Rp58 is essential for the growth and patterning of the...
Transcript of Rp58 is essential for the growth and patterning of the...
DEVELOPMENT AND STEM CELLS RESEARCH REPORT 1903
Development 139, 1903-1909 (2012) doi:10.1242/dev.075606© 2012. Published by The Company of Biologists Ltd
INTRODUCTIONThe structure and organization of the cerebellum are crucial forthe proper execution of its functions in motor control andcoordination and cognition. The adult mammalian cerebellarcortex contains three main cell layers (Goldowitz and Hamre,1998; Herrup and Kuemerle, 1997; Sotelo, 2004), from outsideto inside: the molecular layer, the Purkinje layer (PL) and theinternal granule layer (IGL). These layers are generatedthroughout embryonic to postnatal stages from two maingerminative zones: the ventricular zone (VZ) and the upperrhombic lip (URL) (Fig. 1A). The VZ gives rise to GABAergicneurons including Purkinje neurons (PNs), whereas the URLgives rise to all glutamatergic neurons including granule neurons(Butts et al., 2011; Carletti and Rossi, 2008). In the VZ, Ptf1a isessential for the generation of cerebellar GABAergic neurons(Hoshino et al., 2005). URL cells express Atoh1 (Math1) andgive rise first to deep nuclei projection (DNP) neurons and latergenerate granule neuron progenitors (GNPs) (Machold andFishell, 2005; Wang et al., 2005) (reviewed by Butts et al., 2011;Carletti and Rossi, 2008). DNP neurons are generated from URLcells that migrate along the subpial stream to form the nucleartransitory zone (NTZ) and sequentially express Pax6 and Tbr1as they differentiate (Fink et al., 2006). Following their
specification, GNPs migrate tangentially to cover the surface ofthe cerebellum to form the external germinal layer (EGL), wherethey undergo amplification during the early postnatal weeks(Chedotal, 2010). When GNPs become postmitotic they moveinwards and differentiate in the IGL. The transcriptional programleading to correct differentiation of VZ and URL progenitors intopostmitotic neurons, which includes regulation by Ptf1a, Atoh1and Pax6, is still poorly understood.
Cerebellum hypoplasia is likely to result from abnormalprogenitor behavior. Deletion of the distal end of humanchromosome 1 (1q) is associated with cerebellar vermishypoplasia (e.g. Boland et al., 2007; Hill et al., 2007; van Bonet al., 2008). The gene or genes contributing to this phenotypeare still unknown, but a critical region containing the gene RP58(also known as ZNF238) has recently been defined (Boland etal., 2007; Hill et al., 2007; van Bon et al., 2008). Rp58 isrequired for neocortex development (Okado et al., 2009; Xianget al., 2012), acting on neuronal differentiation partly via itsdirect regulation of neurogenin 2 (Ngn2) and Neurod1 (Xiang etal., 2012). Our study demonstrates that Rp58 is crucial forcerebellum growth and organization and identifies Rp58 as anessential regulator of the early development of both GABAergicand glutamatergic cerebellar neurons.
MATERIALS AND METHODSMiceMice carrying loxP-flanked Rp58 alleles (Rp58fl/fl) were described previously(Xiang et al., 2012). Rp58 conditional knockouts (Rp58 cKO) with Nestin-Cre (Tronche et al., 1999) were obtained from crossing Rp58fl/+;Nestin-Creand Rp58fl/fl. Rp58+/– heterozygotes (Xiang et al., 2012) were crossed togenerate Rp58–/– homozygotes. Rp58fl/fl;Atoh1-CreER mice were bred withRp58fl/fl to generate Atoh1-CreER-driven mutants with tamoxifen treatment.For proliferation assays, BrdU was injected at 40 mg/kg body weight into
The Wistar Institute, 3601 Spruce Street, Philadelphia, PA 19104, USA.
*Present address: University of Pennsylvania School of Medicine, Department ofNeurosurgery, Philadelphia, PA 19104, USA‡These authors contributed equally to this work§Author for correspondence ([email protected])
Accepted 15 March 2012
SUMMARYCerebellum development depends on the correct differentiation of progenitors into neurons, a process controlled by atranscriptional program that remains poorly understood. Here we show that neural-specific deletion of the BTB/POZ zinc-fingertranscription factor-encoding gene Rp58 (Znf238, Zfp238) causes severe cerebellar hypoplasia and developmental failure ofPurkinje neurons, Bergmann glia and granule neurons. Deletion of Rp58 in mouse embryonic Atoh1+ progenitors leads to strongdefects in growth and foliation owing to its crucial role in the differentiation of granule neurons. Analysis of the Rp58 mutant atE14.5 demonstrates that Rp58 is required for the development of both glutamatergic and GABAergic neurons. Rp58 mutantsshow decreased proliferation of glutamatergic progenitors at E14.5. In addition, Rp58 ablation results in a reduced number ofGABAergic Pax2+ neurons at E16.5 together with defects in the transcriptional program of ventricular zone progenitors. Ourresults indicate that Rp58 is essential for the growth and organization of the cerebellum and regulates the development of bothGABAergic and glutamatergic neurons.
