Repro Duks i
-
Upload
purwo-raharjo-insyaf -
Category
Documents
-
view
15 -
download
1
Transcript of Repro Duks i

FISH REPRODUCTION
Naruepon Sukumasavin, Ph.D. Technical Group, Inland Fisheries Research and Development Bureau,
Department of Fisheries
Background Induced fish breeding has been practised in Thailand since 1933, when the Thai
Department of Fisheries successfully induced natural breeding in the common carp. In 1958, the technique of using pituitary hormones to induce spawning in fish was introduced to Thailand. This technique has been used effectively to induce spawning in several fish species: i.e., striped catfish, Pangasianodon hypophthalmus (Boonbrahm, 1959), walking catfish, Clarias macrocephalus (Tongsanga, 1961) and tawes, Barbodes gonionotus (Sidthiimunka, 1962). Recently, the technique of using gonadotropin releasing hormone analogues (GnRHAs), and a dopamine antagonist such as domperidone has been developed for fish species indigenous to Thailand (Sukumasavin and Leelapatra, 1988). This has proven to be very effective. Now, more than 20 species, which could not be effectively induced by pituitary gland extract, have been successfully induced to spawn using the GnRHA and domperidone approach. Broodstock Management
Teleosts are poikilothermic animals whose sexual maturity depends on water temperature. Under tropical conditions, most fishes become sexually mature within the first year (Horvath et al., 1984). For example, Barbodes gonionotus matures sexually at the age of 8 months, when the length is only 8.5 cm (TL) and the weight 9 g (Paohorm, 1969). Although the fish is mature, the fertility of the eggs is poor. Thus, a low survival rate of the larvae has been reported from female B. gonionotus weighing 100 g. Therefore, the appropriate size of this species for breeding purposes is about 200 g (Joragun, 1978). In some tropical freshwater species, sexual maturation takes longer than one year: for example, 2 years in Morulius chrysophekadion (Unsrisong et al., 1990), 3 years in Osphronemus gouramy (Pasukdee, personal communication), 4 years in Probarbus jullieni (Rodrarung et al., 1990) and more than 10 years in Pangasianodon gigas (Sukumasavin, personal observation). In hatchery operations, large broodstock are difficult to handle and require a longer period for adapting to captive conditions. Horvath et al. (1984) have suggested the following guidelines for the management of broodstock in captivity: 1. Select healthy fish with good physical characteristics. 2. Feed them with good quality food of the correct dietary composition. 3. Keep the broodstock at a low stocking density. 4. Identify the sex of the broodstock and keep separately if possible, because mixed stocks
are inclined to spawn naturally. 5. Replace unspawned broodstock because the broodstock should not only tolerate but
actually respond positively to induce spawning. 6. Keep spent (spawned) fish separately from the other broodstock and feed with protein-rich
feed at 2-5% body weight per day, in order to promote recrudescence of eggs and sperms. 7. Produce natural feed by adding fertilizers regularly. 8. Select deep ponds for keeping broodstock and supply with adequate water, in order to
ensure favourable water quality and to stimulate gonadal development. 9. Before each spawning season, add some trash fish into the feed, in order to promote
gonadal development as well as recovery. 10. Stock new spawners with old spawners, for the replacement of broodstock.
Advanced Freshwater Aquaculture: Fish Reproduction

134
Fish reproduction is generally initiated by environmental factors (Fig.1 and Fig. 2), for example: temperature, rainfall, water quality, photoperiod, and food quality as well as food availability. The fish receives these signals through the brain and interprets them, in order to determine whether the environmental conditions are suitable for spawning. The message is then sent to the hypothalamus, which produces gonadotropin, releasing the hormone (GnRH). This is passed to the pituitary gland, which is located underneath the brain. The pituitary gland then produces gonadotropins (GtHs). Both GnRH and GtHs cause tiny incipient gonads to develop, mature and finally release gametes at the end of the process. This is a slow and long process, influenced by environmental temperature. In tropical countries, the process is faster than in temperate areas, where yolk formation slows down or almost stops during the winter season (Harvey and Carolsfeld, 1993).
Advanced Freshwater Aquaculture: Fish Reproduction

135
Fig. 1: Events in the reproductive endocrine control of maturation and ovulation, amongst female teleosts
Advanced Freshwater Aquaculture: Fish Reproduction

136
Fig. 2: Events in the reproductive endocrine control of maturation and spermiation, amongst male teleosts
Advanced Freshwater Aquaculture: Fish Reproduction

137
Female gonadal development In the female, GtH I stimulates steroidogenesis in the ovary to produce testosterone in
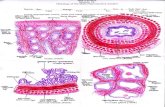
the theca cells, where it is is then converted to estrogen by the granulosa cells of the oocyte follicle. The estrogen triggers the liver to start producing vitellogenin (yolk protein), which is sent via the blood and deposited in oocytes. The oocytes then develop slowly until mature. At that stage, the concentration of GtH I peaks. The ovary stops producing estrogen and instead starts producing a maturation-inducing steroid (MIS), under the control of GtH II. The MIS stimulates the germinal vesicle of the mature oocyte to migrate to the periphery of the oocyte and then break down at final oocyte maturation. Next, prostaglandin causes ovulation that result in free ova (Fig. 1). This process is called oogenesis and is similar in most teleosts. However, details may vary considerably in different species. During oogenesis, oocytes are divided into various stages depending on the morphology of the nucleus, cytoplasm and follicle. These stages may be grouped into the previtellogenic, vitellogenic, maturation, and atresia phases. Details of each stage are completely documented in Selman and Wallace (1989). Previtlellogenic phase: Oogonia undergo mitotic proliferation and enter the first meiotic division. The nucleus of the oocyte contains only one nucleolus (primary oocyte, stage 1). The primary oocyte is arrested in the ovary until it enters a growth phase (stage 2). The stage 2 oocyte increases in size progressively, surrounded by a layer of granulosa cells. The nucleus has several nucleoli at this stage, which are positioned in the peripheral region of the nucleus. RNA and mRNA of the oocytes are transported from the nucleus into the oocyte cytoplasm. They are called yolk nuclei or Balbiani bodies. The Balbiani bodies, which cause the oocyte cytoplasm to be stained basophilic, are initially located at the nuclear membrane (stage 2a) and subsequently migrate through the periphery of the cytoplasm (stage 2b, 2c). During this growth phase, the volume of the oocyte increases about one thousand-fold. Ovaries in this stage contain immature oocytes and have a gonadosomatic index (GSI) of less than 2% (Selman and Wallace, 1989). Vitellogenic phase: The oocytes increase in size due to endogenous and exogenous vitellogenesis. At the end of endogenous vitellogenesis, the Balbiani bodies disappear from the oocyte cytoplasm completely (stage 3). Next is a presentation of cortical alveoli at the oocyte cytoplasm (stage 4). The cortical alveoli are carbohydrate vesicles that stain for proteins and carbohydrates: for example; alcian blue, toluidine blue and periodic acid & Schiff reagents. The staining with the periodic acid & Schiff reagents results in pink cortical alveoli (stage 4) at the oocyte cytoplasm. However, the cortical alveoli are difficult to preserve. Thus, they loose their staining properties and assume a vacuolar appearance (Wallace et al., 1987). Subsequently, the oocytes develop through exogenous vitellogenesis (stage 5), which is a long growth process under the control of pituitary gonadotropins. The enlargement of the oocytes is mainly due to accumulation of yolk that contributes 80-90% of egg dry weight (Wallace et al., 1987). During stage 5, there is an increase of hepatic synthesis and a secretion of vitellogenin, lipophosphoprotein, and yolk protein precursor in response to the circulation of estrogen. The vitellogenin is delivered to the oocytes and forms yolk bodies by micropinocytosis. The yolk bodies accumulate in yolk globules and form yolk pellets. The nucleus of the stage 5 oocyte is reduced in size but still located at the centre of the oocyte. Maturation phase: The nucleus of the vitellogenic oocytes starts migrating to the animal pole (germinal vesicle migration; GVM; stage 6). Next, the nuclear envelope of the oocyte breaks down (GVBD; stage 7). The oocytes have then reached the final maturation phase. The process of oocyte maturation is usually rapid and accomplished within 24 hours (Selman and Wallace, 1989). Finally, the mature oocytes are hydrated and released into the ovarian cavity (ovulation).
Advanced Freshwater Aquaculture: Fish Reproduction

