II receptors, c-myc, and c-jun in myocytes afler myocardial infarction and ventricular failure. Am J Physiol. 1 993 ;264 (3 Pt 2):H760-769.
Reiss K, Meggs LG, Li P, Olivette G, Capasso JM, Anversa P. Upregulation of IGF1 -receptor and late growth related genes in ventricular myocytes acutely after infarction in rats. J Cell Physiol. 1994;158(1):160-168.
Riabowol KT, Vosatka RJ, Ziff EB, Lamb NJ, Feramisco IR. Microinjection of fos-specific antibodies blocks DNA synthesis in fibroblast cells. Mol Cell Biol. l988;8: 1 670- 1676.
Rohrer D, Dillmann WH. Thyroid hormone markedly increases the mRNA coding for sarcoplasmic reticulum c~ '+-ATP~s~ in rat heart. J Bi01 Chem. 1988;263:694 1-6944.
Sadoshima J, Xu Y, Slayter HS & Izumo S. A u t o c ~ e release of angiotensin II mediates stretch- induced hypertrophy of cardiac myocytes in vitro. Cell. 1993; 75:977-984.
Saiki RK, Gelfand DH, Stoffel S, Scharf SJ, Higuchi R, Horn GT, Mullis KB, Erlich HA. Primer- directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science 1988;239:487-49 1.
Sambrook I, Fritsch EF, Maniatis T: Molecular cloning. New York, Cold Spring Harbor Laboratory Press, 1989.
Sapp JL, Howlett SE. Density of ryanodine receptors is increased in sarcoplasmic reticulum from prehypertrophic cardiomy opathic hamster heart . J Mol Cel Cardiol. 1 994;26(3) :325-3 34.
Sasayama S, Nonoogi H, Miyazaki S, et al. Changes in diastolic properties of the regional myocardium during pacing-induced isc hemia in hurnan subjects. J Am Col1 Cardiol. 1 98 5;5:599.
Schiaffino S. Samuel JL, Sassoon D, et al. Uncoordinated accumulation of a-skeletal actin and 126

rnyosin heavy chah mRNAs during early stages of pressure overload-induced cardiac hypertmphy demonstrated b y in situ hybridization. Cire Res. 1 989; 6 W 3 7-948.
Schneider MD & Olson EN. Control of myogenic differentiation by cellular oncogenes. Molec h'eurobiol. l988;2: 1.
Schneider MD & Parker TG. Cardiac myocytes as targets for peptide growth factors. Circulation. 1 9W;8 1 : 1443.
Schwartz K. (1990) Phenoconversion and mechanogenic transduction of the mamalian hem. Medicindscience 6564-673.
Schwartz K, Boheler KR, de la Bastie D,et al. Switches in cardiac muscle gene expression as result of pressure and volume overload. Am J Physiol. 1 994; 262:R364-369.
Schwartz K, Chassagne C, Boheler KR. The rnolecular biology of heart failure (Review). J Am Col1 Curdiol. 1 993;22(suppl A):30A-33 A.
Schwartz K, de la Bastie D, Bouveret P, et al. a-Skeietal actin -As accumulate in hypertrophiecl adult rat hearts. Circ Res. l986;59:5S 1-555.
Schwartz K, Lompre Am, Lacombe G, Bouveret P, Wisnewsky C, Whalen RG, D'Albis A, Swynghedaw W. Cardiac rnyosin isozymic transitions in mammals. In: Alpert NR, ed. Perspectives in CardiovascuIur Reseurch. New York, NY: Raven Press Publishers; 1983;7:345- 358.
Schwrtzkopff B, Uhre B, Ehle B, Losse B, Frenzel H. Variability and reproducibility of morphologie findings in endomyocardial biopsies of patients with hypertrophie obstructive cardiomyopathy. Zeir~chrifi fur kurdiologie. 1987;76:suppl3:26-32.
Schwinger RH, Bohm M, Schmidt U, Karczewski P, Bavendiek U, Flesch M, Krause EG, Erdniann E. Unchanged protein Ievels of SERCA II and phospholamban but reduced CaZC uptake and c~' '-ATP~s~ activity of cardiac sarcoplasmic reticulum from dilated cardiomyopathy patients compared with patients with nonfailing hearts. Circulation. 1995;92(11):3220-3228.
Sen S, Tarazi RC, khairallah PA,Bumpus FM. Cardiac hypertrophy in spontaneously hypertensive rats. Cardiovarc Res. 1974; 35:775-781.
Shen J, Mirsalimi SM, Weiler JE, Julian RJ, O'Brien PJ. Effects of mild cardiac hypertrophy, induced by volume overload in turkeys, on myocardial sarcoplasmic reticulum calcium-pump and calcium-channel activities and on the creatine kinase system. Am J Vet Ra. 1 99 1 ;52: 1527- 1530.
127

