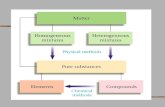
Properties of Matter Chapter 2 Pure Substances ELEMENTS Cannot be broken down into simpler...
-
Upload
charleen-briggs -
Category
Documents
-
view
213 -
download
1
Transcript of Properties of Matter Chapter 2 Pure Substances ELEMENTS Cannot be broken down into simpler...

Properties of Matter
Chapter 2

hydr ogen
element s
wat er
compounds
pur e subs t ances
salt wat er
homogenous
conc r et e
het er ogeneous
mix t ur es
M A T T E R

Pure Substances
• ELEMENTS• Cannot be broken
down into simpler substances.
• Can be found as solids, liquids, or gases
• Represented by symbols
• COMPOUNDS• 2 more elements
chemically joined together
• Can be broken down through chemical means
• Represented by a formula

Mixtures
•Homogenous• Appear the same
throughout.• Well-mixed• Examples:
• Heterogeneous• Can see different parts• Not very well mixed• Examples:

• The best mixed mixture.
• One substance is dissolved in another.
• Cannot be separated by filtering
• Particles are too small to be seen

A heterogeneous mixture that will separate over time
The particles in a suspension are larger than the particles dissolved in a solution
Cloudy appearance
Examples:
muddy water, oil & vinegar salad dressing

• Intermediate sized particles. Smaller than suspension but larger than solution.
• Particles are permanently suspended
• Will scatter light

Physical Properties
• A characteristic of matter that can be directly observed without changing the identity of the substance

Viscosity• The resistance of flow
in a liquid• How “thick” or “thin”
a liquid is• Examples of substance
that have high viscosity:

Conductivity
• The ability to conduct heat or an electric current
• High conductivity: metals
• Low conductivity: wood, rubber

Malleability vs ductility
• MALLEABLE• Ability to be
hammered into a thin sheet.
• Example:
• DUCTILE• Ability to be drawn
into a thin wire.• Example:

Melting and Boiling Points
• Very useful in determining unknown substances.

Density
• The amount of mass per unit volume
• D = M/V

Separating Mixtures
• Filtration = using a strainer or filter to separate on the basis of size of the particles.

Distillation
• A means of separating substances in a mixture or solution by differences in their boiling points.

Chemical properties
• Flammability : a materials ability to burn in the presence of oxygen
• Reactivity : how easily a chemical reacts with other substances.
• Health : degree of danger to humans

Signs of a chemical reaction
1. Change in color
2. Production of a gas
3. Formation of a precipitate

Assignment:
Pages 63-64
1-10,12,15,26,27
Page 65
1-6