KEY WORDS: GABAergic, Mouse knockout, Rp58 (Znf238, Zfp238), Cerebellum hypoplasia, Glutamatergic, Granule neuron progenitors,Neuronal differentiation
Rp58 is essential for the growth and patterning of thecerebellum and for glutamatergic and GABAergic neurondevelopmentValérie Baubet*,‡, Chaomei Xiang*,‡, Aliah Molczan, Laura Roccograndi, Svetlana Melamed and Nadia Dahmane*,§
DEVELO
PMENT
1904
pregnant dams 30 minutes before embryos were collected. All experimentalprocedures were approved by the Wistar Institute Institutional Animal Careand Use Committee (IACUC).
Immunohistochemistry and in situ hybridization (ISH)Embryos were dissected and fixed in 4% paraformaldehyde (PFA) on icefor up to 2 hours or overnight. Postnatal mice were perfused using 4%PFA, then post-fixed in PFA for 1 hour. Immunohistochemistry wasperformed as described (Xiang et al., 2012) and antibodies are listed insupplementary material Table S1.
ISH was performed as previously described (Tiveron et al., 1996). DIG-labeled antisense riboprobes were generated from linearized plasmids:Ascl1 (Lo et al., 1991), Atoh1 (Helms and Johnson, 1998), Corl2 (Minakiet al., 2008), cyclin D2 (Ciemerych et al., 2002), Neurod1 (Allen BrainAtlas, http://mouse.brain-map.org/experiment/show/75650865), Ngn1 (Maet al., 1996), Ngn2 (Gradwohl et al., 1996), Pax2 (Nornes et al., 1990),Ptf1a (Pascual et al., 2007), Rp58 (Tatard et al., 2010) and Zic1 (Aruga etal., 1996).
These analyses were performed on cerebellar sagittal sections.
Area measurement and cell countingAlthough the cerebellum is very different in size between control and Rp58mutants, the extent of the mediolateral axis is similar. For each cerebellumat various stages (mostly E14.5 to P2), we collected a similar number ofsections/slide, which made it easier to select sections at comparable levelsfor Rp58 mutant and control.
Area measurements were carried out on sagittal cerebellar sections afterNissl staining. At least three sections/cerebellum located around the vermisarea were analyzed with NIH ImageJ software. For each genotype, threeembryos from three different litters were used.
Cell counting was performed after processing sections forimmunohistochemistry. For quantification on total cerebellum, for eachstaining all positive cells were counted on all the serial sections/slide/cerebellum. For quantification at the vermis level, at least threesections around the vermis area per embryo were chosen and all cellspositive for the specific staining were counted. In each case, weperformed the quantification on three embryos of each genotype fromdifferent litters.
Statistical analysisStudent’s t-test was applied to determine the significance of the differencesbetween controls and mutants.
Quantitative RT-PCRTotal RNA was extracted from whole E14.5 cerebellum in TRIzol reagent(Invitrogen) and reverse transcribed into cDNAs with Superscript reversetranscriptase II (Invitrogen). Real-time PCR was performed with SYBRGreen probe on an ABI Prism 7000 according to the manufacturer’sparameters. Gene expression was assessed in three embryos of eachgenotype from three different litters with normalization to the expressionof the housekeeping gene Gapdh. Primers are listed in supplementarymaterial Table S2.