138
Atresia: Atresia is a degenerative process in which the oocytes at various stages stop growing and undergo rupture and resorption in situ by granulosa cell phagocytosis. Yolks are liquefied, forming irregular drops and staining acidophilic. Yolk residual may be seen as brown or black bodies. Atresia commonly occurs in fish ovaries during all development phases. In fact, there is no difference between the sizes of developing and atretic oocytes. Thus, fecundity estimates based on oocyte size may be affected by atresia (Macer, 1974). Female gonadal maturation may occur only once or else several times a year, depending on the species (reproductive cycle) and on environmental conditions (season, water and food). Male gonadal development
Male gonadal development is called spermatogenesis. The process involves mitotic proliferation to produce large numbers of sex cells and meiotic division to generate genetic diversity and half the chromosome number. The process is initiated by environmental factors. The brain receives the signals and sends them to the hypothalamus. The hypothalamus releases GnRH to the pituitary gland, resulting in the production of GtHs. Initially, GtH I stimulates the Leydig cells of the testis to produce testosterone. The testosterone causes spermatogonia (2n) to undergo mitotic division to primary spermatocytes (2n). The primary spermatocyte enlarges in size and reduces the chromosome by half at the first meiosis, which results in secondary spermatocytes (n). The secondary spermatocytes then develop into spermatids at the end of the meiotic division. Next, GtH II peaks and stimulates the Leydig cells to produce 11-ketotestosterone, which induces spermatids to form a tail and become spermatozoa. The process is called spermatogenesis. The spermatozoa are the mature sperms in the lumen of the testis. A steroid called 17α, 20β-dihydroprogesterone, which stems from GtH II, causes the spermatozoa to dilute with seminal fluid and results in a sperm suspension called milt. This process is called spermiation. In nature, mature males with ripe gonads can be found over a longer period than mature females with ripe gonads. The spermatozoa may be ready for several weeks during the spawning season (Fig. 2). Staging of gonadal development
The staging of gonadal development is necessary for propagation by hormonal administration. Effective hormone treatment requires the correct gonadal stage, which depends on the reproductive characteristics of the sex and species. Staging of the female gonad
Gonadal development can be staged by various methods. Some methods may be quick and easy while other methods may be complex, expensive and time-consuming. Each method has some advantages as well as disadvantages. Female gonadal development can be staged in the following way. External appearance of abdomen
In the female, a large, soft belly and swollen genital papilla indicates readiness for hormonal induction. External examination requires skill and experience for good results. Selection of broodstock based on these characteristics is fast, easy and causes little stress. However, the method is less useful for inexperienced farmers, and for the examination of new fish species, which might have different abdominal appearances. Ovarian biopsy
Biopsy techniques can be used to sample eggs for staging by size (diameter) and appearance (color). The biopsy techniques are: 1) cannulating gonoduct with a fine polyethylene tube and aspirating a few dozen oocytes
by mouth or by using a syringe. It is necessary to sample oocytes from the same part of the ovary in each fish to reduce sampling bias. This technique works in carps, catfish, milkfish, mullet, rabbitfish, seabass and grouper etc (Harvey and Carolsfeld, 1993).
Advanced Freshwater Aquaculture: Fish Reproduction

139
2) puncturing the abdominal wall with a hypodermic needle and withdrawing some oocytes. The technique is effective and quick in less ripe gonads and in some species with fragile oviducts. The technique is less damaging than cannulation.
3) incising the abdominal wall and removing ovarian tissue. The technique is applied to large fish where the other biopsy techniques do not work. The sampled oocytes are cleared with clearing solution (40% formalin, 40% ethanol and 20% glacial acetic acid) to find the position of the germinal vesicle or nucleus of the oocytes, which may be classified into central (stages 2-5), migrating (stage 6) and peripheral (stage 7) germinal vesicles. Readiness for hormonal induction is indicated when one third of the oocytes have germinal vesicles in migrated and peripheral stages, while the rest have a central nucleus. Using clearing solution, it takes 1-2 minutes to clear up the oocyte yolk. The nucleus will only be visible for 4-5 minutes (Harvey and Carolsfeld, 1993).
Oocyte diameter and distribution The diameter of the oocytes can be measured by expelling the sample into a fixative
solution of 5% phosphate-buffered formalin placed in a petri-dish. Using a microscope with an ocular micrometer, about 50 oocytes are measured. The average oocyte diameter as well as the size distribution can be used to predict the appropriate timing for hormonal induction, when compared to a reference. In milkfish for example, an average oocyte diameter of 750μm or more is the critical size for GnRHa treatment. Diameter distribution is useful in species where the diameter of the oocyte does not significantly increase after the completion of vitellogenesis (Harvey and Carolfeld, 1993). Ovary weight and oocyte morphology
The technique needs a representative sample of the broodstock. Thus, a few fish (3-5) are sacrificed and ovaries dissected. The ovaries are weighed for calculation of the gonadosomatic index (GSI (%) = ovary weight/body weight x 100) and then processed for histological sectioning. The GSI can be used to predict the gonadal stages of the fish, when compared to a reference. The histo-morphological structure of oocytes in the ovary is the most accurate method for oocyte staging. However, there are many oocytes in an ovary. Thus, the average oocyte stage is required to decide the ovarian stage. The stereological method is based on the Delesse Principle, which assumes that a random 2-dimentional section (histological slide) can be used to quantify the composition of a 3-dimentional object (ovary). Thus, the stereological method presents a composition of the ovary, which is occupied by oocytes at each stage. Although the method is effective, it requires expensive equipment, knowledge of stereology and is time-consuming. The method has been used to quantify ovarian composition and the maturity of some aquatic animals (Lowe et al., 1982; MacDonald and Thompson, 1986). Staging of the male gonad
The staging of the male gonad is simpler and easier than the female. The ripe male can be staged in the following ways. Secondary sexual characteristics
Mature males show secondary sexual characteristics such as: 1) pearl organs on the bodies and pectoral fins of cyprinids, 2) distinct body colors, like dark and bright colors on the chins of tilapias or black spots
on the pectoral fins of Giant gourami (Srisakultiew et al. 1994), 3) protruding genital papillae in Clarias spp.
Spermiating male Males generally produce milt in captivity. The spermiating male has sperm in the
lumen of the testis and sperm duct. During the final stage of maturation in the male (hydration), seminal fluid is released in the lumen. The volume of sperm increases dramatically, and sperm become more fluid and diluted. The male may remain in this
Advanced Freshwater Aquaculture: Fish Reproduction

140
condition for several months without spawning (Harvey and Carolsfeld, 1993). Ripe males are easily distinguished by the release of milt, when the belly is pressed or squeezed. Breeding techniques
Some cultured species spawn in captivity easily but some may not spawn at all. For those that do spawn, it may be asynchronously, or in a season not necessarily desired by the farmer for optimal management. Thus, breeding techniques play an important role for large-scale fry production. Breeding techniques can be divided into 2 categories. Natural spawning
Males and females are released together in a spawning pond where external cues are manipulated, (e.g. temperature, water exchange, water quality, photoperiod and presence of the nest), to stimulate the fish into spawning naturally. The spawners may be left to incubate their eggs, or else they may be separated, depending on their brooding behaviour. In Thailand, there are approximately 11 species where natural spawning is used (Table 1). This technique is also called uncontrolled breeding or semi-controlled breeding. Table 1. Freshwater species that are reproduced by natural spawning Common name Scientific name References Giant gourami Osphronemus
gouramy Srisakultiew et al., 1994
Nile tilapia Oreochromis niloticus Pongsuwan and Sithimungka,1989
Catfish Clarias macrocephalus
Pongsuwan and Sithimungka,1989
Catfish Clarias batrachus Pongsuwan and Sithimungka,1989
Royal featherback Chitala blanci Supachalust, 1988 Spotted Featherback Chitala ornata Rodrarung and Meewan,1996 Swamp eel Monopterus albus Kwanmuang et al., 1993 Freshwater Garfish Xenentodon cancila Juntubtim, 1996 Drumfish Boesemania
microlepis Pimolbutr and Pasugdee, 1994
Sand Goby Oxyeleotris marmorata
Amatayakul et al., 1995
Hormonally induced spawning A hormone is administered, in order to stimulate the fish to spawn either naturally or
artificially. This method is usually applied to cultured fish that have matured but lack the ability to spawn in captivity. However, the technique has become routine practice on fish farms because it is relatively easy, efficient and practical. The hormones used for manipulating fish maturation and ovulation include pituitary extract, HCG, and GnRH/LHRH plus their analogues, in combination with dopamine antagonist. Details of the hormones used in aquaculture are explained below. Fish pituitary extract (hypophysation)
This method was established in 1931 by Houssay, who used fish pituitary extract or hypophysation to induce fish spawning (Harvey and Hoar, 1979). Fish pituitary glands are obtained from sexually maturing or mature donors either of the same or of a different species. The gland may be used fresh or stored in absolute alcohol or acetone. General computation of the hypophysation working dose is based on the fresh weights of the donor and the recipient:
Working Dose =Weight of donor fish (kg)
Weight of recipient fish (kg)
Advanced Freshwater Aquaculture: Fish Reproduction

