PRESSURE ULCER EVALUATION
Transcript of PRESSURE ULCER EVALUATION

PRESSURE ULCER EVALUATION CLINICAL RESOURCE GUIDE

Wound, Ostomy, and Continence Nurses Society™ (WOCN®)
1
Pressure Ulcer Evaluation: Clinical Resource Guide
The Wound, Ostomy, and Continence Nurses Society™ (WOCN®) suggests the following format for bibliographic citations: Wound, Ostomy and Continence Nurses Society. (2016). Pressure Ulcer Evaluation: Clinical Resource Guide. Mt. Laurel: NJ. Author. Copyright© 2016 by the Wound, Ostomy, and Continence Nurses Society™ (WOCN®). Date of Publication: 3/16/2016. No part of this publication may be reproduced, photocopied, or republished in any form, in whole or in part, without written permission of the WOCN Society.

Wound, Ostomy, and Continence Nurses Society™ (WOCN®)
2
Table of Contents Acknowledgments………………………………………………………………………………………………………………………………………………………….3 Introduction………………………………………………………………………………………………………………………………………………………………… 4 Purpose……………………………………………………………………………………………………………………………………………………………………..4 Comprehensive Pressure Ulcer Evaluation……………………………………………………………………………………………………………………………..4
History………………………………………………………………………………………………………………………………………………………………4 Frequency of pressure ulcer evaluation………………………………………………………………………………………………………………………...5 Table 1. Pressure Ulcer Evaluation……………………………………………………………………………………………………………………………..6 Table 2. Considerations for Assessment of Pressure Ulcers in Special Patient Groups: Pediatric, Geriatric, and Surgical Patients………………25
Glossary……………………………………………………………………………………………………………………………………………………………………28 References………………………………………………………………………………………………………………………………………………………………...31 Appendix A: Differential Assessment of Pressure Ulcers from other Common Lesions/Wounds……………………………………………………………….36 Appendix B: Pressure Ulcer Classification Systems………………………………………………………………………………………………………………….39

Wound, Ostomy, and Continence Nurses Society™ (WOCN®)
3
Acknowledgments
Pressure Ulcer Evaluation: Clinical Resource Guide This document was developed and completed by the WOCN Society’s Pressure Ulcer Evaluation Task Force in August 2015.
Carolyn Crumley, Chair, DNP, RN, ACNS-BC, CWOCN Coordinator-CNS Program of Study University of Missouri Sinclair School of Nursing Columbia, Missouri Clinical Educator – Wound Care Team Saint Luke’s East Hospital Lee’s Summit, Missouri Barbara Dale, RN, CWOCN, CHHN Director of Wound Care Quality Home Health Livingston, Tennessee Joel Graeter, BSN, RN, CWOCN Wound, Ostomy & Continence Nurse East Texas Medical Center Specialty & Rehab Hospital Tyler, Texas Anne Jinbo, PhD, APRN, MPH, CWOCN, CPNP Nurse Practitioner Hawaii Wound, Ostomy & Continence Services Honolulu, Hawaii

Wound, Ostomy, and Continence Nurses Society™ (WOCN®)
4
Pressure Ulcer Evaluation: Clinical Resource Guide Introduction The Wound, Ostomy and Continence Nurses Society™ (WOCN®) recognizes and supports pressure ulcer evaluation as one aspect of a comprehensive patient assessment. This assessment includes, but is not limited to obtaining a history and physical examination, identifying risk factors and comorbidities associated with pressure ulcer development, identifying the individual patient’s goals and expectations, and a thorough evaluation of the pressure ulcer and documentation of the findings. This clinical resource guide updates the previous Pressure Ulcer Evaluation: Best Practice for Clinicians (WOCN, 2008) document, and focuses only on the evaluation of the pressure ulcer. A pressure ulcer is a localized injury to the skin and/or underlying tissue, usually over a bony prominence, as a result of pressure or pressure in combination with shear (National Pressure Ulcer Advisory Panel [NPUAP], European Pressure Ulcer Advisory Panel [EPUAP], & Pan Pacific Pressure Injury Alliance [PPPIA], 2014). A number of contributing or confounding factors are also associated with pressure ulcers; however, the significance of these have yet to be elucidated. Pressure ulcers may be either acute or chronic (i.e., present for 3 to 4 weeks and/or not responding to conventional therapy). Often, wounds of other etiologies are mistakenly identified as pressure ulcers. Purpose The purpose of this clinical resource guide is to facilitate the evaluation and documentation of pressure ulcers in a variety of clinical practice settings. It is intended for use by all levels of healthcare practitioners. The focus of the document is on the evaluation of the pressure ulcer. Table 1 of this document provides an overview of key parameters to assess and factors to consider in the evaluation of pressure ulcers including: location, shape, dimensions, tissue type(s), wound edges, periwound skin, exudate, odor, wound pain, bacterial burden/infection, onset/course/duration, and pressure ulcer classification. Other factors to consider for assessment of pressure ulcers in selected special groups (i.e., pediatric, geriatric, surgical patients) are provided in Table 2. Differentiating pressure ulcers from other types of wounds can be challenging. Various wounds/lesions can be mistakenly identified as pressure ulcers. Pressure can be a contributing factor in wounds/lesions of various etiologies including, but not limited to moisture-associated skin damage (MASD), which includes incontinence-associated dermatitis (IAD) and intertriginous dermatitis (ITD); and ulcers due to arterial, venous, and neuropathic/diabetes disease, and skin tears (NPUAP et al., 2014; Perry et al., 2014). Therefore, it is important to correctly determine the etiology of wounds before initiating treatment (NPUAP et al., 2014). To facilitate differential assessment of pressure ulcers, an overview of common wounds/lesions that may have pressure as a component in their etiology, or are sometimes mistakenly identified as pressure ulcers is provided in Appendix A. Note: Wounds that develop on mucosal membranes are not classified as pressure ulcers (NPUAP et al., 2014), and are not included in this document. Comprehensive Pressure Ulcer Evaluation A comprehensive evaluation of a pressure ulcer and accurate documentation of the findings is the key to providing appropriate, individualized wound care and determining if the care/treatment is effective or needs to be modified. A thorough evaluation and documentation of the findings are also necessary for meeting regulatory requirements, obtaining reimbursement, and preventing litigation. Protocols for pressure ulcer evaluation may vary depending on the care setting, institutional guidelines, skill level of the available caregiver(s), and the overall goals for the individual patient. A thorough evaluation includes findings obtained from a history and physical examination. History. Obtaining a focused history from the patient, in conjunction with a comprehensive assessment of the pressure ulcer(s), provides background information about risk factors and comorbidities that contributed to the development of the pressure ulcer and/or delayed wound healing, the effectiveness of prior treatments, and the individual patient’s goals and expectations. The history should include: a pertinent medical/surgical history

