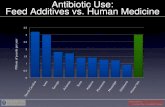
Sample Webinar Slides
description
Transcript of Sample Webinar Slides

Sample Webinar Slides
From ACUP Consultingwww.acupconsulting.com/webinars.htm
l

Compliance Inspections
PAM, Mock-USDA and Medical Record Audits
Melissa Hunsley, PhD, CPIA
www.ACUPconsulting.com
• 1-hour webinar • 60 slides • $75 per institution

Today’s Webinar
Inspection basics Where & when to inspect Inspection criteria Preparing for a visit from the USDA
Notes on “Adequate Veterinary Care” Medical Records Audits Other records that should be inspected and maintained
IACUC records & details “written narrative” in the IACUC protocol
Tips for IACUCs Review & Final Recommendations

“Basic” areas to inspect:
Centralized housing Decentralized housing & satellites Procedure areas (in & out of facility) Surgery areas (in & out of facility) Imaging and irradiation facilities Behavioral testing areas Feed, bedding & equipment storage Cage wash

When preparing for:
IACUC, AAALAC (the Guide)“Basic” areas plus: Occupational health program for all animal users Protocols using biohazards reviewed by Biosafety Professionals
If job requires respirator: fit testing Pre-employment screening (if allowed)
Training records for staff & students
All vertebrate animal use areas laboratory-bred Mus mice, Rattus rats, and birds
Fish & amphibians

When preparing for: USDA (AWA, AWR)
“Basic” areas plus Detailed review of USDA-species animal medical records• Compare to approved protocols
Disposition records for dogs and cats Environmental enrichment plan for NHPs Exercise plan for dogs
NO cold-blooded vertebrates, laboratory-bred Mus mice or Rattus rats, or birds

Procedure Areas- General
Drugs, agents, medical materials are: not expired listed on protocol stored appropriately
• Controlled substances locked up
• All subs stored away from detergents or chemicals
No corrugated cardboard, unsealed wood or cloth chairs
Stored feed and water is covered and not expired
Carcasses stored appropriately (i.e. freezer)
Compressed gas tanks secured to wall

Medical Record Audits Must be complete

Medical Records Audits Treatment Sheets (or Post-Procedural Care sheets) Record of each analgesic dose listed in the protocol, even if it’s during non-working hours•“every 6 hours” means there should be a dose late at night or early in morning

Designing & Implementing a PAMProgram for Your Institution
• 1-hour webinar • 56 slides • $75 per institution

Financial Incentives NIH
Enforcing their Policy on Allowable Costs for Grant Activities Involving Animals when Terms and Conditions are not Upheld•Institutions may have to give back grant money for the times when the protocol was not followed!
USDA 2008 Farm Bill authorized USDA to increase the maximum financial penalty to $10,000 per citation

Blueprint for a PAM Program Goals Design Staff Tasks Formalizing Training Scheduling Implementing

Design How large is your animal program?
# of protocols # of USDA-covered species # of different use locations
How complex/invasive are the protocols? What is the technical level of the personnel? Highly trained technicians vs. novice grad students
What is your budget? Will staff be dedicated to PAM
Private vs. Public institutions Level of public scrutiny, sunshine laws

Examples Small private company, 1000 cages of mice, highly trained techs, mostly breeding & euthanasia protocols Don’t need dedicated PAM staff Vet tech or high level husbandry can do PAM
Large public university, 30K cages of mice, USDA species, students & postdocs, invasive protocols 2+ dedicated staff, sufficient authority & support
Vet tech or MS/PhD IACUC or Animal Resources staff

Protocol Audits: Method Read protocol thoroughly, paying attention to: Surgical procedures Drugs and other agents administered Monitoring parameters Experimental manipulations and adverse effects
Other painful procedures Unexpected outcomes Endpoints Euthanasia