141
Fish are induced to spawn by administering the working dose once or twice (Table 2, p 16). For example, a single injection, of 1.4-2.0 times the working dose of the hypophysation, induced B. gonionotus to spawn 4-6 hours after administration (Tavarutmaneegul et al., 1992). This method is practical in the field, because simple equipment and small amounts of materials are needed. The whole pituitary gland contains other pituitary hormones in addition to the gonadotropins, which may increase the efficacy of hypophysation (Donaldson, 1986). The method however, has some disadvantages: • Many donor fish have to be killed to obtain pituitary glands. • The pituitary extract is unreliable, due to poor standardization. • In China, there is some evidence that fish may develop an immune reaction to repeated
injections. • When fresh pituitary extract is used, the donor fish may transmit a disease to the recipient. • The weight of the donor fish may be unknown, when stored or dried pituitary glands are
used.
Using dried pituitary glands, Rowland (1983) reported that a very low dose (1 mg. carp pituitary gland per kg. of the recipient) induced oocyte maturation without ovulation in the golden perch (Macquaria ambigua), whereas a somewhat higher dose (5 mg/kg) induced 100% ovulation. However, the fertilization rate was more variable at 5 mg/kg than at the relatively high dose of 10 mg/kg. On the other hand, a really high dose (15 mg/kg recipient) reduced hatchability when compared to 10 mg/kg, which appeared to be the optimal dosage for the species. Although the method solves the problem of the unknown weight of the donor, it requires a fine electrical balance for weighing the gland to the nearest milligram. The technique has been improved by using a lyophilised pituitary powder, which is a crude preparation of fish gonadotropin. This powder is more stable, as well as having a longer shelf life and known potency of the gonadotropin content, than the fresh pituitary gland of either a carp or a salmon (Yaron and Levavi-Zermonsky, 1986). Induced spawning with a lyophilised pituitary gland is quite expensive and only efficient when used with closely related species. Furthermore, the hypophysation approach may later face problems as new fish species are introduced to aquaculture, and closely-related fish pituitaries are difficult to obtain (Yaron and Zohar, 1993) Human Chorionic Gonadotropin
Human Chorionic Gonadotropin (HCG) is extracted from the urine of pregnant women. This gonadotropin is a complicated glycoprotein, with a molecular weight of about 30,000 Dalton. It is uniform in a given batch and can therefore be standardized. HCG is also available as a pharmaceutical product. The use of HCG eliminates the need for killing fish and the whole pituitary preserving process (Donaldson, 1986). However it works in some fish gonadotropin receptors, but not in others. Sometimes, the HCG may work on the male but not the female. In addition, the effective dosage of HCG may vary from species to species, depending on how closely related the fish endogenous gonadotropin is to HCG (Lam, 1982). Nonetheless, HCG alone, or in combination with hypophysation, has efficiently induced ovulation in a number of fish species. The method is common practice on many fish farms, despite the fact that HCG is a relatively expensive product. Gonadotropin-Releasing Hormone, or luteinizing hormone-releasing hormone, their analogues and dopamine antagonists
The gonadotropin-releasing hormone (GnRH), or the luteinizing hormone which releases another hormone (LHRH), is a short peptide hormone, composed of 10 amino-acids. The peptide is similar in most teleosts and mammals (Donaldson, 1986). Analogues (GnRHa or LHRHa) are molecules in which some amino-acids have been substituted, in order to
Advanced Freshwater Aquaculture: Fish Reproduction

142
increase the half-life of the molecule in the fish and so increase its capacity to bind with GnRH receptors in the pituitary gland. Thus, the efficacy of the GnRHa/LHRHa will be higher than that of natural forms of the GnRH/LHRH. The use of either GnRH/LHRH or GnRHa/LHRHa for spawning induction has several advantages over the traditional hypophysation technique. The GnRH/LHRH or GnRHa/LHRHa is a small peptide molecule. Thus, it can be synthesized into its native form and also into altered forms (analogues), with a slow rate of degradation. Therefore, lower doses are required when using analogue forms (Zohar et al., 1989). GnRH/LHRH and its superactive analogues (GnRHa/LHRHa) are unlikely to elicit immunological responses, like some heterologous gonadotropins (hypophysation and HCG) do, and are therefore potentially less harmful to the recipient fish. In addition, GnRH/LHRH (GnRHa/LHRHa) stimulates the secretion of the fish's own GtHs. This means that it is not species-specific and can be successfully applied to a great variety of fish species. Studies on the brain’s regulation of hypophysial function by Peter et al. (1986) revealed the presence of two hypothalamic hormones that control the release of GtHs from the pituitary gland. The first is GnRH, which stimulates GtHs release. The second is dopamine, an amine which antagonises the release of the GnRH in most teleosts, and thus reduces the production of GtHs from the pituitary gland. Therefore, injecting either GnRH/LHRH or GnRHa/LHRHa alone is generally ineffective in inducing ovulation in cultured fish, due to the strong inhibitory effect of dopamine on GtHs secretion. This has been found to be the case with goldfish, common carp and Chinese carps (Peter et al., 1986; Lin et al., 1986). Lin and Peter (Peter et al., 1988) developed the “Linpe method”, by injecting a combination of a GnRHa/LHRHa and a dopamine antagonist (domperidone, pimozide or metoclopramide), in order to induce the ovulation and spawning of cultured fish. The Linpe method has been widely and effectively applied in many cultured fishes throughout the world (Lin and Peter, 1996). In Thailand, the Linpe method has also been used successfully in many fish species, as shown in Table 2, p.16. However, it is important to note that the dopamine antagonists vary in their efficacy amongst different fish species (Zohar, 1988; 1989). In some cases, GnRHa is used in combination with carp pituitary extract or HCG, in order to induce the ovulation and spawning of cultured fish species (Peter et al., 1988a; Table 2). In addition, the sustained release of GnRH from slow-releasing vehicles (cholesterol, cholesterol-cellulose or biodegradable polymers), has been successfully used to induce continuously high GtHs levels and/or ovulation in salmonids (Crim and Glebe, 1984), goldfish (Sokolowska et al., 1984), milkfish (Marte et al., 1987), sea bass (Almendras et al., 1988) and gilthead sea bream (Zohar, 1988). The rate of GnRH release can be determined by altering the proportion of cellulose in the cholesterol pellet (Sherwood et al., 1988). Importance of stress from handling
Fish try to avoid capture and struggle violently when restrained. The stress of capture and handling has profound effects on blood chemistry and stimulates the hypothalamus and pituitary effecting blood levels of gonadotropin androgens, and the “stress hormone” cortisol. Contact with nets and workers’ hands strips away the protective mucous layer and promotes infection. The amount of stress varies tremendously; some species arc easily handled whereas others (the milkflsh is a good example) leap violently from the water to avoid capture. Handling may result in reduced feeding, infection, or mortality, and there is very strong evidence that the degree of stress in females affects the. Quality of eggs produced in induced spawning. Even the hidden physiological effects of handling stress are so profound that an jducedspasTm1g procedure using the “right” hormone applied at the “right” time can still fail if broodstock are handled roughly. One long-term way to increase handling tolerance is domestication. Again, milkfish provide an example. In Hawaii, milkfish kept in tanks and handled repeatedly in experiments have become relatively docile. However, this is not a
Advanced Freshwater Aquaculture: Fish Reproduction