Wound, Ostomy, and Continence Nurses Society™ (WOCN®)
5
(including a history of previous pressure ulcers and effective treatments), previous vascular evaluations, nutritional status, functional capacity, and use of any medical devices. Information should also be gathered regarding use of pressure redistributing strategies, and the availability and use of pressure redistribution equipment. It is important to evaluate a history of any wound pain (e.g., type of pain, etiology/source of pain, condition of the wound, topical wound care). Also, an assessment of psychosocial factors (e.g., psychological health, cognition, social and financial support systems, knowledge and beliefs regarding treatment, and the ability to adhere to the treatment plan) can help determine the care needs, values, and treatment goals of the patient (Bryant & Nix, 2012; NPUAP et al., 2014). Frequency of pressure ulcer evaluation. Minimally, wounds should be assessed and documented on admission, and reassessed at least weekly and whenever signs of deterioration occur (WOCN, 2010). The frequency of assessment may also be determined by agency/facility policies, regulatory guidelines, the overall patient’s condition, wound characteristics, care setting, and the patient’s goals/plan of care (NPUAP et al., 2014). A comprehensive reassessment aids in early detection of possible complications and identifies any needs to make adjustments in the plan of care. If the wound is improving, no change in the treatment plan is indicated; if the wound is deteriorating, the treatment plan should be modified (NPUAP et al., 2014). A patient with a serious/complicated wound in a critical care setting may require more frequent reassessment, while a patient with a chronic wound or receiving palliative care services may warrant less frequent reassessment. Documentation. Documentation of the assessment findings is an essential component of a wound care program that facilitates communication among providers and is necessary to evaluate healing. Wound photography may be utilized as a supplement to written documentation. Photographs taken at the initial assessment, and repeated at regular intervals during treatment, can be helpful in monitoring healing of the pressure ulcer over time (Nix, 2012; NPUAP et al., 2014). For additional information about photography, refer to the WOCN Photography in Wound Documentation: Fact Sheet - www.wocn.org/?page=WoundDocumentation Education. Healthcare personnel caring for the patient/resident with/or at risk for pressure ulcers need education regarding assessment and documentation. Educational programs should incorporate evidence-based guidelines, current accepted terminology, and information about reliable and valid documentation and assessment tools to enable healthcare personnel to provide safe, effective care. In developing policies/procedures for effective pressure ulcer prevention and treatment programs, use of the following information and resources is beneficial:
• Clearly defined terminology and definitions from professional organizations such as the International NPUAP/EPUAP Pressure Ulcer Classification System (www.npuap.org/resources/educational-and-clinical-resources/npuap-pressure-ulcer-stagescategories).
• Regulatory guidelines from the Centers for Medicare and Medicaid Services (CMS; www.cms.gov): Minimum Data Set (MDS), and the Outcome and Assessment Information Set (OASIS; see CMS Manuals: www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/).
• Current standardized, clinical practice guidelines from organizations such as the NPUAP (www.npuap.org), EPUAP (www.epuap.org), the WOCN Society (www.wocn.org), and the Agency for Healthcare Research and Quality (www.ahrq.gov).
• Reliable and validated tools for documentation and evaluation of pressure ulcer risk, such as the Braden Scale for Predicting Pressure Sore Risk (www.bradenscale.com).
• Reliable and validated tools for documentation and evaluation of healing such as the Pressure Ulcer Scale for Healing (PUSH Tool; www.npuap.org/resources/educational-and-clinical-resources/push-tool).
• Templates and documentation formats such as flow sheets, anatomical diagrams, photographs, and electronic documentation systems.