RESEARCH REPORT Development 139 (11)
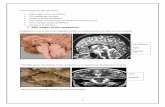
Fig. 1. Loss of Rp58 leads to cerebellum hypoplasia. (A)Schematic of the mouse early cerebellum showing the VZ (green) and URL (blue) zones. (B-D)Rp58 expression was analyzed by RNA in situ hybridization (ISH). At E12.5 and E14.5 (B,C) Rp58 is expressed in the VZ, the NTZ, the URL, the DCNand the EGL. At E16.5 (D), Rp58 is expressed in the EGL, the DCN and central regions. (E,F)Dorsal view of P18 cerebella (outlined) from control andRp58 cKO littermates. The size of the cerebellar hemispheres is much reduced and the vermis is absent (arrowhead) in the mutant (F). (G-R)Nissl stainingof control and Rp58 mutant cerebella at P2 and P8. Mutant cerebella lack noticeable lamination (P,R), exhibit a hypoplastic vermis (arrow in L) and havesmaller hemispheres (arrow in N) as compared with controls. The boxed areas are magnified beneath. (S-X)PNs (calbindin+) and glial cells (Gfap+)visualized on P8 medial sections. In the Rp58 cKO, the PNs are not organized in a monolayer (S,T) and do not present the characteristic maturation oftheir dendritic tree (arrow in U). The glial cell fibers (arrowhead in W) are disorganized. Arrow in X indicates the pial surface. (Y-BB) Rp58 and Zic1 arenormally expressed in a gradient that increases from the outer (oEGL) to the inner (iEGL) layers and in the forming IGL. Cb, cerebellum; DCN, deepcerebellar nuclei; EGL, external germinal layer; IGL, internal granule layer; NTZ, nuclear transitory zone; PL, Purkinje layer; PN, Purkinje neurons; URL,upper rhombic lip; ventricular zone, VZ. Scale bars: 200m in B-D; 500m in G-J; 3 mm in E,F; 1 mm in K-N; 100m in O-T; 50m in U-BB. D
EVELO
PMENT
RESULTS AND DISCUSSIONLoss of Rp58 in nestin-positive progenitorsinduces cerebellar hypoplasia and a defectivefoliation patternAt E12.5, Rp58 is expressed in the VZ, the URL and the subpialstream (Fig. 1B). At E14.5, Rp58 expression is strong in deepcerebellar nuclei (DCN) neurons (Fig. 1C). At this stage,expression in the VZ is still present but reduces at E16.5 as Rp58becomes expressed in progenitor cells, just beside the VZ (Fig.1D). In the EGL, Rp58 is detected at E14.5 and at E16.5 starts toshow a gradient of expression that is higher in the inner than outerEGL (Fig. 1D, supplementary material Fig. S1). Therefore, theRp58 expression pattern suggests a role in the generation and/ordifferentiation of cells originating from the VZ and URL.
To decipher the role of Rp58 in cerebellum development, weused a recently generated conditional Rp58 knockout (Rp58 cKO)model (Xiang et al., 2012). Ubiquitous loss of Rp58 (Rp58 KO)causes late embryonic/early postnatal lethality (Okado et al., 2009;Xiang et al., 2012), whereas Rp58 cKO mice, obtained by crossingRp58fl/fl with the Nestin-Cre line, die at ~3 weeks and displaydefects in neocortex development (Xiang et al., 2012). Comparedwith control littermates at P18, the gross morphology of mutantbrains showed a much smaller cerebellum, no vermis and adecreased number of lobules (Fig. 1E,F).
The size of the Rp58 cKO cerebellum is much reduced at P2(Fig. 1G-J) and this decrease is more pronounced at P8 (Fig. 1K-N), with the most drastic reduction being at the level of thevermis (Fig. 1K,L). At P2, cerebellar lobules became clearlydiscernible in the control (Fig. 1G,I) but not in the Rp58 cKO(Fig. 1H,J). At P8, few lobules were present in the mutant (Fig.
1Q,R), suggesting a delay in establishing the primary fissures.However, the single lobules in the Rp58 cKO wereunderdeveloped or absent in the vermis (Fig. 1L,P). Loss ofRp58 in the cerebellum also led to defective lamination, whichwas clearly observable at P8 (Fig. 1P,R).
Examination of cell populations in the Rp58 cKO cerebellarcortex revealed defects in its organization and lamination. Themutant PNs migrate but do not form a monolayer as in the normalcerebellum (Fig. 1S,T). Besides a reduced number of postnatal PNsin the mutant (supplementary material Fig. S2), their maturation isdefective as the dendrites fail to develop (Fig. 1U,V). TheBergmann glia fibers are disorganized (Fig. 1W,X). Defective PNdifferentiation might also be due to the abnormal glial scaffold, asthis has been shown to be important for PN dendritogenesis (e.g.Lordkipanidze and Dunaevsky, 2005). Similar to Rp58, expressionof Zic1, which is normally detected in GNPs as they start todifferentiate and in mature IGL neurons (Aruga et al., 1998), ishighly reduced (Fig. 1Y-BB). Additionally, the Zic1 gradientobserved in the control is absent from the mutant EGL (Fig.1AA,BB), further indicating defects in GNP differentiation. Theseresults demonstrate that Rp58 is essential for cerebellumdevelopment, acting on various cell types including the PNs andGNPs.
To investigate whether the reduction of cerebellum size is anearly event in the Rp58 KO phenotype, we quantified the cerebellararea at the vermis level of E16.5 and E18.5 embryos. The Rp58 KOcerebellar area is less than half that of the control (supplementarymaterial Fig. S3), suggesting that Rp58 is required for thegeneration of embryonic cerebellar cells.