143
typical production setup. In sea cages, milk- fish that have been captive for years but handled infrequently still react violently to netting. In any event, for most fish-culture operations it is just not practical to spend time training broodstock to accept handling. Fortunately, even simpler methods will reduce stress and these deserve more attention than they now get. Straigh-forward nonchemical methods of reducing stress include the following:
•Avoid 0vercrowding the fish during capture. A mass of struggling broodstock partially exposed to the air in a seine net is impressive but is likely to seriously affect reproduction. A solution that has worked for fast-swimming, delicate tuna is to construct barriers and funnels in the holding tank to allow careful herding of fish into individual capture bags, where they remain during further handling (Kaya et at. 1984). With some trial and error, this idea could be applicable to a variety of holding facilities.
•Never throw fish hack into tanks or ponds: they should be carefully placed in the water and then released.
•Moisten hands and all cloth nets or holding slings (hapas) before handling fish, to minimize scale and mucous loss.
•Cover the fish’s eyes with a wet cloth whenever possible. •Develop a secure holding technique that minimizes the effect of struggling; often a
single handhold at the centre of the fish is much safer than a hand on the tail and one on the head. Fish tend to be more docile when inverted than when upright or on their side.
•Minimize noise during all handling: many fish have a very acute sense of hearing. •transport all fish, even freshwater species, in slightly salty water (1-2% NaCl for
freshwater species and 80% sea water for marine species) and anesthetize them lightly. Add oxygen if fish density is high or the transport is long. Using anesthetics to reduce stress
Anesthesia in fishes is usually described as a series of stages, from light sedation where fish are easily captured but still responsive to touc1i, to surgical anesthesia where there is no response to stimuli. Fish that are going to be injected for induced reproduction do not need to be anesthetized, only sedated enough for weighing, biopsy, and injection -usually a matter of a few minutes- and, for this reason, finding the right dose of an anesthetic for a given species must be done by trial and error using published reports as a guide. Excitement profoundly affects ease of sedation, and a dose that works on the first fish removed from an enclosure may have to be increased for fish that have already been stressed as animals are chased and captured. Descriptions of sedating fish by “placing the fish in an anesthetic bath” omit an essential point: capture of the fish from its tank or pen. Yet, for most large broodstock fish used in aquaculture, most of the stress occurs during this first step. This point is often overlooked because many published anesthetic procedures are for small fish, which suffer relatively less from netting; a brood- stock female weighing 5 kg, however, is a powerful animal and can injure itself struggling. The real challenge is for culturists to develop ways of exposing these large fish to the anesthetic before they are removed from the tank or cage. For a few freshwater species, it is possible to hand-inject a small volume of concentrated anesthetic solution directly into the mouth of a fish that has been cornered but not yet removed from the tank. Metomidate hydrochloride, a new anesthetic, is very effective this way because it acts so rapidly that sedation occurs in seconds after the solution passes over the gills. In most anesthetic baths, progressive changes in blood chemistry mean that sedation deepens the longer fish are in the bath; if a fish is left too long, breathing can stop. Fish should be removed to clean water as soon as possible after weighing and injection. Humans should limit their exposure to anesthetic drugs: all drugs are potentially hazardous. Little information is available on the effects of long-term exposure to fish
Advanced Freshwater Aquaculture: Fish Reproduction

144
anesthetics, and the best practical advice to culturists is to avoid contact with the eyes and mucous membranes, wear long rubber gloves, and limit the number of people touching the powdered or dissolved drug. All anesthetics leave residues in the flesh of fish; broodstock that die during the induced-breeding process should not be eaten. Anesthetics available for fish-culture work Only a few anesthetics are available to fish culturists, and all of them are used the same way: dissolved in a small-volume holding bath into which the fish is placed after capture. Tricaine methane sulfonate (MS 222) 2-phenoxyethanol, and quinaldine are all effective on most fishes, although quinaldine is so irritating to fish and humans that it should not be used at all (a less irritating derivative, quinaldine sulfate, has a low margin of safety with many fish and is also not recommended). Chlorobutanol, once widely used, is carcinogenic and should also be avoided. Carbon dioxide bubbled through the water produces sedation and anesthesia in fish and leaves no chemical residues in the tissues, but its action is slow, the margin of safety is narrow, initial excitation is high, and the apparatus required is impractical for sedation of broodstock. Doses of all anesthetics reported in the literature vary widely with the size of the fish, degree of sedation or anesthesia desired, and water temperature, and should be used as guidelines only. Response to anesthetizing drugs differs widely between species, and these differences are not always just in physiological effect: tilapias, for example, have an unusual (and annoying) ability to shut their mouths in an anesthetic bath, reducing anesthetic flow over the gills and greatly prolonging time to sedation. One of the most familiar and widely used anesthetics in fish culture is tricaine methane sulfonate (MS 222 tricaine, TMS). It is available from many suppliers of aquaculture chemicals, and is the only compound registered for use as an anesthetic for food fishes in any country (USA). It is highly soluble in water but is photosensitive and degrades on standing, and is used at 50-100 ppm (mg/l). Tricaine has many drawbacks, and is certainly not the cheapest drug for sedating fish. Unbuffered fresh water baths of tricaine are acidic and irritate fish, and the long list of physiological effects includes hypoxia and changes in blood electrolytes, hormones, cholesterol, urea, and lactic acid. Some of the irritating effects of tricaine can be eliminated by neutralizing the anesthetic bath using sodium bicarbonate. The buffer should only be added to the working solution; it will precipitate in more concentrated stock solutions and eliminate the anesthetic effect. Sodium bicarbonate at 200-250 mg/ml of 100 mg/l stock solution used is generally suitable; any cloudiness that develops in this bath should disappear with agitation. A cheaper alternative to tricaine is 2-pheiioxyethanol, an oily liquid that dissolves in water with shaking and is also used as an antibacterial, antifungal bath. 2-Phenoxyethanol produces anesthesia at 200-500 ppm, and remains effective as a working solution for several days. Some people have reported skin irritation after putting their bare hands in anesthetic baths of 2-phenoxyethanol. Two newer or less-used anesthetics for fish that are excellent alternatives to tricaine and 2-phenoxyethanol are metomidate hydrochloride and benzocaine. Metomidate hydrochloride (Marinil Hypnomidate now being registered as an anesthetic for food fish in Canada and the USA, is a highly soluble analogue of the medical anesthetic etomidate. Its main advantage is extremely rapid action at low concentrations (5-20 ppm), and it can be very effective when squirted directly into the mouth of a fish that has been cornered. It is also one of the few fish anesthetics that is also effective when injected intramuscularly. Recovery time is, however, longer than with tricaine or 2-phenoxyethanol. Metomidate also has promise as a long-term sedative at lower concentrations for transport of fish, and should be considered as a tool for limiting stress when broodstock must be shifted from one location to another. No information is yet available on irritation to humans.
Advanced Freshwater Aquaculture: Fish Reproduction

145
Benzocaine is chemically related to tricaine but is cheaper and less stressful to fish because it is neutral in solution. It must, however, be dissolved in ethanol (100 g/l) to form a stable stock solution; it is used at about the same concentration as tricaine. Benzocaine is also available as the water-soluble hydrochloride salt, but is irritating to fish in this form because of the low p11 of the resulting anesthetic bath, and solutions of the drug must be chemically neutralized. Information on irritation to users is not available, but the drug is commonly used as a topical human anesthetic (in throat lozenges, for example). Methods of hormone administration
Spawning hormones are administered at present by two general methods: injecting a water or saline solution or implanting a slow-release pellet. Traditionally, both have been done either intramuscularly (IM) or intraperitoneally (in the abdominal body cavity, or IP. Oral (in the food) and topical (from the water) uptake of some hormones have been shown experimentally; although these routes are not yet practical, they offer intriguing advantages and should be tried on a larger scale. Injection
Some workers choose to inject IM, and some to inject IP, without any justifications other than tradition. No controlled studies with fish justify choosing one technique over the other. Whatever the route of injection, it should not be varied if consistent results are desired. One advantage of IM injection is standardization it is easy to inject at the same spot and to the same depth in many fish. Using the finest possible needle leaves the smallest possible hole for the solution to leak out through and, in large fish, the muscle mass is thick enough to allow placing the liquid deeply. It also helps to keep injection volume as low as possible, and to withdraw the needle slowly after waiting a few seconds for the liquid to find its way between muscle fibres. A good general rule is to keep volume under 0.5 mh1kg, and to inject in several locations if the total is much greater than 1 ml. IP injections are often tolerated by unanesthetized fish, although an IP injection into the body cavity can easily end up in the intestine or gonad, and drug absorption is hard to standardize without careful needle placement. Experience with a given species helps to alleviate this problem. Larger diameter needles can be used in IP injections, facilitating the injection of suspensions of pituitary powder or domperidone and of more viscous solutions, such as Ovaprim Larger volumes (2-3 ml/kg) can also be used. IP injection should be done behind the pelvic or pectoral fin. The number of injections naturally varies with the hormone used and the state of readiness of the animal. Methods that work with a single injection GnRHa-dopamine antagonist mixtures frequently do — have the great advantage of minimizing handling; in excitable species (particularly marine ones), the extra stress of a second capture may cancel out the benefit of a second injection. Implantation Drugs injected after dissolving them in saline solution enter the general circulation within minutes and are then metabolized and excreted. In some special cases, it is useful to make the “hormone available to the animal over weeks or months, and this can been done by mixing the drug with a binding material from which it is slowly released (silicone rubber or Silastic, normally used to deliver steroids) or which slowly breaks down allowing the hormone to escape (cholesterol or cholesterol-cellulose). Although implants work well in some situations, they are not commercially available in sizes and doses for fish culture, and must be manufactured by hand according to published methods (see references for methods of making cholesterol or cholesterol-cellulose pellets, the only type feasible to manufacture in the laboratory). The special equipment required and the need to handle relatively large quantities of powdered hormone so far makes this technique impractical except for research. Hormone dosages for all forms of implant exceed
Advanced Freshwater Aquaculture: Fish Reproduction