Wound, Ostomy, and Continence Nurses Society™ (WOCN®)
6
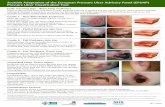
Table 1. Pressure Ulcer Evaluation
Wound Evaluation and Documentation
Parameter Assessment and Documentation Considerations
Location • Identify the anatomical location of the pressure ulcer.
o Use proper terminology in documentation. Terms such as anterior/posterior, medial/lateral, proximal/distal should be used.
o Anatomical drawings or photography may be used.
• Examine the skin under and around medical devices for injury twice a day, or more often if the patient is prone to fluid shifts or edema (NPUAP et al., 2014).
• Pressure ulcers can occur in any anatomical site; most often are found over bony prominences (Black et al., 2010).
• Using proper terminology for the anatomic location of the pressure ulcer can provide information about the causative/contributing factors for the wound (Nix, 2012).
o Anatomic location helps to determine and guide the plan of care (Nix, 2012).
o Consistent numbering of multiple ulcers (with each assessment) helps in identification of each specific ulcer when documenting.
• Medical device-related pressure ulcers:
o Can develop at any location due to pressure from a device designed and applied for diagnostic or therapeutic purposes; the pressure ulcer generally corresponds to the pattern or shape of the device (NPUAP et al., 2014).
o Potential sources of device-related injury include: Tracheostomy faceplates/securement devices, nasogastric tubes, masks for non- invasive positive pressure ventilation, endotracheal/nasotracheal tubes, oximetry probes, oxygen tubing/nasal cannulas, cervical collars, halo devices, helmets, external fixators, immobilizers, plaster casts, Foley catheters, fecal containment devices, surgical drains, central venous/dialysis catheters, intra-aortic balloon pumps, intermittent pneumatic compression device sleeves, graduated compression stockings, and restraints (Bryant & Nix, 2012; Ham, Schoonhoven, Schuurmans, & Leenen, 2014; NPUAP et al., 2014).
o In spinal cord injured patients, cervical collars and backboards can make repositioning difficult due to the need to log-roll the patient and concerns about neurological damage, and devices hinder regular skin assessment (Ham et al., 2014).
o Medical device-related pressure ulcers are common in adults and children in the acute care setting, and may

Wound, Ostomy, and Continence Nurses Society™ (WOCN®)
7
be unavoidable in some circumstances (Pittman, Beeson, Kitterman, Lancaster, & Shelly, 2015).

Wound, Ostomy, and Continence Nurses Society™ (WOCN®)
8
Wound Evaluation and Documentation
Parameter Assessment and Documentation Considerations
o The risk for a medical device-related injury may increase as a result of impaired sensation, moisture under the device, poor perfusion, altered tissue tolerance, poor nutrition, edema, and over sedation/confusion resulting in an inability to communicate discomfort (Bryant & Nix, 2012; NPUAP et al., 2014)
o Rapid deterioration to a suspected deep tissue injury, or a Stage III, IV, or unstageable pressure ulcer is often seen (Bryant & Nix, 2012).
• Bariatric patients.
o Pressure ulcers may occur in atypical locations due to increased tissue weight and subsequent vascular occlusion (NPUAP et al., 2014).
o Common atypical locations include the lateral fleshy part of the buttocks (rather than in the midline at the sacrum), skin fold areas under the breasts (both genders), beneath the pannus, in perineal and gluteal folds, in the lumbar and mid back areas, and on the posterior neck (Camden, 2012; NPUAP et al., 2014).
• Differentiate between MASD (i.e., IAD, ITD) and Stage I and II pressure ulcers (NPUAP et al., 2014; Yates, 2012).
Shape • Document the shape of the pressure ulcer using adjectives such as round, oval, irregular, etc. (Nix, 2012).
o Tracing of shapes or photographs may be helpful.
o Device-related pressure ulcers will be apparent because they will replicate the shape of the device (Nix, 2012).
• Documenting shape provides an accurate clinical description, and can provide clues to the wound’s etiology (Nix, 2012).
• In some settings, such as long-term care hospitals (LTCH), pear- shaped, purple ulcers that occur in the sacrococcygeal area prior to death may be considered Kennedy Terminal Ulcers, and are not coded as pressure ulcers on the LTCH Care Data Set for quality reporting (CMS, 2013).

Wound, Ostomy, and Continence Nurses Society™ (WOCN®)
9
Dimensions • Select and utilize a uniform, consistent method for measuring wound dimensions: Length, width, and depth; and the wound area (NPUAP et al., 2014).
o When possible, place the patient/resident in the same position each time the wound is measured to promote consistency.
o Linear two-dimensional (length, width) and three-dimensional (length, width, depth) measurements are most commonly used and recorded in centimeters (Nix, 2012). – Length is measured by placing the ruler at the point of greatest length―head to toe; width is measured by placing the ruler at the point of greatest width―side to side, and perpendicular (90° angle) to the length (Langemo, Anderson, Hanson, Hunter, & Thompson, 2008; Nix, 2012).
• Communication of size is useful for other members of the healthcare team, regulatory agencies, and payers to determine progress.
• A consistent measurement method allows for more accurate comparisons and evaluation of healing over time (Langemo et al., 2008).
• The greatest length (head-to-toe) and greatest width (side-to- side) method of linear measurement is a quick, noninvasive method of measurement that is the current clinical standard for measuring wounds (Langemo, Spahn, & Snodgrass, 2015).
• The greatest length and greatest width method is currently utilized for documentation on OASIS, MDS, and the NPUAP’s PUSH Tool. Other measurement methods have been reported to result in greater overestimation of the wound area (Langemo et al., 2008).

