PRIDE-HD on behalf of the HSG PRIDE-HD Investigators
-
Upload
huntington-study-group -
Category
Health & Medicine
-
view
276 -
download
0
Transcript of PRIDE-HD on behalf of the HSG PRIDE-HD Investigators

PRIDE-HDon behalf of the HSG PRIDE-
HD InvestigatorsHSG Presentation of Trial
ResultsNovember 3, 2016

HART
on behalf of the HSG HART Investigators
HDCRS Presentation of Trial Results
October 16, 2010

Pridopidine represents a novel therapeutic class of agents: dopamine stabilizers
High
Low
Normal
psychomotor activityPridopidine
High
Low
Normal
psychomotor activityStandard antipsychotic

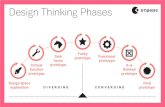
Phase II Trial in Huntington Disease
• Randomized, double-blind, placebo controlled trial
• n = 58• Pridopidine 50 mg/d (n = 28)• Followed for 28 days
*Component of Modified Motor Score
Components of the Total Motor Scale of the UHDRS, with
Modified Motor Score Highlighted
Ocular Pursuit
Saccade Initiation
Saccade Velocity
Dysarthria*Tongue Protrusion*Finger Taps*Pronate/Supinate Hands*Luria (Fist-hand-palm test)*Rigidity – arms*Body bradykinesia*Maximal Dystonia
Maximal Chorea
Gait*Tandem Walking*Retropulsion Pull Test*

Clinical motor improvement by pridopidine – HART and MermaiHD studies results
5
• Phase III study with 437 patients in eight European countries
• Significant effects on TMS after 26 weeks; -3.0 points (p = 0.004)
• The primary endpoint (mMS) was not met
• Phase IIb study with 227 patients in the United States and Canada
• Significant effect on TMS after 12 weeks; -2.8 points (p = 0.039)
• Primary endpoint (mMS) was not met
HART studyMermaiHD study
Huntington Study Group HART Investigators. Mov Disord. 2013 Sep;28(10):1407-15. Yebenes JG, et al. Lancet Neurol. 2011 Dec;10(12):1049-57.

Disclaimer
–Data presented here is based on topline phase 2 analysis
–Further analysis is being undertaken
–Additional data will be presented at forthcoming meetings
–Teva plans to submit results for publication
6

Initial PRIDE-HD design: Evaluation of safety, tolerability and efficacy of higher doses (≥45mg bid) for symptomatic (motor) effects
– Global Ph2, dose-ranging study to build on HART and MermaiHD’s findings– Study Duration: 26 wks; adequate for evaluation of motor effects– 4 doses (45, 67.5, 90, 112.5) and placebo – x2.5 higher that HART and MermaiHD– Population: 400 patients no HD stage restrictions – subjects had minimal TMS ≥
25– 52 sites– Endpoints: focused on motor symptoms (TMS, mPPT)
7

8
PRIDE-HD Chronology of Events:
–26-week protocol finalization: May 2013–Type C meeting: July 2013–First subject first visit: Jan 2014–52-week amendment: October 2014–Enrolment Completion: June 2015–26-week readout: January 2016–52-week readout: August 2016–Press release: September 2016

Based on new insights into Pridopidine MoA
9
Pridopidine may have broader effect beyond symptomatic relief
Phase 2 protocol modified to explore long-term effect on
function and rate of progression (52wks) & safety and tolerability
Total Functional Capacity* is an established endpoint to assess function and disease progression in HD: pre-specified and collected at 26 and 52 weeks
* Shoulson et al. Neurology 1981

PRIDE-HD recruited all stages of HD
10
• Steepest rate of natural decline• Most sensitive to current clinical measures• HD-CAB and TRACK-HD assessments designed
specifically for this period and earlier
• Difficulty completing assessments• Floor and ceiling effects limit ability to track
change• Very significant brain tissue loss
yrs
3
5
5-8
>8

No significant effect on TMS in the Full Analysis Set (FAS) at week 26
11
• All groups showed improvement from baseline
• Large placebo effect was observed
• Similar effects seen at 52 wks

Total Functional Capacity (TFC)

Total Functional Capacity is the most widely accepted tool for assessing functional decline in HD
13
• A well established endpoint for trials aiming to slow clinical progression
• Range: 0-13• Floor and ceiling effects make this more
sensitive to change in early HD, than late HD
Source: Shoulson et al. Neurology 1981

Annual rates of decline in TFC are higher in earlier stages of disease
14 Source: K. Marder et al. Neurology 2000;54:452
HD1 (11-13) HD2 (7-10) HD3 (3-6) HD4 (1-2)