146
those used for injection at present and, if fish have already reached the stage of final maturation, injection is usually preferred. Silicone or cholesterol-cellulose pellets can be implanted intramuscularly or intraperitoneally. IM allows positioning of the pellet and release of the drug can be standardized, although the muscle mass must be great enough to accommodate the pellet. An antibiotic cream should be applied to the IM implantation site to reduce the chance of infection. Dietary administration It is widely believed that, because many polypeptides and proteins are ineffective when taken orally by humans, the fish gut must similarly degrade protein hormones in the diet. This is almost certainly an oversimplification because enzymatic degradation of proteins varies with species and diet; many fish, including the large cyprinid family, are agastric (“stomachless”), and drug absorption in these species does not follow the mammalian pattern. Oral delivery of relatively stable GnRHa has the great advantage of eliminating handling stress, a factor that makes many induced-breeding procedures fail.
Recent studies with several cold-water species point the way to a third alternative to injection and implantation of GnRHa. In sablefish, a handling-sensitive fish that, responds to injected GnRH, oral intubation (tube feeding) of the hormone at 1 mg/kg resulted in spawning (Solar et al. 1990). Experiments with species of sea trout show that mammalian GnRHa can be absorbed from the fish gut when present in the food and, although 10 times as much hormone was used as with intramuscular injection, the oral treatment did induce ovulation and spontaneous spawning (Thomas and Boyd 1989). Much work needs to be done to find the lowest effective doses and to determine whether the technique works for long-term administration as well as for final maturation and ovulation; however, the way is certainly open for some interesting experiments. When bulk prices for GnRHa and the savings in labour and brood- stock are considered, the oral method may prove highly cost-effective. In Taiwan, at least one milkfish farmer is mixing GnRHa with broodstock diet and obtaining good spawning success. Techniques for artificial fertilization Fish that have been induced to ovulate and spermiate with hormones are often strip-spawned; that is, the gametes are removed by gently compressing the abdomen and then combined artificially. With more and more emphasis being put on environmental manipulation as a way of inducing maturation, natural spawning in enclosures is becoming more important (and culturists are having to think about mechanical solutions to the problem of egg collection). Nevertheless, artificial fertilization has unique advantages and will continue to be used in many situations. Some of these advantages are that:
•Spawning enclosures are not required; •Handling of fertilized eggs is easier; •Milt can be used efficiently when it is scarce (by dilution or preservation); •Mixing of gametes for genetic improvement is easier; and •Interspecific or intergeneric hybrids can be produced.
Fish vary enormously in gamete characteristics: some eggs are sticky and some are
buoyant; some are less than 1 mm across whereas others are five times as large. Artificial mixing of eggs and sprmiatozoa can produce very high fertility - over 90% - but techniques vary from fish to fish, and have to be worked out by trial and error. Spermatozoa are kept immotile (unmoving) in the testis by high concentrations of potassium. When they are shed into the 5urrounding water, the potassium is diluted and the sperm cells are activated (start to swim or become motile). Motility usually lasts less than 1 minute, and fertilization itself requires only a few seconds of contact between eggs and
Advanced Freshwater Aquaculture: Fish Reproduction

Advanced Freshwater Aquaculture: Fish Reproduction
147
spermatozoa. ‘To complicate matters further, the eggs of many species are activated by contact with water and must be fertilized immediately. The dry method is the best basis for a fertilization technique, because it takes advantage of these aspects

Advanced Freshwater Aquaculture: Fish Reproduction
148
Table 2. Doses and results of hormonally induced spawning amongst some Mekong fish species in Thailand. Dose Species Hormone I1 Hr2 II3 Spawning
time (hr post injection)
% spawn
Cirrhinus jullieni PG + HCG 0.5-1+50-100
6 0.5-2+30-200
2-6 80-100
Barbodes gonionotus BUS + DOM 10 + 5 - - 4.5-6 100
Probarbus jullieni BUS + DOM 10 + 10 6 30 + 10 8.5 ?
Morulius chrysophekadion
BUS + DOM 15 + 10 - - 7-9 100
BUS + DOM 5 + 10 6 15+10 4-5 100 Leptobarbus hoevenii BUS + MET 15 + 5 - - 5-6 ?
BUS + DOM 10 + 10 - - 4-6 ?
Osteochilus hasselti BUS + DOM 3 + 5 6 10 + 10 5-6 88-100 Osteochilus melanopleurus
BUS + DOM 5 + 5 4 15+10 6 ?
Puntius orphoides BUS + DOM 15 + 10 - - 5 – 6 ?
Trichogaster pectoralis BUS + DOM 5-30 + 10 - - ? ? Pangasianodon gigas BUS +DOM 10 + 10 12 20 + 10 6-12 ?
Catlocarpio siamensis BUS+DOM+PG
40+10+0.5 - - 9 ?
Note PG = working dose of pituitary gland ; HCG = human chorionic gonadotropin in IU/kg fish; BUS = buserelin acetate (a GnHRa) in μg/kg fish ; DOM = domperidone in mg/kg fish; MET = metoclopamide in mg/kg fish; ? = no data; 1 = first injection; 2 = hours between first and second injection; 3 = second injection; 4 = % fertilization
148

149
References Almendras, J. M., Duenas, C., Nacario, J., Sherwood, N. M., Crim, L. W. 1988.
Sustained hormone release-III: Use of gonadotropin releasing hormone analogues to induced multiple spawnibgs in sea bass, Lates calcarifer. Aquaculture. 74: 97-111.
Amatayakul, C., Leelapatra, W., Sumanochitraporn, S., Viputthanumas, T., Sripatrprasite, P. and Kulbul, S. 1995. Sand goby Oxyeleotris marmorata (Bleeker). Inland Fisheries Division. Department of Fisheries, Ministry of Agriculture and Cooperatives. 89 pp.
Boonbrahm, M. 1959. Report on experiment of artificial fertilization of catfish (Pangasius pangasius). Thai Fish. Gaz. 12(1): 15-18.
Crim, L. and Glebe, B. D. 1984. Advancement and synchrony of ovulation in Atlantic salmon with pelleted LHRH analog. Aquaculture. 43: 47 – 56.
Donaldson, E. M. 1986. Lecture on induced spawning, monosex, chromosome set manipulation and growth acceleration in teleosts. Technical Report No.12. CIDA/DOF Northeast Fisheries Project. 112p.
Harvey, B. and Carolsfeld, J. 1993. Induced breeding in tropical fish culture. International Development Research Centre. Ottawa. 144 pp.
Harvey, B. J. and Hoar, W. S. 1979. The Theory and practice of induce breeding in Fish. IDRC-TS21e. 48 pp.
Horvath, L., Tamas, G. and Tolg, I. 1984. Special methods in pond fish husbandry. Halver Corporation. Seattle. 147 pp.
Joragun, J. 1978. Biology of Puntius gonionotus. Annual Report 1978, Kanchanaburi Inland Fisheries Station. Inland Fisheries Division. Department of Fisheries. Ministry of Agriculture and Cooperatives. 52-64 pp.
Juntubtim, W. 1996. Breeding and embryonic development of freshwater garfish (Xenentodon cancila Buchanan). Technical Paper No. 35/1996, Inland Fisheries Division. Department of Fisheries, Ministry of Agriculture and Cooperatives. 58 pp.
Kaya, C.M., Queenth, M.K.K., Dizon, A.E., 1984. Capturing and restraining technique for experimental work on small tuna in larg laboratory holding tanks. Progressive Fish Culturist, 46,288-290.
Kongratanakosol, W and Chesoh, S. 1995. Embryonic development and nursing of Pink-Tail Barb, Leptobarbus hoevenii (Bleeker). Technical Paper No. 17/1995, Pattani Inland Fisheries Development Center, Inland Fisheries Division, Department of Fisheries. Ministry of Agriculture and Cooperatives. 43 pp.
Kwanmuang, S., Maunsa, B., Ajanakitti, J. and Rattanarungsi, S. 1993. Preliminary study on some biological aspects and experiment on breeding of swamp eel, Fluta alba. Technical Paper No. 54/1993, Inland Fisheries Division. Department of Fisheries, Ministry of Agriculture and Cooperatives. 37 pp.
Lam, T. J. 1982. Applications of endocrinology to fish culture. Can. Fish. Aquat. Sci., 39: 111-137.
Lin, H. R. and Peter, R. E. 1996. Hormones and spawning in fish. Asian Fisheries Science. 9:21-33.
Lin, H. R., Van Der Kraak, G., Liang, J. Y., Peng, C., Li, G. Y., Lu, L. Z., Zhou, Z. L., Chang, M. L. and Peter, R. E. 1986. The effect of LHRH analogue and drugs which block the effects of dopamine on gonadotropin secretion and ovulation in fish cultured in china. In: Billard, R. and Marcel, J. (eds.) Aquaculture of Cyprinids. INRA. Paris, France. 139-150 pp.
Advanced Freshwater Aquaculture: Fish Reproduction