Wound, Ostomy, and Continence Nurses Society™ (WOCN®)
10
Wound Evaluation and Documentation
Parameter Assessment and Documentation Considerations
– The most common method of obtaining wound depth is by inserting a cotton-tipped applicator into the wound bed at the greatest depth and placing a mark on the applicator at the level of the skin (Nix, 2012).
o An estimate of the area of the wound can be calculated by multiplying length by width for area in centimeters squared (Langemo et al., 2008).
• Identify and describe the presence of sinus tracts/tunneling, and/or undermining (Nix, 2012):
o To evaluate tunnels and undermining, GENTLY insert a sterile cotton- tipped applicator as far as it will go without force, mark the applicator, and measure the length upon removal.
o Use the clock face orientation, with 12 o’clock indicating the head and 6 o’clock indicating the feet, to document the location of sinus tracts, undermined, or tunneled areas.
o In large wounds with irregular borders, the area derived from multiplying length times width will be larger than the actual area (Langemo et al., 2008; Langemo, Spahn, Spahn, & Pinnamaneni, 2015).
o In clinical practice using the ruler method, clinicians tend to vary from measuring the length head-to-toe and the width side-to-side (perpendicular to the length). Instead, the measurements are taken at the longest length and width, irrespective to the head-to-toe orientation, and the width measurement is not perpendicular to the length.
o Recent investigations have shown that thermal (infrared) and visual wound imaging technology might be an option to increase the accuracy of measuring the area of the wound (Langemo, Spahn, & Snodgrass, 2015; Langemo, Spahn, Spahn, et al., 2015).
• Sinus tracts/tunneling, and/or undermining indicate tissue destruction resulting in dead space, which has the potential of forming an abscess.
Tissue Type(s)
• Identify and describe the color/type, amount, and location of tissue in the open wound.
o Describe the tissue color: Red, pink, yellow, tan, gray, green, brown, or black.
o The Red-Yellow-Black (RYB) system has been used to classify wounds according to the color of tissue in the wound (DeLaune & Ladner, 2010; Stotts, 1990). See Appendix B for a description of the RYB system.
• When the wound bed contains a combination of tissue types, describe each type of tissue in percentages, and identify/describe any visible foreign bodies (Black et al., 2010; Nix, 2012).
• Identify and describe the presence/absence of viable/healthy tissue (Black et al., 2010).
Granulation tissue: Bright, beefy red, moist, shiny, granular/bumpy with a velvety appearance; bleeds easily; darkens with pressure; and is pale with ischemia (Bates-Jensen, 2016; Black et al., 2010). Epithelialization: New dry skin; color can vary based on skin pigmentation from white to pearly gray or light pink. Underlying structures may be visible in open wounds (Black et al., 2010):
• For accurate assessment, it is necessary for clinicians to differentiate healthy from nonviable tissue, and recognize the anatomical and physiological characteristics of normal tissue and structures in an open wound (Black et al., 2010).
o The presence or absence of epithelialization and/or granulation tissue can help determine the severity of tissue damage, duration of the wound, stage of healing and effectiveness of current treatment (Nix, 2012).
o The presence of nonviable tissue in the wound is often associated with altered tissue oxygenation, wound desiccation, or increased bacterial burden (Nix, 2012).
o Healing wounds are characterized by increasing amounts of viable tissue (e.g., granulation tissue) and decreasing amounts of nonviable tissue (e.g., eschar or slough).
o A wound may be clean, but not granulating. Nongranulating wounds that are plateaued and not healing have tissue that is pink/red, smooth, and slick versus the healthy, beefy red, berry-like, granulation tissue seen in healing wounds (Bates-Jensen, 2016).
o Stage II pressure ulcers heal by epithelialization, rather

Wound, Ostomy, and Continence Nurses Society™ (WOCN®)
11
than granulation. The dermis would remain intact, with a pink base.