1905RESEARCH REPORTRp58 controls cerebellum growth
Fig. 2. Loss of Rp58 impairs glutamatergic and GABAergic lineage differentiation. (A-H)Rp58 expression is not detected (A-D) and theexpression of Atoh1 in the URL and EGL is not significantly changed (E-H) in the Rp58 KO mouse. (I-P)Pax6+ and Tbr1+ cells (arrows) as detected byimmunofluorescence are strongly decreased in the Rp58 KO cerebellum. (Q,R)Quantification of the total number of Pax6+ or Tbr1+ cells in controland Rp58 KO cerebellum. (S-HH) Ptf1a (arrows), Pax2 and Corl2 expression was examined by ISH at E14.5 (S-DD) and E16.5 (EE-HH). (II,JJ) Quantification of Pax2+ cells after immunostaining (arrows indicate individual cells). *, P<0.05. Error bars indicate s.e.m. (R) or s.d. (Q,JJ). Scalebars: 200m, except 100m in I-L. D
EVELO
PMENT
1906
Development of glutamatergic and GABAergicneurons is affected by the loss of Rp58 duringembryogenesisThe loss of embryonic cerebellar area prompted us to investigatethe role of Rp58 in the generation of glutamatergic andGABAergic cerebellar neurons. First, we examined theexpression of Atoh1, Pax6 and Tbr1 at E14.5. Atoh1 is detectedin the URL and in the developing EGL with no apparent changesin expression levels between control and mutant brain (Fig. 2A-D,E-H). Pax6+ cells are detected in the URL, NTZ and VZ (Fig.2I-L, supplementary material Fig. S4) and their EGL number isreduced by more than half in the mutant (Fig. 2Q). As theyundergo differentiation in the URL at ~E10.5, DNP progenitorsdownregulate Pax6 expression and begin to express Tbr1 (Finket al., 2006). Pax6+ cells are detected in greater numbers in themutant NTZ (supplementary material Fig. S4), suggesting thatthese cells fail to progress toward timed differentiation. Indeed,the number of Tbr1+ cells is severely decreased in the Rp58 KOcompared with the control cerebellum (Fig. 2M-P,R).
Given the expression pattern of Atoh1 and Rp58 duringglutamatergic neuron differentiation, our results suggest that Rp58,which was recently identified as a potential Atoh1 direct target(Klisch et al., 2011), is required downstream of Atoh1 forglutamatergic differentiation.
GABAergic neurons are produced from the VZ, with PNs beingproduced between E10.5 and E12.5 and other GABAergicinterneurons between E13.5 and P7. Ptf1a is essential forGABAergic differentiation (Hoshino et al., 2005; Pascual et al.,2007). At E14.5, we observe a slight decrease in Ptf1a expressionin the lateral part of the mutant cerebellum, with no apparentchange of expression in the medial part (Fig. 2S-V). Corl2 (Skor2– Mouse Genome Informatics) expression, which labels PNs asthey become postmitotic (Minaki et al., 2008), is also slightlyreduced in the E14.5 and E16.5 Rp58 mutant (Fig. 2AA-HH). Thelocalization of the Corl2 expression domain indicates that themigration of PNs occurs normally following loss of Rp58 (Fig.2EE-HH). Pax2 expression, which labels inhibitory interneuronsgenerated from the Ptf1a+ population (Maricich and Herrup, 1999;
RESEARCH REPORT Development 139 (11)
Fig. 3. Deletion of Rp58 in Atoh1+ GNPs leads to growth and foliation defects. (A-F)Nissl staining shows the characteristic foliation patternof normal P16 mouse cerebellum and the strong staining of IGL granule cells (A). Folia are indicated by I to X. The Rp58fl/fl;Atoh1-CreER cerebellumis hypoplastic with hardly distinguishable lamination and IGL layer (B). Rp58 (C,D) and Zic1 (E,F) expression is highly reduced in the mutantcerebellum. (G-J)The layering of PNs (calbindin+, G,H) and glial cells (Gfap+, I,J) is severely affected in the anterior lobules of the Rp58fl/fl;Atoh1-CreER mutant. (K,L)Nissl staining of E19.5 sections shows the cardinal lobes (arrows in K): anterobasal (AB), anterodorsal (AD), central (C), posterior(P) and inferior (Inf). A secondary fissure in the AB lobe is not present in the Rp58fl/fl;Atoh1-CreER cerebellum. (M-R)Rp58 and Zic1 mRNA wereanalyzed by ISH. EGL Rp58 expression is abolished in the Rp58fl/fl;Atoh1-CreER cerebellum (M,N). Note also the faint Rp58 expression in the IGL.Zic1 expression is reduced in the EGL and IGL (O-R). (S,T)PH3 staining indicates reduced proliferation in the mutant EGL. (U,V)Cyclin D2expression, as analyzed by ISH, is reduced in the mutant EGL. (W-Y)Relative cerebellar area at the vermis level was quantified for control and Rp58mutant cerebella (W, see K,L). Quantification of EGL thickness based on Zic1 expression (X, see bars in Q,R) shows a significant decrease in theRp58 mutant compared with control. The number of PH3+ EGL cells/section (Y, see S,T) is significantly reduced in the Rp58 mutant. GNPs, granuleneuron progenitors. *, P<0.05; **, P<0.01. Error bars indicate s.d. Scale bars: 1 mm in A-F; 100m in G-J; 500m in K-P,S,T; 50m in Q-R,U,V. D
EVELO
PMENT
Weisheit et al., 2006), is decreased at E14.5 (Fig. 2W-Z). Thisdefect becomes stronger at E16.5 (supplementary material Fig. S5).Quantification of Pax2+ cells at E16.5 indicates a 20% decrease inthe number of these neurons in the Rp58 KO (Fig. 2II,JJ).
