Richard A. Feely, Ph.D. NOAA Pacific Marine Environmental Laboratory/NOAA
description
Transcript of Richard A. Feely, Ph.D. NOAA Pacific Marine Environmental Laboratory/NOAA

Richard A. Feely, Ph.D.NOAA Pacific Marine Environmental Laboratory/NOAA
NOAA Ocean Climate Climate Observation 7th Annual System Review28 October 2008
Developing an Ocean Acidification Observing Network to Study the Other CO2 Problem
PMEL PIs: R. Feely, C. Sabine S. Alin, L. Juranek, A. Sutton, S. Hankin AOML PIs: R. Wanninkhof, T.-H. Peng, D. Gledhill, D. Manzello, J.-Z. Zhang University PIs: U. Send (SIO), A. Dickson (SIO), B. Hales (OSU), J. Salisbury (UNH), S. Lohrenz (USM), W. Cai (UG)

Conceptual Diagram of Ocean Acidification

Oce
an C
O2 C
hem
istry
Ocean Acidification OHCO 22 32COH 3HCOH 3
23 HCOCOH 32
232 2HCOOHCOCO
2−[CO3 ][CO2]
150−200% 50%
2100
8.2
8.1
8.0
7.9
7.81800 1900 2000 2100
50
40
30
20
10
0
300
240
180
120
60
0
pH
μmol kg
−1
Year
pH
CO2(aq)
CO32−
50% acidity16% [CO3 ]
2000
2−
Wolf-Gladrow et al. (1999)


Oce
an C
O2 C
hem
istry
Saturation State
calcium carbonate calcium carbonate
CO2 CO32 H2O 2HCO3
Ca2 CO32 CaCO3
Saturation State
W phase =Ca2[ ] CO3
2[ ]Ksp,phase
*
W>1= precipitationW=1=equilibriumW<1=dissolution

Change in Aragonite Saturation with CO2
Steinacher et al. Biogeosci., 2009
-Saturation state declines across all latitudes
-Undersaturated conditions appear for aragonite in high latitudes


WOCE/JGOFS/OACES Global CO2 Survey
~72,000 sample locations collected in the 1990sDIC ± 2 µmol kg-1
TA ± 4 µmol kg-1Sabine et al (2004)
2005/2006, 1991

Difference of present-day levels minus pre-industrial (year 1800)
Half trapped in upper 400m
Equivalent to about a third of all historical carbon emissions
Penetration of Anthropogenic CO2 into Ocean
Sabine et al. Science 2004

Oce
an C
O2 C
hem
istry
Observed aragonite & calcite saturation depthsFeely et al. (2004)
The aragonite saturation state migrates towards the surface at the rate of 1-2 m yr-1, depending on location.

DIC change due to ventilation and respiration processes
Total DIC change over 15 years in the Pacific
Sabine et al. (in prep)
DIC change due to uptake of anthropogenic CO2

Shoaling of aragonite saturation horizon of ~1-2 m yr-1
Large-scale decreases of aragonite saturation in the upper 1000m
Feely et al. (in prep)
Δ Sa
tura
tion
Dep
th (m
)

“The further development of current biogeochemical sensors and the development of new sensors is critical to the ongoing development of an integrated ocean observing system. Reliable sensors for autonomous platforms is an important research and development focus for pCO2 and other carbon sensors including DIC and total alkalinity. These sensors along with certified reference material would enable the ocean carbonate system to be constrained.”

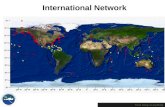
An International Ocean Acidification Observing Network

An International Ocean Acidification Observing Network
from Feely et al., 2010

Ocean acidification Ocean Carbon Observatory Network
Updated 10/5/10

Coastal Moorings Operated by SIO
All real-time, with meteorological, physical, chemical, and biological variables
CCE1
CCE2
Del Mar

California Current Ecosystem (CCE) moorings
Pt.Conception
Gliders (CORC,LTER, Moore)
CalCOFI/LTER
CCE-1 (SIO/SWFSC/PMEL)
The power of CCE1/2 comes from the context of other measurements
- Ships sample many variables and provide ground truth- Gliders provide cross-shelf sampling with a few variables- Moorings give full time sampling, wide range of variables
CalCOFI line 80
CCE-2 (SIO/SWFSC/PMEL)
Chlorophyllshown on surface;salinity on cross-section

CCE-2Real-timedataexample
Send & Ohman
with Demer, Martz, Sabine, Feely, Dickson, Hildebrand
upwelling upwelling

SBE-37
Durafet
ISE Reference
Aanderaa 3835 Optode
SBE 5M pump
Flow manifold
Copper intake
SeapHOx

CCE-1 surface pH (Jan – Sept. 2010)
Figure provided by T. Martz,U. Send, M. Ohman, SIO
pH measured using a modified Honeywell Durafet and estimated from pCO2 using TA = f(S,T) provided by Simone Alin (PMEL).
Examining the agreement between the three different pH values provides a useful QC on sensor data.
7.98
8.00
8.02
8.04
8.06
8.08
8.10
8.12
8.14
D J F M A M J J A S
pH (t
otal
scal
e)
pH(FET|INT)
pH(FET|EXT)
pH(pCO2,TA)
-0.03
-0.02
-0.01
0.00
0.01
0.02
0.03
D J F M A M J J A S
ΔpH
ΔpH (EXT-pCO2/TA)ΔpH (INT-pCO2/TA)ΔpH (EXT-INT)

An Ocean Acidification Observing Network What tools do we need to address ocean acidification?

Autonomous Underwater GlidersInnovating Technology
High resolution data
J. Barth, K. Shearman & A. Erofeev
CTDdissolved oxygenchlorophyll fluorescenceCDOM fluorescencelight backscatter
cross-margin transect twice per week since April 2006

Algorithms to predict Ωarag and pH in the N. Pacific
Juranek, Feely et al. (in prep)
Algorithm development data:CLIVAR P16 (March, 2006)STUD08 (Sept., 2008)
Data from 40-55°N, 50-500 db:Ωarag: R2=0.99, RMSE=0.05pH: R2=0.99, RMSE=0.017
(Ω,pH)

Conclusions and ChallengesSince the beginning of the industrial age surface ocean pH (~0.1), carbonate ion
concentrations (~16%), and aragonite and calcite saturation states (~16%) have been decreasing because of the uptake of anthropogenic CO2 by the oceans, i.e., ocean acidification. By the end of this century pH could have a further decrease by as much as 0.3-0.4 pH units.
An observational network of repeat surveys, moorings, floats and gliders for ocean acidification is under development as a strong collaboration between federal, state and private institutions using state-of-the-art technologies and new proxies.
Special Thanks to: Joan Kleypas, Uve Send, Sarah Cooley, and Scott Doney.
Moored and glider sensors for Dissolved Inorganic Carbon, Total Alkalinity and pH need development. Near real-time data transmission and uniform data management infrastructure is required with public availability.









![Report from the US Collaboration Panel Rik Wanninkhof NOAA/AOML, Miami [For the 4 th time] On behalf of Richard Feely, Associated US representative SSC.](https://static.fdocuments.in/doc/165x107/56649ebd5503460f94bc5e6d/report-from-the-us-collaboration-panel-rik-wanninkhof-noaaaoml-miami-for.jpg)