Wound, Ostomy, and Continence Nurses Society™ (WOCN®)
12
Wound Evaluation and Documentation
Parameter Assessment and Documentation Considerations
- Superficial fascia: Tissue directly below the dermis; has a thin, glistening, “spider-web” appearance.
- Subcutaneous tissue: Fat located just beneath the skin; tissue is pale yellow, waxy, globular, oily, and glistens.
- Muscles: Appear shiny, red, with visible striations; are vascular and may bleed easily.
- Tendons: Appear shiny and white with striations; connect muscles to bones; will move when the joint is moved.
- Ligaments: Appear ribbon-like, striated and pearly white; are broader and flatter than tendons; connect bone to bone; and are seen in or near joints.
- Nerves: Appear as long, white, tubular, and rubbery structures. Avoid touching nerves, which could cause damage.
- Bursa: A fluid-filled sac of white fibrinous tissue and synovial fluid; if opened, may leak sticky, mucus-like fluid.
- Bones: Are creamy white, hard, and covered with shiny periosteum; smooth and slick to touch.
- Deep fascia: Dense, fibrinous connective tissue covering muscles, bones, nerves and blood vessels; appears as a glistening surface on muscles.
• Identify and describe the presence/absence of nonviable/necrotic tissue.
o Eschar: Desiccated granulation tissue, skin, fat, tendon or muscle (Black et al., 2010); adherence to the wound bed should be noted (Nix, 2012).
o Slough: Soft, moist, avascular tissue; may be white, yellow, tan, or green; thickness and adherence to the wound bed should be noted (Nix, 2012).
• Identify and describe any additional abnormal tissue/findings.
o Hypergranulation tissue (“proud flesh”): Overgrowth of granulation tissue above the skin level, which inhibits epidermal cell movement across the wound bed to resurface the wound.
o Foreign bodies: Sutures, orthopedic hardware, environmental debris, maggots, etc.
o Avascular/ischemic muscle: Appears dull red, cyanotic, pale, and mushy (Black et al., 2010).
• For closed wounds (Stage I or suspected Deep Tissue Injury), it is important to note the color, texture and temperature of the intact skin and the presence of a serum or blood filled blister/bullae.
• A wound with exposed muscle will not heal until covered with granulation tissue (Black et al., 2010).
• Pressure ulcers in the trochanter, ischium, elbow or knee may leak synovial fluid (Black et al., 2010).
• Bone that is exposed or allowed to dry/desiccate may die (Black et al., 2010).
• Dry adherent eschar can progress to moist, soft, brown, necrotic tissue that lifts/loosens (demarcates) from the wound base; and then may progress to yellow slough that can be firm and adherent, or moist and stringy (Nix, 2012).
• Slough varies in color and consistency (Black et al., 2010):
o White slough indicates a low level of bacterial colonization.
o Yellow or green slough indicates a higher bacterial count.
o As slough ages, it becomes thicker.
o Slough can be confused with tendons, ligaments, fascia, joint capsules, or bursae.
• Diagnosis of osteomyelitis in a patient with a pressure ulcer cannot be based on the clinical exam alone; the condition of bone (firm versus soft) may not correlate with diagnosis of active infection (Larson, Gilstrap, Simonelic, & Carrera, 2011).

Wound, Ostomy, and Continence Nurses Society™ (WOCN®)
13
o Infected bone/osteomyelitis: Bone is gray and flaky with soft fragments (Black et al., 2010).

Wound, Ostomy, and Continence Nurses Society™ (WOCN®)
14
Wound Evaluation and Documentation
Parameter Assessment and Documentation Considerations
Wound Edges
• Identify and describe the condition of wound edges in open wounds.
o Attached edges: Wound edges should be attached, moist, and flush with the wound base so that epithelial cells can migrate from the wound edges across the surface of the wound bed (Nix, 2012).
o Unattached/rolled/closed wound edges (i.e., epibole):
- Undermining is present between the dermis and subcutaneous tissue (Nix, 2012).
- The edge of the wound is raised and more pale or pink than the surrounding tissue (Black et al., 2010).
- Edges are non-proliferative.
o Undermining:
• Tissue destruction that occurs under intact skin around the wound perimeter (Nix, 2012).
• A gap in the edge of the tissue; creates a lip or overhang of the tissue (Black et al., 2010).
• The rim of the wound or wound edge provides information regarding epithelialization, chronicity, and etiology of open wounds (Nix, 2012).
• Epibole results when squamous cells migrate along the base, wall(s), and edge(s) of the wound, preventing migration of epithelial cells and closure of the wound (Nix, 2012).
• Undermining is typically associated with shearing forces (Black et al., 2010; Nix, 2012).
Periwound Skin (Note: Some factors addressed for wound edges also apply to periwound skin as they may represent an extension of the same process.)
• Observe and describe the periwound skin, which should be intact.
• Identify and describe the condition/appearance of the skin within 4 centimeters of the wound edges (WOCN, 2010):
o Color: Redness, pallor (white to blue), blanchable erythema, non- blanchable erythema/red, or purple discoloration.
o Temperature: Use the back of the hand to assess the skin temperature for warmth and coolness.
o Turgor: Assess “tenting” by gently pinching the skin and observing the time it takes for the skin to return to normal, which should be less than 3 seconds.
o MASD; maceration.
o Callus or hyperkeratosis: Hard, white/gray tissue.
o Tenderness or pain.
o Induration: To evaluate, gently press and palpate the skin. If the area is indurated, the skin and underlying tissue will feel abnormally firm and have a definite margin.
o Edema:
- Pitting or non-pitting edema.
• Observation of the periwound skin can help determine the cause of the wound and the response to treatment.
• Skin color is based on anatomical and physiologic phenomena, including four pigments: Melanin, carotene, oxygenated hemoglobin, and reduced hemoglobin (Everett, Budescu, & Sommers, 2012).
• Assessing darkly pigmented skin can be challenging.
o Erythema may be difficult to assess in darkly pigmented individuals. Skin assessment should compare skin temperature and tissue consistency to adjacent skin.
o Post-inflammatory hypopigmentation or hyperpigmentation can occur in individuals with darkly pigmented skin (Wysocki, 2012).
o Dark skinned individuals rarely have a blanch response indicative of adequate tissue perfusion, as seen in lighter skinned individuals (Everett et al., 2012).
o Early skin damage, as well as inflammatory redness associated with cellulitis, may be under-detected in dark skinned individuals (NPUAP et al., 2014).
o Indications of possible skin/tissue damage in darkly

Wound, Ostomy, and Continence Nurses Society™ (WOCN®)
15
- Edema with inflammation/warmth:
▪ Measure the extent of the edema around the wound in centimeters, and/or
pigmented individuals include: Skin discoloration, induration, warmth, edema, pain, or tenderness (Bates-Jensen, 2016; NPUAP et al., 2014).