150
Lowe, D. M., Moore, M. N. and Bayne, B. L. 1982. Aspects of gametogenesis in the marine mussel Mytilus edulis L. J. Mar. Biol. Ass. U. K. 62:133-145.
MacDonald, B. A. and Thompson, R. J. 1986. Influence of temperature and food availability on the ecological energetics of the giant scallop, Placopecten magellanicus. III. Physiological ecology, the gametogenic cycle and scope for growth. Mar. Biol. 93:37-48.
Macer, C. T. 1974. The reproductive biology of the horse mackerel Trachurus trachurus (L.) in the North Sea and English Chanel. J. Fish. Biol. 6: 415-438.
Marte, C. L., Sherwood, N. M., Crim, L. W. and Harvey, B. 1987. Induced spawning of maturing milkfish (Chanos chanos Forsskal) with gonadotropin-releasing hormone (GnRH) analogues administered in various ways. Aquaculture. 60: 303-310.
Paohorm, S. 1969. Study on biology, breeding method and embryonic development of Puntius gonionotus. Annual Report 1969, Chiang Mai Inland Fisheries Station. Ministry of Agriculture and Cooperatives. 16-41 pp.
Pennapaporn, P., Nukua, A., Ritthitham, S. and Gannaarong, M. 1991. Breeding of Osteocheilus hasseltii (Cul and Val). Technical Paper No. 1, Khon Kaen Inland Fisheries Development Center, Department of Fisheries, Ministry of Agriculture and Cooperatives. 21 pp.
Peter, R. E., Chang, J. P., Nahorniak, C. S., Omeljaniuk, R. J., Sokolowska, M., Shih, S. H. and Billard, R. 1986. Interactions of catecholamines and GnRH in regulation gonadotropin secretion in teleost fish. Recent Prog. Horm. Res. 42: 513-548.
Peter, R. E., Lin, H. R. and Van Der Kraak, G. 1988. Induced ovulation and spawning of cultured freshwater fish in China: Advances in application of GnRH analogues and dopamine antagonists. Aquaculture. 74: 1-10.
Pimolbutr, S. and Pasugdee, S. 2537. Natural breeding and embryonic development of Drumfish, Boesemania microlepis (Bleeker). Technical Paper No. 48/2537, Inland Fisheries Division. Department of Fisheries, Ministry of Agriculture and Cooperatives. 43 pp.
Pholprasith, S. and Tavarutmaneegul, P. 1998. Biology and Culture of the Mekong Giant catfish, Pangasianodon gigas (Chevey, 1930). Extension Paper No. 31, National Inland Fisheries Institute. Thailand. 79 pp.
Pongsuwan, A. and Sithimungka, A. 1989. Handbook of aquaculture in the Northeast Region. Northeast Fisheries Development Project. Department of Fisheries. 388 pp.
Purakkiet, C., Ratanatriwong, V. and Durayab, S. 1990. A preliminary experiment on breeding Black shark (Morulius chrysophekadion) by using suprefact and motillium. Annual Report 1990, Phrae Inland Fisheries Station. Department of Fisheries. 84-91 pp.
Rodrarung, D. and Meewan, A. 1996. Study on spawning ability of Spotted featherback (Notopterus chitala Hamilton). Technical Paper No. 19/1996, Inland Fisheries Division, Department of Fisheries, Ministry of Agriculture and Cooperatives. 18 pp.
Rodrarung, D. and Janesirisak, S. 1990. Induced spawning of earthy pond reared Jullien carp. Annual Report 1990, Nongkai Inland Fisheries Station, Udornthani Inland Fisheries Development Center, Inland Fisheries Division, Department of Fisheries, Ministry of Agriculture and Cooperatives. 114-117 pp.
Advanced Freshwater Aquaculture: Fish Reproduction

151
Rowland, S. J. 1983. The hormone-induced ovulation and spawning of the Australian freshwater fish golden perch, Macquaria ambigua (Richardson) (Percichthyidae). Aquaculture. 35:221-238.
Selman, K. and Wallace, R. A. 1989. Review: Cellular aspects of oocyte growth in teleosts. Zool. Sci. 6:211-231.
Sherwood, N. M., Crim, L. W., Carolsfeld, J. and Walter, S. M. 1988. Sustained hormone release. I. Characteristics of in vitro release of gonadotropin-releasing hormone analogue (GnRH-A) from pellets. Aquaculture. 74: 75-86.
Sidthiimunka, A., Pinyoying, S. and Polasen, P. 1962. Experiments on breeding of Puntius gonionotus (Bleeker). Thai Fish. Gaz. 15(3): 277.
Sokolowska, M., Peter, R. E., Nahorniak, C., Chang, P. J. P., Crim, L. W. and Weil, C. 1984. Induction of ovulation in goldfish, Carassius auratus by pimozide and analogues of LHRH. Aquaculture. 65: 337-34.
Solar, I.I., McLean, E., Baker, I.J., Sherwood, N.M. and Donaldson, E.M., 1990. Induced ovulation of sable fish (Anoplopoma fimbria) following oral administration of des Gly10-(D-Ala6) LHRH ethylamide: short communication. Fish Physiology and Biochemistry, 8 (6), 497-499.
Srisakultiew, P., Pasukdee, S., Ritjarung, S., Mahawong, N. and Pongchawee, K. 1994. Giant gourami Osphronemus gouramy Lacepede. Inland Fisheries Division, Department of Fisheries, Department of Fisheries, Ministry of Agriculture and Cooperatives. 36 pp.
Sukumasavin, N. and Leelapatra, W. 1988. The application of gonadotropin releasing hormone analogue and domperidone for fish seed production in Northeast Thailand. Northeast Fisheries Project Technical Report-15. 31 pp.
Sukumasavin, N. and Leelapatra, W. 1993. Comparison on the biological activities of gonadotropin releasing hormone and its analogues in combination with domperidone on the induction of gonadotropin secretion and spawning in the Thai carp, Puntius gonionotus Bleeker. Thai Fish. Gaz. 46(6): 511-518.
Supachalust, M. 1988. Life history of Notopterus blanci. Annual Report 1988, Nongkai Inland Fisheries Station. Inland Fisheries Division. Department of Fisheries, Ministry of Agriculture and Cooperatives. 59-60 pp.
Tavarutmaneegul, P., Nukwan, S. and Lawonyawut, K. 1992. Induced spawning of some economics freshwater fish species of Thailand. Extension Paper No. 22, National Inland Fisheries Institute. Department of Fisheries. Thailand. 32 pp.
Tongsanga, S. 1961. The effect of pituitary hormones on the spawning of catfish (Clarias macrocephalus). Thai Fish. Gaz. 14(2): 145-162.
Unakornsawat, Y. and Aupakaratna, N. 1996. Embryonic development of giant carp Catlocarpio siamensis (Boulenger). Technical Paper No. 6/1996, Inland Fisheries Division, Department of Fisheries, Ministry of Agriculture and Cooperatives. 23 pp.
Unsrisong, K., Jittakorn, A., Sriwattanawarunyu, and Thienchareon, P. 1990. A culture of Morulius chrysophekadion (Bleeker) spawners in earthy ponds. Annual Report 1990, Chiangmai Inland Fisheries Research Center. Inland Fisheries Division, Department of Fisheries, Ministry of Agriculture and Cooperatives. 86-99 pp.
Vongkamolchoon, C. and Chatchavanthatri, T. 1995. Study on the breeding of red cheek barb, Puntius orpholdes (Cul and Val.). Technical Paper No. 15/1995, Inland Fisheries Division, Department of Fisheries, Ministry of Agriculture and Cooperatives. 29 pp.
Advanced Freshwater Aquaculture: Fish Reproduction