Shubeita HE, Martinson E, Van Bilson M, Chien KR, Brown IH. Expression of a constitutively active protein kinase C gene activates the transcription of the ANF and MLC-2 genes in culhired myocardial cells. Proc Nutl Acad Sci USA. 1 W2;89: 1 305- 1 309.
Sin FM, Krueger J, Nordin C, Ming 2, Aronson RS. Depresseci intracellular calcium trmsients and contraction in myocytes fiom hypertrophied and fading guinea pig hearts. Am J Physiol. 1 99 l;26 1 :H5 14-H530.
Skelton CL, Su JY, Pool PE. Influence of hyperthyroidism on glycerol-extracted cardiac muscle h m rabbits. Cardiovasc Res. 1976; 10:380-384.
Sommer IR, Jennings RB. Ultrastructure of cardiac muscle. In: Fovard HA, haber E, Jemings RB, Katz AM, Morgan HE, eds. The Heurt and Cardiovnsnrlm System. 2nd ed. New York, NY: Raven Press Publishers; I 992:3-50.
Suko J. The calcuim pump of cardiac sarcoplasmic reticulum: FMctional alteratsons at different levels of thyroid state in rabbits. J Physiol flond). 1973;228:563-582.
Sutoh K. Identification of myosin-binding sites on the actin sequeme. Biochemirtry. l982;2 1 :3657-366 1.
Takahashi T, Allen PD, 1-0 S. Expression of A-, B-. and C-type natriuretic peptide genes in failing and developing human ventricles:correlation with expression of the c~ ' - -ATP~s~ gene. Circ Res, 1 WZ;7 1 :9- 1 7.
Takahashi T, Allen PD, Lacro RV, Marks AR, Dennis AR, Schoen FJ. Grossman FJ, Grossman W, Marsh JD, Izumo S. Expression of dihyfropyridine receptor (ca2- channel) and calsequeshin genes ni the myocardium of patients with end-stage heart failure. J Clin Invat. 1992;90:927-935.
Takahashi T, Schunkkert H, Isoyarna S, et al . Age-related differences in the expression of proto- oncogenes and contractile proiein genes in response to pressure overload in the rat rnyocardim. J Clin Invest. 1992; 89:936-946-
Tanigawa G, Jarcho JA, Kass S, Solomon SD, Vosberg H-P, Seidman JG, Seidman CE (1990). A molecular basis for familial hypertrophie cardiomyopathy: an C@ cardiac rnyosin heavy chain hybrid gene. Ce11 62:99 1 -998.
Thorburn J, Carlson M, Mansour SJ, Chien KR, Ahn NG & Thorburn A. Inhibition of a signaling pathway in cardiac muscle cells by active mitogenactivated protein kinase kinase. Mol Bi01 Cell. 1 M ; 6 : 147% 1490.