Wound, Ostomy, and Continence Nurses Society™ (WOCN®)
16
Wound Evaluation and Documentation
Parameter Assessment and Documentation Considerations
▪ Consider drawing a line around the red areas with an indelible marker and note the date to monitor if the area is increasing or decreasing in size.
• Fluctuance (bogginess).
• Lesions: Papules, pustules, bullae, or rashes.
• Skin denudation/erosion.
• Skin stripping: Medical adhesive-related skin injury known as MARSI (McNichol, Lund, Rosen, & Gray, 2013).
• Evidence of healed pressures ulcers/scar tissue.
• Absence of hair (lower extremities).
• Altered pigmentation, compared to adjacent tissue.
• Presence of staples/sutures.
• Increased skin temperature may indicate inflammation and tissue damage; red or purple tissue may indicate deeper tissue injury; and decreased temperature may indicate poor perfusion.
• Decreased turgor/tenting may be the result of aging or dehydration.
• Maceration makes the skin vulnerable to friction and shearing injuries.
• Callus can indicate repetitive stress.
• Tenderness or pain may indicate inflammation or infection.
• Induration may indicate further tissue damage or infection.
• Edema prolongs the healing process; may indicate vascular and/or lymphatic compromise.
• Warmth and swelling indicates soft tissue inflammation that is not always due to infection (e.g., might be an allergic/or sensitivity reaction), but is considered a sign of infection if there is advancing warmth and swelling.
• Lesions/rashes might be a symptom of an allergic reaction to adhesives or topical wound therapy.
• Denuded or eroded skin might be associated with moisture, friction or shear.
• A healed pressure ulcer/scar tissue has decreased tensile strength, which makes the area more vulnerable to recurrent pressure ulcers.
• Skin stripping may indicate inappropriate adhesive removal.
• Lack of hair on the legs may indicate ischemia.
• Increased or changed pigmentation may indicate scars, new epithelial tissue, or chronic injury (e.g., hemosiderin staining due to lower extremity venous disease).

Wound, Ostomy, and Continence Nurses Society™ (WOCN®)
17
Exudate • Identify and observe the color, type, consistency, and amount of exudate.
• Describe the color/type:
o Clear pink/serosanguinous: Typical wound drainage.
o Red or sanguineous: May be normal. Copious bloody drainage may indicate impaired clotting or another disease process, and should be reported immediately to the primary healthcare provider.
o Cloudy, yellow, tan, purulent: Can indicate wound debris, or infection; can be the result of breakdown of slough with autolytic debridement.
o Green: Could indicate bacterial overload.
o Brown or gray: Contains dead cells and debris; may be cloudy and viscous.
• Describe the consistency/viscosity: Liquid-like; thick.
• Describe the amount: None, light, moderate, or heavy.
• Type of exudate can be an indicator of clotting status, infection, or a response to treatments.
• Type/color of exudate will be influenced by the tissue on the wound bed, presence of various microbes, the dressing material and the hydration state of the patient.
• Amount of exudate will vary depending on the stage of healing.
o An increase in exudate may coincide with hyperplasia, critical colonization, biofilm, or wound infection.
o Frequent saturation and strike-through of the dressing may indicate the need for another type of dressing to better control the drainage. It is important to document the type of dressing used and the effect it has on the fluid environment of the wound.

Wound, Ostomy, and Continence Nurses Society™ (WOCN®)
18
Wound Evaluation and Documentation
Parameter Assessment and Documentation Considerations
o To determine the amount, observe the wound bed and tissue, and the dressing used on the wound.
o Observe the wound bed for moisture and determine if the wound and edges are desiccated, moist, or full of fluid. Estimate/quantify the amount of drainage by determining how soiled or wet the dressing is (e.g., slightly moist to completely saturated).
o Determine the length of time it took for the dressing to become saturated, the frequency of dressing changes, and identify if there is persistent periwound maceration.
Odor • Identify and describe any odor from the wound and/or exudate.
• Odor can be recorded as absent, faint, moderate, or strong (Nix, 2012).
• Wound odor should be assessed after removing the old dressing and cleaning the wound.
• Extremely odorous, purulent drainage indicates infection, but not all odor is indicative of infection (Nix, 2012):
o Odor will vary based on the wound moisture, type of dressing, type and number of microbes, location of the wound, and personal and wound-related hygiene.
o Odor can be the result of dressings that promote autolytic or enzymatic debridement.
Wound Pain
• Determine the presence/absence of pain associated with the wound or wound treatment.
• Observe for/and describe the characteristics of the pain (Krasner, 2012; NPUAP et al., 2014):
o Location, onset, and duration.
o Intensity and quality.
o Type of pain: Spontaneous, induced, or positional.
o Alleviating and aggravating factors.
o Effects of pain on quality of life.
o Body language and non-verbal cues.
• Utilize valid, reliable pain scales to assess pain that are appropriate for the patient population: Neonate, child, adult, or cognitively impaired/non-verbal (Krasner, 2012; NPUAP et al., 2014).
• Examples of valid/reliable pain scales include:
o Visual Analog Scale (VAS) or 0 to 10 Numeric Rating Scale (NRS): Valid/reliable single item scales for adults able to self-report pain intensity (Krasner, 2012).
o Wong-Baker FACES Pain Rating Scale: Appropriate for
• The condition of the wound affects wound pain, and the wound condition is frequently changing; pain assessment must be done regularly.
• Unrelieved pain can slow recovery, create an extra burden for the patient/resident and their family members, and increase the costs of health care. An acute change in the level of pain may indicate infection (NPUAP et al., 2014).
• Self-report of pain is the most reliable indicator of the existence and intensity of acute pain (Krasner, 2012).