152
Wallace, R .A., Selman, K., Greeley, M. S., Jr Begovac, P. C., Lin, Y-W. P. and McPherson, R. 1987. Current status of oocyte growth. Proceeding of the third International Symposium on the Reproductive Physiology of Fish. St. John’s, Newfoundland, Canada. 167-177 pp.
Watanadirokul, K., Kitisuwan, P. and Boonpew, L. 1983. Life history of Osteochilus melanopleurus (Bleeker) in Ubolratana Reservoir. Annual Report 1983, Ubolratana Reservoir Fisheries Research Station. Inland Fisheries Division. Department of Fisheries, Ministry of Agriculture and Cooperatives. p 15.
Watanadirokul, K. and Kongship, S. 1987. Breeding of Pink-Tail Barb. Proceeding of the Seminar on Fisheries 1987. Department of Fisheries, Inland Fisheries Division, Department of Fisheries, Ministry of Agriculture and Cooperatives. p. 9
Watanadirokul K., Kongcheap, S. and Nabandit, Y. 1987. Life history of Pla Soi Khaw, Cirrhinus jullini Sauvage. In Pla Soi Khaw, Cirrhinus jullieni Sauvage, from Ubolratana Reservoir. Technical Report No. 1/1987, Ubolratana Reservoir Fisheries Research Station. Inland Fisheries Division. Department of Fisheries, Ministry of Agriculture and Cooperatives. 1-20 pp.
Woynarovich, E., Horvath, L., 1980. The artificial propagation of warm water finfishes –a manual for extension. Food and Agriculture Organization of the United Nations, Rome, Italy, Fisheries Technical Paper 201, 183 pp.
Yaron, Z, and Levavi-Zermonsky, B. 1986. Fluctuations in gonadotropin and ovarian steroids during the annual cycle and spawning of the common carp. Fish Physiol. Biochem. 2: 75-86.
Yaron, Z. and Zohar, Y. 1993. An Overview. I. Application of comparative endocrinology to fish culture. In: Muir and Roberts (eds.) Recent Advances in Aquaculture IV. pp. 3-10.
Zohar, Y. 1988. Gonadotropin releasing hormone in spawning induction in teleosts: Basic and applied considerations. In: Zohar, Y. and B. Breton (eds.) Reproduction in Fish: Basic and applied Aspects in endocrinology and genetics. INRA, Paris, France. pp. 47-62.
Zohar, Y. 1989. Endocrinology and fish farming: Aspects in reproduction, growth, and smoltification. Fish Physiol. Biochem. 7: 395-405.
Zohar, Y., Goren, A., Tosky, M., Pagelson, G., Leibovitz, D. and Kock, Y. 1989. The bioactivity of gonadotropin releasing hormones and its regulation in the gilthead seabream, Sparus aurata: in vitro and in vitro studies. Fish. Physiol. Biochem. 7: 59-67
Advanced Freshwater Aquaculture: Fish Reproduction

153
Sperm cryopreservation for application in aquaculture
Basic knowledge
Cryopreservation is the long–term preservation of biological material (e.g. milt or sperm) through freezing using ultra low temperature, usually at –196 °C (Liquid Nitrogen (LN2) temperature). At this temperature cellular viability can be stored in a genetically stable form.
Major benefits of sperm cryopreservation
1. Potentially greater efficiency for selective breeding through storage of gametes from genetically improved fish stocks obtained by classical selective techniques, genetic manipulation (e.g. polyploids&androgenesis) or transgenic.
2. Essential for cross–fertilization between/within related species with non-overlapping breeding seasons, hermaphrodites (e.g. gilthead seabream (Sparus auratus), eels and grouper etc.).
3. Extension of the breeding season in many species. 4. Good choice for conservative programs e.g. protect genetic variation in
natural and farm stocks (Ex–situ preservation or gene bank). 5. Ease for transportation between country.
Fish’s sperm properties
Almost freshwater fish’s sperm have been non–motile (inactivated state) in testis or reproductive tract. Sperm become motile when they are released into water while spawning occur. The major role induces sperm motility is the osmolarity (total solutes/ml) reduction or hypotonic shock causes by water dilution. Generally, freshwater fish’s sperm moving between 20 second to 30 minute depend on milt’s quality, species, environments, season, health etc.
The effects of freezing on sperm cell
Sperm cryopreservation is involved freezing. Theoretically, sperm cell are made up of water, dissolved salt, organelles, sugar, protein and lipids, all surround by a semi–permeable membrane. The membrane permits water to flow relatively freely across it, while acts as a barrier to the large molecule solutes. During cooling progress, cell may be subjected to various stress arising from:
1. The reduction of temperature. 2. Physical and mechanical effects of extra and intracellular ice crystals. 3. Concentration of extra and intracellular solution during freezing.
The latest factor is the major cause to cell damage. Normally, water tend to move from low to high concentration. During freezing progress, extracellular water become solid (ice) before intracellular water cause water leave from cell. This effect make damage to cell because cell are shrunken. Thus, concentration within the cell and its surrounding should be balance in order to protect all cell damage.
Freezing rate (°C/min.) also important factor. Some species need high or slow freezing rate or two steps of freezing rate. A slow freezing rate causes larger ice crystal to from, which may damage cell membrane. On the other hand, if freezing rate is high cold shock may damage the cell.
Advanced Freshwater Aquaculture: Fish Reproduction

154
Question : How to make sperm be survived during freezing?
Answer : Use appropriate extender and dilution rate, use chemical call “cryoprotectant” and use appropriate freezing and warming rate.
Chemical & Equipment involved cryoproservation
1. Extenders Extenders are defined as “a solution of salts, sometimes including organic
compounds, which helps maintain viability of cells during refrigeration”. Extenders have been developed for many fish species. Use of extenders provides increased storage time and dilutes the milt to a greater volume, making the milt easier to work with. Specific dilution ratios (1–10 fold dilution) should be optimized for each species. The antibiotics can be added to extenders to reduce the growth of bacteria that reduce sperm viability, but antibiotic in high concentration can be toxic to sperm cells, therefore, concentrations should be optimized for each species.
Generally, extenders have been devided into 2 groups :
- Simple formulations which only one chemical diluted e.g. Normal Saline (0.9%NaCl), 5% Glucose, 6% Fructose etc.
- Complex formulations which more than one chemical diluted e.g. Fish Ringer’s Solution (FRS), Hank Balanced Salt Solution (HBSS), Immobilizing solution (IMMO) etc. Some extenders have been widely used for many species, but some have
been specie specific. Generally, suitable extender for a specie should be:
- Keep sperm in non–motile stage - Osmolarity (mOsmol/kg) resembling (or slightly higher) that of seminal
plasma. - Appropriate pH and buffering system (weak acid/base plus its minaral). - Antibiotic, 500 IU/ml Penicilin & 0.01mg/ml streptomycin (Penosep®) are
widely used.
Advanced Freshwater Aquaculture: Fish Reproduction

155
Table Chemical composition of extenders commonly used for the cryopreservation of fish spermatozoa
Compositions (g/l) extender
NaCl KCl CaCl2 NaHCO3 Urea Su crose
Fruc tose
Glu cose
Tris
pH Osmo larity
(mOsmol/kg)
0.90% NaCl
(general freshwater fish)
modified Fish Ringer’s solution
(Rana and McAndrew, 1989a):
carp & tilapia
modified Zhang and Liu (MZL)
(Zhang and Liu., 1984 b):
common carp
Extender no.1
(Horvath and Urbanyi, 2001c): European carp, perch
6% fructose
Catfishes (Pangasid, Clariid)
5% glucose
Catfishes (Pangasid, Clariid)
fertilization solution
9.0
6.50
3.00
-
-
-
4.00
-
3.00
-
-
-
-
-
-
0.30
-
-
-
-
-
-
0.20
0.50
-
-
-
-
-
-
-
-
-
-
3.00
-
-
-
10.27
-
-
-
-
-
-
-
60.00
-
-
-
-
40.00
-
-
50.00
-
-
-
-
3.63
-
-
-
7.0
7.9
7.7
8.0
7.7
7.9
7.0
266
275
300
381
322
257
-
a Rana, K.J. and B.J McAndrew. 1989. The viability of cryopreserved tilapia spermatozoa. Aquaculture 76: 335-345. b Kurokura, H., R. Hirano, M. Tomita and M. Iwahashi. 1984. Cryopreservation of carp sperm. Aquaculture 37: 267-273 c Horvath, A. and B. Urbanyi. 2001. Cryopreservation of sperm of some European cryprinids and percids. Word Aquaculture 2001: 23-35.
Advanced Freshwater Aquaculture: Fish Reproduction