Thorbum A, Thorburn J, Chen SY, et ni. H-Ras dependent pathways can activate morphological and genetic markers of cardiac muscle ce11 hypertrophy. JBiol Chem. 19931 268:2244-2249.
Tomplisin CW, Godin DB, Rabkin S W. Adnarnycin cardiomyopathy: implication of cellular changes in a canine mode1 with mild impairment of le fi ventricular fùnc tion. Biochem Pharmacol. 1985;34:4033-404 1.
Tsoporis JN, Marks A, Kahn KJ, Butany W. Liu PP, O'Hanlon D, Parker TG. S lOOp inhibits a 1 -adrenergic induction of the hypertrophic p henotype in cardiac myoc ytes. J Biol Chem (in press).
Unverferth DV, Lee SW, Wallick ET. Human myocardial adenosine triphosphate activities m health and heart failure. Am Heart J. 1 988; 1 1 5: 1 39- 146.
Van Bilsen M & Chien KR. Growth and hypertrophy of the heart: towards an understanding of cardiac specific and inducible gene expression. Cardiovasc Res. 1 993; 27: 1 140- 1 149.
Vandekerckhove .J Weber K. The complete amho acid sequence of actins from bovine aorta, bovine heart, bovine fast skeletal muscle, and rabbit slow skeletal muscle. Drfferenliatiorz. 1979; l4:123-133.
Wang AM, Doyle MV, Mark DF. Quantitation of rnRNA by the polymerase chain reaction. h o c Nad Acad Sci U SA,. 1989;86:97 17-972 1.
Wallace AG, Skinner NS, Mitchell JH. Hemodynamic detertninants of the maximal rate of rise of le ft ventricular pressure. Am J Physiol. 1963 ; 205:30.
Weber KT, Clark WA, Janicki JS, Shroff SG. Physiologic versus pathologic hypertrophy and the preswe-overload myocardiurn. J Cardiovasc Pham. 1987; 10:S37-S49.
Weber KT, Janicki JS. The heart as a muscle-pump system and the concept of heart failure. Am Heart J. 1979;98:371.
Weber KT, Janicki JS, Pick R, Capasso J, Anversa P. Myocardial fibrosis and pathologic hypertrophy in the rat with renovascular hypertension. Ani J CardioZ. I990;65: 1 G-7G.
Whitmer JT, Kumar P, Solaro TU. Calcium transport properties of cardiac sarcoplasmic reticulum from cardiomyopathie Syrian hamsters (BI0 53 S8 and 14.6): evidence for a quantitative defect in dilated myopathie hearts not evident in hypertrophic hearts. Circ R a . 1 988; 62:8 1-85.
Zarain-Herzberg A, Afzal N, Elimban V, Dhalla NS. Decreased expression of cardiac sarcoplasmic reticulum ~ a ' ' ~ ~ ~ a s e - ~ u m ~ ATPase in congestive heart failure due to myocardial
129

infàrction. Mo2 Ce2 Biochem. 1996; 16% l64:285-290.
Zarain-Herzberg A, MacLennan DH, Periasamy M. Characterization of rabbit cardiac sarco(endo)plasmic reticulum C ~ ~ * A T P ~ S ~ gene. J Biol Chem. 1 990;265:467O-4677.
Zarain-Herzberg A, Y ano K, Elirnban V, DhalIa NS. Cardiac sarcoplasmic reticulum ca2+-~TPase expression in streptozocin-induced diabetic rat heart. Biochem Biophys Res Comm. 1994;203(l): 1 13-1 20.
Zierhut W, Zimmer HG. Significance of myocardial a- and adrenoceptors in catecholamine- induced cardiac hypertrophy. Circ Ra. 1989; 65: 1417-1425.
Zirnpfer M, Vatner SF. Effects of acute increases in left venûicular preload in indices of myocardial Function in conscious, unrestrained and intact, tranquilized baboons. J Clin Invest. 198 1 ; 67:430.
Zorzato F, Fujii J, Otsu K, et al. Molecular cloning of cDNA encoding human and rabbit forms of the ca2' release channel (ryanodine receptor) skeletal muscle sarcoplasmic reticulum. J Biol Chem, 1 !WO;265:2244-2256.





![Endoplasmic Reticulum Stress and Lipid Metabolism · 2019. 8. 7. · endoplasmic reticulum [8], which is responsible for the syn-thesis and folding of proteins, as well as calcium](https://static.fdocuments.in/doc/165x107/60f7de19756675294413c02d/endoplasmic-reticulum-stress-and-lipid-metabolism-2019-8-7-endoplasmic-reticulum.jpg)