Pure substances Mixtures · 2017. 9. 9. · Pure substances matter with constant composition...
Transcript of Pure substances Mixtures · 2017. 9. 9. · Pure substances matter with constant composition...

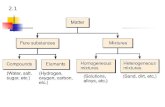
Matter is anything that has mass
and volume
Pure substances matter with constant composition
Elements
substance made up of one kind of atom
Compounds substance made from two or more elements (kinds of atoms)
Inorganic
Compounds
Organic Compounds
are most compounds containing carbon atoms
Mixtures matter with variable composition
Homogeneous
a mixture which uniform composition
Heterogeneous
a mixture which has non uniform composition

Scientists classify matter by looking at the
particles that it contains.
Particles
Atoms Molecules
group of atoms
joined together

This diagram contains two
different kinds of particles.
Each particle is called a single atom.

This diagram contains only
one kind of particle.
Each particle is made up of two
atoms joined together.
Each particle in this diagram is
a molecule.

What can you say about the particles shown in the following diagrams?

What is an element? Elements are the simplest substances.
Each element is made up of just
one particular type of atom,
which is different to the atoms in
any other element.
Atoms usually join together. This is called
bonding.
In some elements, atoms
bond to form small, simple
structures. In other
elements, atoms bond into
giant structures with
millions of atoms.
O
C
N
S
K

METALS NONMETALS
Metals are generally solid (except
mercury) at room temperature
Nonmetals are found in all three
states
Solid: Carbon, Sulfur, Silicon,
Iodine, Phosphorus, Boron
Gaseous: Hydrogen, Oxygen,
Nitrogen, Fluorine, Chlorine
Liquid: Bromine
Metals are heavy (except Na, K,
Mg)
Nonmetals are generally light in
weight
They are hard (except Na, K and
Pb which are soft)
Solid nonmetals are hard, but
brittle (break easily)
They are good conductors of heat
and electricity (except Pb)
They are bad conductors of heat
and electricity (except graphite)

METALS NONMETALS
They are ductile (can be
drawn into wires) and
malleable (can be hammered
into sheets)
They are neither ductile nor
malleable
Their melting and boiling
points are generally high
Their melting and boiling
points are generally low
They are generally lustrous
and shiny (when polished)
They are generally non-
lustrous and dull

Allotropes
Allotropes are forms of the same element, which
show different physical properties.
The different physical properties displayed by
allotropes of an element are explained by the fact
that the atoms are arranged into molecules or
crystals in different ways.
Elements such as carbon, oxygen, phosphorus, tin and sulfur, display allotropy.

What is a compound? A compound is the substance that can be broken down into
simpler substances or elements by a chemical reaction.
A compound is a substance made up of two or more
different types of atoms combined together chemically .
Two elements, hydrogen (H)
and oxygen (O), combine to
make the compound, water.
Which two elements combine
to make the compound
carbon dioxide?

A compound has very different properties to the elements
from which it is made.
carbon dioxide
A colourless gas
which is used
to put out fires.
to make
carbon
A black solid
which can be
used as a fuel.
combines
with
compound elements oxygen
A colourless gas
which is essential
for life.

What are the elements which make up water?
In what ways are the elements different to their compound?
to make combines
with
compound elements water
A liquid which is
essential to our
lives and has many
different uses.
hydrogen
A colourless gas
which is used in
hot air balloons.
A colourless gas
which is essential
for life.
oxygen

Remember: A compound is a substance that is made from more than one element.
atom atom
A compound made up of 2 different elements
A compound made up of 7 different elements