These results suggest that Rp58 is also necessary, most likelydownstream of Ptf1a, for GABAergic neuron development.
Crucial role of Rp58 in GNPs for cerebellar growth,lobule formation and laminationBecause the development of the main cerebellar cortical cell typesis tightly linked, we sought to delete Rp58 in a subset of cells totest its requirement in a spatiotemporal manner. To examine thedefects induced by loss of Rp58 in embryonic GNPs, we chose theAtoh1-CreER line to ablate Rp58 in newly generated GNPs throughadministration of tamoxifen at E14.5 and E16.5 (supplementarymaterial Fig. S6) (Machold and Fishell, 2005).
The effects of this deletion on cerebellum development were firstexamined at P16 (Fig. 3A-J). Nissl staining of control and Rp58conditional mutant (Rp58fl/fl;Atoh1-CreER) sections revealed ahypoplastic cerebellum. The size reduction at the vermis level isnot as severe as that observed in the Nestin-Cre-driven Rp58mutant and suggests that Rp58 might function prior to E14.5 inGNPs and/or in other cell lineages during vermis formation/growth.In addition to growth defects, the foliation pattern is also disruptedin the Rp58fl/fl;Atoh1-CreER cerebellum, with the more drasticdefects observed in folia I-III and VI-VIII (Fig. 3A,B). The fivecardinal lobes are produced by four fissures that occur during lateembryogenesis, followed by secondary fissures that establish theten lobules observed in mice (Sillitoe and Joyner, 2007). Secondaryfissures in the anterobasal lobe that establish lobules III and I-II andfissures that divide the central lobe into lobules VI to VIII failed tooccur in the Rp58fl/fl;Atoh1-CreER cerebellum (Fig. 3A,B).
Furthermore, a dramatic reduction of the IGL layer was observed,indicating substantial loss of granule neurons in the Rp58fl/fl;Atoh1-CreER cerebellum (Fig. 3A,B), which was also confirmed by areduction in Zic1 expression (Fig. 3E,F).
Nissl staining of E19.5 sections showed that the Rp58fl/fl;Atoh1-CreER cerebellum is smaller and presents a defective foliationpattern (Fig. 3K,L, supplementary material Fig. S7). A foliationdefect is observed in a secondary division of the primary lobes intolobules: the fissure in the anterobasal lobe does not form in themutant. Quantification of the cerebellar surface at the vermis levelshowed a 30% decrease in the mutant compared with control (Fig.3W). We examined whether loss of Rp58 in Atoh1+ cells affectsearly GNP differentiation and observed that Zic1 expression isquickly reduced following Rp58 ablation (Fig. 3M-R). In addition,the size of the EGL is reduced by 20% (Fig. 3Q,R,X), suggestinga defect in GNP pool amplification.
We also explored the impact of Rp58 loss in embryonic GNPson other cerebellar cortical cells. In the Rp58fl/fl;Atoh1-CreERcerebella, the PNs fail to fully differentiate and align in amonolayer, in contrast to the control brain (Fig. 3G,H). Similarly,the Bergmann glia fibers are disorganized, showing defects inlamination (Fig. 3I,J). Examination of lobule X indicates that PNsstill exhibit localization and differentiation defects despite a visibleIGL (supplementary material Fig. S8).
These results show that Rp58 in embryonic GNPs plays a crucialrole in the correct development of the cerebellum.
Defects in cell proliferation and in thetranscriptional program of neuronal progenitorsTo examine whether proliferation defects are responsible for thereduction in the generation of glutamatergic and GABAergicneurons, we analyzed proliferation in control and KO E14.5
1907RESEARCH REPORTRp58 controls cerebellum growth
Fig. 4. Rp58 loss-of-function leads to decreasedEGL proliferation and defective gene expressionin EGL and VZ. (A,B)Mouse E14.5 cerebellumsections were examined for BrdU incorporation (A).Quantification of the total number of BrdU+ cells inthe EGL, VZ and URL from control and Rp58 KOcerebella (B). *, P<0.05. (C-E)Expression of Ngn1 (C),Ngn2 (D), Ascl1 and Neurod1 (E) analyzed by ISH atE14.5. Arrowheads indicate ectopic expression ofAscl1. (F) Quantification of expression levels of Ngn1,Ngn2, Ascl1 and Neurod1 in the E14.5 cerebellum.qRT-PCR values were normalized to the expression ofGapdh. Error bars indicate s.d. Scale bars: 200m inA; 100m in C-E.