156
2. Cryoprotectants
Cryoprotectants are necessary for preserving cell at low temperatures and allow cell to survive during freezing and thawing condition. Cryoprotectants reduces the freezing damage to cell by raising the osmolarity of the cell to prevent excessive dehydration during freezing. (During cryopreservation, water external to cell is frozen, raising the osmolarity of the external environment, and causing water to move out of the cell by diffusion), affecting the size and shape of ice crystal, which from during the freezing and thawing process. Cryoprotectants are divided into two groups:
- Permeating (intracellular) e.g. Dimethyl sulfoxide (DMSO), Methanol, Glycerol etc.
- Non–permeating (extracellular) e.g. sugar (Glucose, Fructose), polymers, starch and protein. However, almost permeating cryoprotectants are highly toxic to cell (and user!) at
ambient temperature. Though, the type and concentration of cryoprotectants are very important for cryopreservation success. Generally, suitable cryoprotectant for a specie should low toxic to cell and high cryoprotective efficiency.
Cryoprotectants safety precautions: Cryoprotectants especially permeating (DMSO, Methanol etc.) are highly toxic to human. Always wear gloves and hands cleaning when and after handling cryoprotectants.
Table Chemicals with cryoprotectant properties.
Chemical Molecular formula Type of action
Dimethyl sulfoxide (DMSO)
Methanol (MeOH)
Ethylene glycol (EG)
Polyethylene glycol (PEG)
Glycerol
Glucose, Fructose
Sucrose
CH3SOCH3
CH3OH
CH2OH-CH2OH
CH2OH-CHOH-CH2OH
C6H12O6
C12H22O11
Intracellular
Intracellular
Intracellular
Intracellular
Intracellular
Extracellular
Extracellular
3. Cryosperm containers and storage (dewar)
Cryosperm containers are made by ultra low temperature tolerance plastic grade, vary in shapes and sizes and depend on freezing method, proposes, milt’s volume and budgets. Containers widely used in fish’s cryosperm is “plastic straw”. Straws are designed to hold a specific volume of cryosperm (e.g. 0.25–0.5 ml). Manufacturers already place a sealing powder surrounded by cotton plugs at one end of the straw. This area seals the straw when the sperm mixture is drawn into the powder and the another open end can be sealed with PVC powder, ball or sealing machine. Straws should be handled from the cotton plugged end, and should be wiped dry to keep the straws from sticking together when frozen.
Advanced Freshwater Aquaculture: Fish Reproduction

157
Storage dewars are designed to store cryopreserved samples in LN2 for extended periods of time. They use a vacuum chamber to provide insulation. LN2 within the storage dewar will evaporate over time and must be refilled periodically. They are vary in size, shape depend on manufacture and proposes.
Liquid nitrogen safety precautions: Always wear insulated gloves and safety glasses when handling LN2. Never touch exposed skin to objects cooled by LN2. Use only containers designed for use with LN2. Use proper transfer equipment to move and handle samples. Never use hollow rods or tubes as dipsticks measurement, because LN2 can shoot up out of the open end.
4. Cooling apparatus
There are many types of instruments that have been developed to produce controlled-rate freezing (°C/min.). Cooling apparatus can be divided into two groups, programmable and manual which have benefit and unbenefit. The programmable freezing apparatus is high accurate, reliable freezing rate and ease for control, but they are very expensive and almost need electricity. The manual apparatus is simple, e.g. a styrofoam box fill with LN2 at a certain level or LN2 dewar itself are widely used in many species. Manual freezing devices are suitable for field used, but unsuitable if use many freezing rate step and require well–training&experiences users.
5. Thawing apparatus
Samples should be removed from the storage dewar and transferred immediately to a styrofoam ice chest containing LN2. This ensures that the samples will not thaw prematurely due to handling. Cryosperm are completely thawed in warm water (35 – 40 °C). Generally, optimal warming rates depend on cooling rates and temperature at the time of plunging sample into LN2. Waterbath or hotplate stirrer and beaker with heating controlled and thermometer for monitoring are commonly used.
5. Fertilization (artificial insemination) by thawed sperm
Thawed sperm samples should be added to eggs and thoroughly mixed. The sperm and eggs should be activated with an appropriate activating solution (e.g. fertilizing solution). Fresh sperm samples should be used to fertilize other batches of eggs to serve as a control for egg quality. After approximately 5 min, water should be added to water harden the eggs. Percent fertilization should be determined to evaluate gamete quality.
General cryopreservation protocol
1. Extender & equipments preparation - Prepare new extender proper for milt dilution. - Set up cooling apparatus and label straws. - Prepare milt collecting equipments (styrofoam box, ice, collecting tubes, syringes
etc.) To minimize unintentional activation of sperm due to urine and water
contamination during collection, small volume of extender should be added to collecting tube keep on ice everytime.
Advanced Freshwater Aquaculture: Fish Reproduction

158
2. milt collection
Male fish from aquaculture stocks should be starved before collect 1 day to minimize faces contamination. Anesthetize fish with MS222 or suitable anesthetic. Use dry towel/tissue paper wipe abdomen and urogenital papillae until dry. Generally, collection are divided into two methods:
(1) Hand strip by use fingers press at the abdomen (testis region). Gently massage abdomen to expel milt. Collect milt in a tube, syringe or beaker and avoid contaminants e.g. urine, faces and blood.
(2) Testis removal from sacrificed fish, use sharp blade cut a pair of testis. Remove connecting tissue and blood by sharp blade, scissors, forcep and tissue paper. Collect milt by cut&press testis in small plastic bag or petri disc containing extender. Use pipette collect milt solution into collection tube. Milt which direct from testis are hardly pure because they usually contaminate with blood. Avoid cutting blood vessels will reduce problem.
3. Sperm quality assessment
Use micropipette drop ~2 μl of milt and ~50 μl of activating solution (4.5% NaCl, fertilization solution or freshwater for freshwater fish) nearby on slide. Use tiny glass rod, tip or toothpick mix the two drops for activate sperm to motile, then suddenly look motile sperm under microscope at 100X magnification of a compound microscope. Generally, there are three parameters for measure sperm quality:
(1) % motile : Look motile sperm which straight forward moving (circular moving, only head vibration or moving by water current must be excluded) and non-motile sperm in two or three field under microscope. Estimate % motile (0% = all dead - 100% all motile). Good milt quality for cryopreservation should be more than 70% motile sperm.
(2) Moving time : Check the time (second) which sperm become moving until more than 90% stop. Because moving time is specie specific. Good milt quality should has normal moving time respect to specie e.g. 20-30 second in silver barb and 40-60 second in common carp.
4. Dilute with extender & cryoprotectant and allow equilibration
Dilution ratio is vary, depend on species and spermatozoa density (cell/ml). Generally, the ratio (milt:diluent) begins from 1:1 (1 fold) to 1 : 9. (10 fold) The 1:2 and 1:4 have been common used in freshwater fishes. The concentration of cryoprotectant is 5 – 15% final concentration (e.g. 10% Methanol ; 10 ml of MeOH in 100 ml milt solution for silver barb and tilapia). Minimize toxic of cryoprotectant to sperm by series dilution under cool environment (on ice) are strong recommendation.
After cryoprotectant is added to the milt solution, sperm should be allowed for equilibration at certain temperature and period e.g. 4 °C (on ice) for 15 minute in silver barb. During the equilibration period, sperm solution should be loaded into the containers e.g. vials or straws.
*If design only short storage for 1 – 2 day in refrigerator, adding of cryoprotectant into milt solution is not necessary.
Advanced Freshwater Aquaculture: Fish Reproduction

159
5. Freezing procedure
After equilibration is finished, samples are placed into the chamber of freezing apparatus. The initial temperature should be same as equilibration temperature.
The freezing or cooling rate (°C/min) have been varied widely as mention above, e.g. silver barb is 4 °C at start temp. and –2 °C/min until the sample temp. is –4 °C then cooling at –11 °C/min. until reach –80 °C. The samples are removed from chamber and plunged into LN2 (-196 °C) in storage dewar.
6. Thawing procedure
The cryosperm are thawed by heating apparatus with thermal control, a waterbath or hotplate with stirrer are recommendation. Normally, the temperature for thawing is around 35 – 40 °C. Use forcep pick vial/straw from dewar, then plunge into warm water until milt solution is gradually thawed (~8 sec. in straw and ~1 minute in vial). Now, the thawed sperm will ready for used.
Please remember that thawed sperm has several performances less than sperm from fresh milt. However, the thawed sperm still viability to fertilize as fresh milt, if cryopreservation is succeed.
Additional reading
Tiersch, T.R. and P.M. Mazik, editors. 2000. Cryopreservation in Aquatic Species. World Aquaculture Society, Baton Rouge, Louisiana, U.S.A.
Muir, J.F. and R.J. Roberts, editors. 1995. Recent Advances in Aquaculture IV. Blackwell Scientific Publication. London, U.K.
Advanced Freshwater Aquaculture: Fish Reproduction