Wound, Ostomy, and Continence Nurses Society™ (WOCN®)
19
ages 3 and older, and cognitively impaired individuals (NPUAP et al., 2014; Wong-Baker FACES Foundation, n.d.).

Wound, Ostomy, and Continence Nurses Society™ (WOCN®)
20
Wound Evaluation and Documentation
Parameter Assessment and Documentation Considerations
o FLACC (face, legs, activity, crying, consolability) Pain Scale: Effective for children 2 months to 7 years of age (Krasner, 2012; NPUAP et al., 2014).
o CRIES (crying, requires oxygen, increased vital signs, expression, sleepless) Pain Scale: Effective for neonates up to 6 months of age (Krasner, 2012; NPUAP et al., 2014).
o Neonatal Infant Pain Scale (NIPS): Used for neonates (NIPS, n.d.).
o Critical Care Pain Observation Tool (CPOT): Used for critically ill patients who are unable to self-report pain (Stites, 2013).

Wound, Ostomy, and Continence Nurses Society™ (WOCN®)
21
Bacterial Burden/ Infection
• Identify and observe the presence or absence of clinical indicators of an increased bioburden or infection.
• Describe any signs and symptoms of a localized infection that have been identified in a pressure ulcer (NPUAP et al., 2014):
o Lack of healing for 2 weeks.
o Friable granulation tissue.
o Malodor.
o Increased pain in the ulcer.
o Increased heat in the tissue around the ulcer.
o Increased drainage from the wound; a change in the nature of drainage (e.g., onset of bloody or purulent drainage).
o Increased necrotic tissue.
o Pocketing or bridging (i.e., strands of tissue across the ulcer) in the wound bed.
• Describe any signs and symptoms of a spreading acute infection and/or systemic infection that have been identified in a patient with a pressure ulcer (NPUAP et al., 2014):
o Increased erythema, extending from the edge of the ulcer.
o New or increased pain or warmth.
o Purulent drainage.
o Increased size of the wound.
o Induration, crepitus, fluctuance, or discoloration of the surrounding skin.
o Fever, malaise, enlarged lymph nodes.
o Confusion, delirium, and anorexia (especially in older adults).
• Other characteristics associated with wound infection, not specific to patients with pressure ulcers, include (Stotts, 2012):
o Elevated white blood cell count.
o Elevated blood sugar in patients with diabetes.
• Infection develops when the wound bioburden is sufficient to overwhelm the body’s defenses (Stotts, 2012).
• Infection should be diagnosed in the context of the patient’s history and risk factors, as well as subtle clues such as absent or friable granulation tissue and an unexplained failure to heal (Stotts, 2012).
• Classic signs and symptoms of infection may be muted or absent in patients with poor perfusion and/or an impaired inflammatory response, due to malnutrition, steroid therapy, advanced age, immunosuppression, and/or wound chronicity (Stotts, 2012). Other vague symptoms of infection include confusion, malaise, anorexia, and functional difficulties (NPUAP et al., 2014).
• Odor is a subjective finding and varies according to the microbial species present:
o Not all odor indicates infection.
o Odor can be due to topical therapies/dressings.
o The wound should be thoroughly cleaned before odor is evaluated.
• If a pressure ulcer has been present for 4 weeks or longer, lacks any signs of healing in the previous 2 weeks, and has clinical signs of inflammation, despite evidence-based wound care; and/or it does not respond to antimicrobial therapy, the presence of biofilm should be suspected (NPUAP et al., 2014).

Wound, Ostomy, and Continence Nurses Society™ (WOCN®)
22
Wound Evaluation and Documentation
Parameter Assessment and Documentation Considerations
Onset, Course and Duration of Wound
Determine the onset and duration of the wound to determine if the wound is acute or chronic, and if it is making progress in healing or deteriorating (Nix, 2012; WOCN, 2010). Assess for signs of healing such as decreased exudate, decreased wound size, improved tissue in the wound, etc. (NPUAP et al., 2014; WOCN, 2010). Assess for factors that might impede healing (WOCN, 2010): Medications such as steroids, immunosuppressive agents, anti-cancer drugs, etc. Impaired perfusion status if on a lower extremity. Limited or inaccessible resources/equipment for care. Assess for complications such as fistula, abscess, bacteremia/sepsis, cellulitis, squamous cell cancer/Marjolin’s ulcer, etc. (WOCN, 2010).
• Evidence of healing should be observed for partial thickness ulcers within 1 to 2 weeks and in 2 to 4 weeks in full thickness ulcers (WOCN, 2010).
o A pressure ulcer that has been present for 4 weeks or more without any improvement, despite proper therapy, suggests the presence of other co-morbidities or factors such as unrelieved pressure, malnutrition, critical colonization, biofilm, infection, osteomyelitis, or cancer (Nix, 2012; NPUAP et al., 2014).
o Lack of any healing within 2 weeks of appropriate care or deterioration of the wound warrants an evaluation of the treatment plan, and referral for additional diagnostics, interventions, or adjunctive therapies (NPUAP et al., 2014).
• Valid and reliable assessment scales are available to facilitate assessment of pressure ulcer healing, such as the Bates-Jensen Wound Assessment Tool (BWAT) and the Pressure Ulcer Scale for Healing (PUSH) Tool (Bates-Jensen, 2016; Nix, 2012; NPUAP et al., 2014; WOCN, 2010).