DEVELO
PMENT
1908
cerebella. BrdU incorporation was much reduced in the mutantEGL but not significantly modified in the VZ or URL (Fig. 4A,B).Proliferation defects were also observed in the Rp58fl/fl;Atoh1-CreER EGL: phosphohistone H3 (PH3) staining, which labelsmitotic cells, was strongly reduced in the mutant EGL (Fig.3S,T,Y), together with loss of cyclin D2 expression (Fig. 3U,V).By contrast, detection of apoptotic cells by TUNEL assay orcleaved caspase 3 immunostaining did not reveal any significantdifference between control and Rp58 mutant cerebella at E14.5 orP2 (supplementary material Fig. S9).
Our results suggest that defects in cell proliferation are involvedin the reduction of neurons following Rp58 deletion. Furthermore,although we did not detect defects in cell apoptosis at the stagestested, we cannot exclude the possibility that apoptosis is alsoinvolved in the drastic Rp58 cKO cerebellar hypoplasia.
Rp58 directly regulates neocortical Ngn2 and Neurod1expression (Xiang et al., 2012). We examined whetherneurogenic gene expression was altered in the Rp58 KO. Ngn2expression in the VZ is reduced but is maintained in progenitorcells as they undergo neurogenesis (Fig. 4D). A similar result isobserved for Ascl1 (Fig. 4E), which is involved in specificationof GABAergic neurons in the cerebellum (Grimaldi et al., 2009;Sudarov et al., 2011). Ngn1 expression is almost undetectable inthe mutant GABAergic progenitors (Fig. 4C), and expression ofNeurod1, which is required for GNP differentiation (Pan et al.,2009), is strongly reduced in differentiating EGL cells and alsoin the VZ (Fig. 4E). These results, as confirmed by quantitativeRT-PCR (Fig. 4F), suggest that Rp58 acts on neuronaldifferentiation, and thus on cerebellum growth and patterning, byregulating, directly and/or indirectly, the transcriptional programof early cerebellar glutamatergic and GABAergic progenitors.
ConclusionsOur study establishes the transcription factor Rp58 as a novelessential regulator of cerebellum development. Its loss leads tocerebellar hypoplasia, defective lamination and a defective foliationpattern. Our results demonstrate that Rp58 is required for both earlyglutamatergic and GABAergic neuronal differentiation, placing thisgene at the core of cerebellar development and neuronaldifferentiation. The precise mechanisms by which Rp58orchestrates neuronal differentiation and cerebellar growth, inparticular the neuronal subtype-specific transcriptional programthat it controls, remain to be refined in future studies. Since humanRP58 and mouse Rp58 are highly conserved and syntenic at 1qter,deletion of which causes human cerebellar hypoplasia, we proposethat RP58 regulates the size of the human cerebellum.
AcknowledgementsWe thank Drs Rachel Brewster, Douglas Epstein, Ellen Heber-Katz, PaulLieberman and Sharmistha Pal for comments on the manuscript; Drs JunAruga, Douglas Epstein, Judith Grinspan, François Guillemot, Jane Johnson,Yuichi Ono and Piotr Sicinski for reagents; and the Wistar Microscopy Facility(James Hayden and Frederick Keeney) for the images shown in Fig. 1E,F (NIHCore Grant CA010815).
FundingThis work was supported by grants from the American Cancer Society [RSG-08-045-01-DDC] and the National Brain Tumor Society to N.D.
Competing interests statementThe authors declare no competing financial interests.
Supplementary materialSupplementary material available online athttp://dev.biologists.org/lookup/suppl/doi:10.1242/dev.075606/-/DC1
ReferencesAruga, J., Nagai, T., Tokuyama, T., Hayashizaki, Y., Okazaki, Y., Chapman, V.
M. and Mikoshiba, K. (1996). The mouse zic gene family. Homologues of theDrosophila pair-rule gene odd-paired. J. Biol. Chem. 271, 1043-1047.
Aruga, J., Minowa, O., Yaginuma, H., Kuno, J., Nagai, T., Noda, T. andMikoshiba, K. (1998). Mouse Zic1 is involved in cerebellar development. J.Neurosci. 18, 284-293.