A ‘significant’ slowing of functional decline as measured by TFC is seen
15
45 mg bid 67.5 mg bid 90 mg bid 112.5 mg bidN 59 54 56 58Wk52 ∆ to placebo 1.16 0.36 0.71 0.27p value 0.0003 0.2704 0.0239 0.4144
Impr
ovem
ent
Adj. means ± SEM
Placebo 45 mg bid 67.5 mg bid 90 mg bid 112.5 mg bid
-1.6
-1.2
-0.8
-0.4
0
0.4
0.8
Chan
ge fr
om B
asel
ine
Week 52
45 mg bid 67.5 mg bid 90 mg bid 112.5 mg bidN 75 79 81 81Wk52 ∆ to placebo 0.87 0.11 0.19 0.24p value 0.0032 0.7042 0.5099 0.4061
Impr
ovem
ent
Adj. means ± SEM
Placebo 45 mg bid 67.5 mg bid 90 mg bid 112.5 mg bid
-1.6
-1.2
-0.8
-0.4
0
0.4
0.8
Chan
ge fr
om B
asel
ine
Week 52
Full Analysis Set - pre-specified Early Stage (HD1 and HD2) - post-hocTFC annual decline in placebo as expected

The effect on TFC first observed at 26wks
16

17
Responder Analysis: significant difference in the proportion of subjects that showed no decline in TFC over 52wks between Pridopidine and placebo arms
Responder Analysis Questions Observed Data Analysis45mg bidN=37
PlaceboN=41
1. What proportion of early stage subjects had no deterioration on TFC (score ≥0) at 52 weeks?
30(81%) 20(49%)
Nominal P-value (Chi-Square) 0.0031. What proportion of early stage subjects
had an improvement of ≥1 points on TFC at 52 weeks?
10 (27%) 5 (12%)
Nominal P-value (Chi-Square) 0.099

Timed Up and Go Test (sec): Early HD at 52 wks
18

Summary: Pridopidine shows potential effect on functional decline as measured by TFC
• TFC - an accepted endpoint for functional decline in HD
• Effect on TFC was more pronounced in early HD, and also was observed in responder analysis
• The placebo arm declined as expected
• Effects were observed primarily with 45mg bid and 90mg bid, suggesting a non-linear dose response
• Improvement in ambulation may be contributing to TFC effect
19

Safety and Tolerability

PRIDE-HD: Summary of safety and tolerability
No new findings – Safety and tolerability profile fully compatible with Phase 3 developmentECG• QTcF analysis ongoing
Vital Signs• Dose dependent increase of heart rate• No effect on blood pressure
Labs• No clinically significant abnormalities observed

Psychiatric AEs: Summary & Conclusions
• Psychiatric events reported in all arms
• Irritability (most frequently observed psychiatric event) was more prevalent in placebo group (9%)
• Depression (relatively small number of events) reported in all arms
• Suicidality (relatively small number of events) reported in active arms • Columbia suicidality score change from “Negative” to “Positive” reported in all arms
including placebo
• Rates consistent with published literature

: Summary: Pridopidine shows effect on functional decline measured by TFC, an index of clinical progression
• First of 12 trials to show slowing of functional decline as measured by TFC - the gold standard scale for measuring HD clinial progressionEffect on TFC was significant at 52wks in the full analysis set and in early stage HD at
both 26 and 52wks
• Preclinical data generated in the last 3yrs support the effects on functional decline
• Large placebo response masked motor effects in the full analysis set
• Motor effects were observed in early HD subpopulations
• No new safety and tolerability issues
• PRIDE-HD supports further development23

Acknowledgements
–Patients and their families
–The clinical investigators and sites
–The PRIDE-HD Steering Committee: Karl Kieburtz, Bernhard Landwehrmeyer, Ralf Reilmann, Andy McGarry
–Teva clinical development team led by Spyros Papapetropoulos: Katie Blatt, Igor Grachev and Juha Savola
24

Investigators Slide 1 or 2
12 Countries, 52 sites:Australia
Andrew ChurchyardAnita GohClement LoyPeter Panegyres
AustriaKlaus SeppiRaphael Bonelli
CanadaBlair LeavittMark GuttmanTilak Mendis
DenmarkAnette Torvin MollerLena Hjermind
FranceAnne Catherine Bachoud-LeviChristophe VernyClemence SimoninFabienne CalvasJean-Philippe AzulayPierre Krystkowiak
GermanyCarsten SaftJosef PrillerMichael OrthRalf Reilmann
ItalyFerdinando SquitieriGiuseppe De MichelePaola SoliveriSandro Sorbi

Investigators Slide 2 or 2
Netherlands United StatesNasir Ahmad Aziz Andrew Feigin
Poland Christopher RossDaniel Zielonka Claudia TestaGrzegorz Witkowski David ShprecherJaroslaw Slawek Francis WalkerMonika Rudzinska Jody Corey-Bloom
Russia Karen AndersonAlexander Gustov Karen MarderSergey Illarioshkin Kevin BiglanZuleykha Abdullazarovna Zalyalova Pinky Agarwal
United Kingdom Susan PerlmanAndrea Nemeth Valarie SuskiAnne Rosser Victoria SegroDavid CraufurdHugh RickardsOliver QuarrellRoger BarkerSuresh Kumar Komati