Protocol Audits: Method Evaluation of training records Meeting with all personnel on protocol to ask and answer questions
Observation of procedures Inspection of surgery areas Inspection of procedure areas Inspection of animals in facility Evaluation of medical & surgical records•Compare what is documented with what is listed in protocol

Common Non-Compliances 2• Failure to euthanize animals when they reach endpoints specified in protocol– Exhibiting specific clinical signs– Tumor size– Lesion appearance
• Training– Knowledge of euthanasia techniques listed in protocol (e.g. cervical dislocation)
– Recognize pain or distress in their species

The Guide, 8th edition
Highlights, Key Points and Implications for Animal Care and Use Programs
Melissa S. Hunsley, PhD, CPIA
• 1-hour webinar • 69 slides+8 bonus • $75 per institution

Guide, 8th ed. Webinar now on Demand!
Watch our informative webinar that discusses highlights, key points and significant changes, along with implications for your animal care and use program, all organized by chapter and page number, at any time on your computer. For more information:www.acupconsulting.com/webinars.htmwww.shop.animalresearchconsulting.com

This Presentation
Focuses on the changes from the 1996 Guide
Key additions, deletions and changes Implications for animal research facilities
Musts are highlighted
Chapter # page #

Ch. 1 Key Concepts
Added definitions The Three Rs Humane care Engineering, performance & practice standards
Policies, principles, procedures (including SOPs)
Clarification of the words Must = “imperative” and “mandatory” Should = “a strong recommendation”
• Require justification for not following May = “a suggestion to be considered”Chapter 1 p 6-8

Ch. 2 Animal Care and Use Program
Definitions and responsibilities of attending veterinarian (AV), institutional official (IO) and Institutional Animal Care and Use Committee (IACUC)
AV responsibilities and authority are outlined The AV “should” oversee other aspects of the Program
Authority of the AV is defined more clearlyChapter 2
p 13-14

Occupational Health Managing Occupational Health Risks Risk management process should include Health and Safety Specialists•AAALAC expects periodic review of risk
BMBL should be consulted when biohazards are used
Chapter 2 p 18-19

Protocol Review Additions
“a clear and concise sequential description” of the animal procedures
The impact of the procedures on the animals’ well-being
Plan for the care of long-lived species after the study is completed
“Description and rationale for the anticipated or selected endpoints” (animal)
Use of hazardous materialsChapter 2 p 25-26

Major and Minor Procedures
“Whether a procedure is major or minor should be evaluated on a case-by-case basis by the IACUC and veterinarian” (p 30) Implication: Some “majors” may not be required to be major (craniotomy on mice?), but
Implication: Some “minors” probably should be considered major if considerably painful (extensive tissue dissection)
Definition of penetrating a body cavity is removed in this location, however, it is used on pg 117
Chapter 2 p 30

Pharmaceutical-Grade Substances
Should be used when available
When not available, their use should be justified in the protocol Implication: All protocols using MS-222 and Avertin should include a justification
Implication: Researchers should be trained on this requirement- many order their drugs/compounds from SigmaChapter 2 p 31

Genetically Modified Animals (GMAs)
“Carefully designed breeding strategies and accurate genotype assessment” can reduce unwanted animals
When new genotypes are generated, they should be carefully monitored. New phenotypes that “negatively affect well-being” should be reported to the IACUC.
Chapter 3 p 77

Surgery
Investigators must have surgical training, including asepsis, hemostasis, use of instruments, etc.
IACUC and AV responsible for verifying proper training Implication: personnel performing rodent surgery may need formal general surgery training from ACUP
Chapter 4 p 115-16

Key Points Summary
Recurrent themes Social housing is the default Rodents treated less like “exceptions” GMAs need increased monitoring Disaster plan should consider all animals (aquatic, irreplaceable) and potential situations
Definition of major/minor surgery debatable
Staff may need training on signs of animal well-being, reporting recurring problems
Investigators may need further training (asepsis, pharmaceutical-grade drugs, etc.)

Additional Slides
Bonus Material!