Boland, E., Clayton-Smith, J., Woo, V. G., McKee, S., Manson, F. D., Medne,L., Zackai, E., Swanson, E. A., Fitzpatrick, D., Millen, K. J. et al. (2007).Mapping of deletion and translocation breakpoints in 1q44 implicates theserine/threonine kinase AKT3 in postnatal microcephaly and agenesis of thecorpus callosum. Am. J. Hum. Genet. 81, 292-303.
Butts, T., Chaplin, N. and Wingate, R. J. (2011). Can clues from evolutionunlock the molecular development of the cerebellum? Mol. Neurobiol. 43, 67-76.
Carletti, B. and Rossi, F. (2008). Neurogenesis in the cerebellum. Neuroscientist14, 91-100.
Chedotal, A. (2010). Should I stay or should I go? Becoming a granule cell. TrendsNeurosci. 33, 163-172.
Ciemerych, M. A., Kenney, A. M., Sicinska, E., Kalaszczynska, I., Bronson, R.T., Rowitch, D. H., Gardner, H. and Sicinski, P. (2002). Development of miceexpressing a single D-type cyclin. Genes Dev. 16, 3277-3289.
Fink, A. J., Englund, C., Daza, R. A., Pham, D., Lau, C., Nivison, M.,Kowalczyk, T. and Hevner, R. F. (2006). Development of the deep cerebellarnuclei: transcription factors and cell migration from the rhombic lip. J. Neurosci.26, 3066-3076.
Goldowitz, D. and Hamre, K. (1998). The cells and molecules that make acerebellum. Trends Neurosci. 21, 375-382.
Gradwohl, G., Fode, C. and Guillemot, F. (1996). Restricted expression of anovel murine atonal-related bHLH protein in undifferentiated neural precursors.Dev. Biol. 180, 227-241.
Grimaldi, P., Parras, C., Guillemot, F., Rossi, F. and Wassef, M. (2009). Originsand control of the differentiation of inhibitory interneurons and glia in thecerebellum. Dev. Biol. 328, 422-433.
Helms, A. W. and Johnson, J. E. (1998). Progenitors of dorsal commissuralinterneurons are defined by MATH1 expression. Development 125, 919-928.
Herrup, K. and Kuemerle, B. (1997). The compartmentalization of thecerebellum. Annu. Rev. Neurosci. 20, 61-90.
Hill, A. D., Chang, B. S., Hill, R. S., Garraway, L. A., Bodell, A., Sellers, W. R.and Walsh, C. A. (2007). A 2-Mb critical region implicated in the microcephalyassociated with terminal 1q deletion syndrome. Am. J. Med. Genet. A 143A,1692-1698.
Hoshino, M., Nakamura, S., Mori, K., Kawauchi, T., Terao, M., Nishimura, Y.V., Fukuda, A., Fuse, T., Matsuo, N., Sone, M. et al. (2005). Ptf1a, a bHLHtranscriptional gene, defines GABAergic neuronal fates in cerebellum. Neuron47, 201-213.
Klisch, T. J., Xi, Y., Flora, A., Wang, L., Li, W. and Zoghbi, H. Y. (2011). In vivoAtoh1 targetome reveals how a proneural transcription factor regulatescerebellar development. Proc. Natl. Acad. Sci. USA 108, 3288-3293.
Liour, S. S., Dinkins, M. B., Su, C. Y. and Yu, R. K. (2005). Spatiotemporalexpression of GM1 in murine medial pallial neural progenitor cells. J. Comp.Neurol. 491, 330-338.
Lo, L. C., Johnson, J. E., Wuenschell, C. W., Saito, T. and Anderson, D. J.(1991). Mammalian achaete-scute homolog 1 is transiently expressed byspatially restricted subsets of early neuroepithelial and neural crest cells. GenesDev. 5, 1524-1537.
Lordkipanidze, T. and Dunaevsky, A. (2005). Purkinje cell dendrites grow inalignment with Bergmann glia. Glia 51, 229-234.
Ma, Q., Kintner, C. and Anderson, D. J. (1996). Identification of neurogenin, avertebrate neuronal determination gene. Cell 87, 43-52.
Machold, R. and Fishell, G. (2005). Math1 is expressed in temporally discretepools of cerebellar rhombic-lip neural progenitors. Neuron 48, 17-24.
Mandel, S., Rechavi, G. and Gozes, I. (2007). Activity-dependentneuroprotective protein (ADNP) differentially interacts with chromatin toregulate genes essential for embryogenesis. Dev. Biol. 303, 814-824.
Maricich, S. M. and Herrup, K. (1999). Pax-2 expression defines a subset ofGABAergic interneurons and their precursors in the developing murinecerebellum. J. Neurobiol. 41, 281-294.
Minaki, Y., Nakatani, T., Mizuhara, E., Inoue, T. and Ono, Y. (2008).Identification of a novel transcriptional corepressor, Corl2, as a cerebellarPurkinje cell-selective marker. Gene Expr. Patterns 8, 418-423.
Muzumdar, M. D., Tasic, B., Miyamichi, K., Li, L. and Luo, L. (2007). A globaldouble-fluorescent Cre reporter mouse. Genesis 45, 593-605.
Nornes, H. O., Dressler, G. R., Knapik, E. W., Deutsch, U. and Gruss, P. (1990).Spatially and temporally restricted expression of Pax2 during murineneurogenesis. Development 109, 797-809.
Okado, H., Ohtaka-Maruyama, C., Sugitani, Y., Fukuda, Y., Ishida, R., Hirai,S., Miwa, A., Takahashi, A., Aoki, K., Mochida, K. et al. (2009). Thetranscriptional repressor RP58 is crucial for cell-division patterning and neuronalsurvival in the developing cortex. Dev. Biol. 331, 140-151.
RESEARCH REPORT Development 139 (11)
DEVELO
PMENT
Pan, N., Jahan, I., Lee, J. E. and Fritzsch, B. (2009). Defects in the cerebella ofconditional Neurod1 null mice correlate with effective Tg(Atoh1-cre)recombination and granule cell requirements for Neurod1 for differentiation.Cell Tissue Res. 337, 407-428.
Pascual, M., Abasolo, I., Mingorance-Le Meur, A., Martinez, A., Del Rio, J.A., Wright, C. V., Real, F. X. and Soriano, E. (2007). Cerebellar GABAergicprogenitors adopt an external granule cell-like phenotype in the absence ofPtf1a transcription factor expression. Proc. Natl. Acad. Sci. USA 104, 5193-5198.
Sillitoe, R. V. and Joyner, A. L. (2007). Morphology, molecular codes, andcircuitry produce the three-dimensional complexity of the cerebellum. Annu.Rev. Cell Dev. Biol. 23, 549-577.
Sotelo, C. (2004). Cellular and genetic regulation of the development of thecerebellar system. Prog. Neurobiol. 72, 295-339.
Sudarov, A., Turnbull, R. K., Kim, E. J., Lebel-Potter, M., Guillemot, F. andJoyner, A. L. (2011). Ascl1 genetics reveals insights into cerebellum local circuitassembly. J. Neurosci. 31, 11055-11069.
Tatard, V. M., Xiang, C., Biegel, J. A. and Dahmane, N. (2010). ZNF238 isexpressed in postmitotic brain cells and inhibits brain tumor growth. Cancer Res.70, 1236-1246.
Tiveron, M. C., Hirsch, M. R. and Brunet, J. F. (1996). The expression pattern ofthe transcription factor Phox2 delineates synaptic pathways of the autonomicnervous system. J. Neurosci. 16, 7649-7660.
Tronche, F., Kellendonk, C., Kretz, O., Gass, P., Anlag, K., Orban, P. C., Bock,R., Klein, R. and Schutz, G. (1999). Disruption of the glucocorticoid receptorgene in the nervous system results in reduced anxiety. Nat. Genet. 23, 99-103.
van Bon, B. W., Koolen, D. A., Borgatti, R., Magee, A., Garcia-Minaur, S.,Rooms, L., Reardon, W., Zollino, M., Bonaglia, M. C., De Gregori, M. et al.(2008). Clinical and molecular characteristics of 1qter microdeletion syndrome:delineating a critical region for corpus callosum agenesis/hypogenesis. J. Med.Genet. 45, 346-354.
Wang, L., Zhang, Z. G., Gregg, S. R., Zhang, R. L., Jiao, Z., LeTourneau, Y.,Liu, X., Feng, Y., Gerwien, J., Torup, L. et al. (2007). The Sonic hedgehogpathway mediates carbamylated erythropoietin-enhanced proliferation anddifferentiation of adult neural progenitor cells. J. Biol. Chem. 282, 32462-32470.
Wang, V. Y., Rose, M. F. and Zoghbi, H. Y. (2005). Math1 expression redefinesthe rhombic lip derivatives and reveals novel lineages within the brainstem andcerebellum. Neuron 48, 31-43.
Weisheit, G., Gliem, M., Endl, E., Pfeffer, P. L., Busslinger, M. and Schilling,K. (2006). Postnatal development of the murine cerebellar cortex: formation andearly dispersal of basket, stellate and Golgi neurons. Eur. J. Neurosci. 24, 466-478.
Xiang, C., Baubet, V., Pal, S., Holderbaum, L., Tatard, V., Jiang, P., Davuluri,R. V. and Dahmane, N. (2012). RP58/ZNF238 directly modulates proneurogenicgene levels and is required for neuronal differentiation and brain expansion. CellDeath Differ. 19, 692-702.
1909RESEARCH REPORTRp58 controls cerebellum growth
DEVELO
PMENT