Wound, Ostomy, and Continence Nurses Society™ (WOCN®)
23
Pressure Ulcer Classifica- tion
• Classify pressure ulcers according to established classification systems as directed by the facility and/or regulatory requirements for specific settings.
• Pressure ulcers may be staged according to various classification systems:
o The International NPUAP/EPUAP Pressure Ulcer Classification System can be used to stage pressure ulcers, including medical device- related pressure ulcers (NPUAP et al., 2014).
- See Appendix B for the definitions of the stages/categories. Note: Definitions are subject to change. Please refer to the NPUAP’s website for verification of current definitions and images of the different stages (www.npuap.org).
- Mucosal pressure ulcers are not staged using the pressure ulcer staging system because anatomical tissue comparisons cannot be made (NPUAP et al., 2014). Therefore, in some settings such as long-term care hospitals (LTCH), medical device-related mucosal ulcers (e.g., related to rectal tubes) are not coded on the LTCH CARE Data Set for quality reporting (CMS, 2013).
o Red-Yellow-Black Classification System (DeLaune & Ladner, 2010; Stotts, 1990):
- Classifies the wound according to color of tissue.

Wound, Ostomy, and Continence Nurses Society™ (WOCN®)
24
Wound Evaluation and Documentation
Parameter Assessment and Documentation Considerations
- See Appendix B for further description of the system.
o Superficial Skin Changes and Deep Pressure Ulcer Framework (Sibbald, Krasner, & Woo, 2011):
- Differentiates superficial from full thickness wounds based on etiology.
- See Appendix B for further description of the system.

Wound, Ostomy, and Continence Nurses Society™ (WOCN®)
25
Table 2. Considerations for Assessment of Pressure Ulcers in Special Patient Groups: Pediatric, Geriatric, and Surgical Patients
Considerations for Assessment of Pressure Ulcers in Special Patient Groups
Group Factors to Consider Comments
Pediatric Patients
• Carefully assess the most common areas where pressure ulcers are found in children (Coha, Wysocki, Bryant, & Nix, 2012):
o Head (particularly the occiput), ears (e.g., under oxygen tubing and cannula), bridge of the nose (e.g., under bilevel positive airway pressure [BIPAP] masks).
o Coccyx and iliac crest.
o Fingers and toes (e.g., under pulse oximetry probes); heels, ankles and elbows from pressure on the bed.
o Skin under splinting devices and casts, or any site where tubes contact with skin (e.g., gastrostomy or tracheostomy tubes).
• Multiple factors and comorbidities are associated with pressure ulcers in pediatric patients.
o Due to the relatively large size of the infant’s head in proportion to the rest of the body, pressure ulcers in pediatric patients most commonly occur on the head (Bernabe, 2012; Coha et al., 2012).
o Undeveloped bowel and bladder control in infants are risks for pressure ulcers in infants (Coha et al., 2012).
o Children with spina bifida and/or cerebral palsy and other disorders are affected by decreased mobility, insensate areas (especially the lower extremities), fecal and urinary incontinence, difficulty in communicating, and feeding problems (Coha et al., 2012).
o Critically ill infants and children with impaired perfusion, altered nutrition, unstable hemodynamic status, limited mobility, immunosuppression, and multiple medications are at increased risk for pressure ulcers.
o Pediatric patients are particularly susceptible to device-related pressure ulcers (Coha et al., 2012; NPUAP et al., 2014):
- Due to the varying sizes and the growth patterns of children, ill-fitting medical devices can be a source of pressure.
- Orthotic devices may rub on insensate areas, or become moist from perspiration (e.g., lower extremity devices or thoraco-lumbo-sacral orthoses).
- Wheelchairs can cause ulcers behind the knees and/or ankles.
- In neonates, skin texture immaturity and endotracheal intubation have been significantly associated with pressure ulcers (NPUAP et al., 2014).

Wound, Ostomy, and Continence Nurses Society™ (WOCN®)
26
Geriatric Patients
• Consider the individual’s cognitive status when assessing pain and selecting a pain assessment tool (NPUAP et al., 2014).
• Ensure pressure ulcers are correctly differentiated from other skin injuries, especially IAD or skin tears (NPUAP et al., 2014).
• Perform a vascular assessment (e.g., pedal pulses, ankle brachial index) if the pressure ulcer is on the lower limb/foot/heel to rule out arterial disease (WOCN, 2014).
• Elderly individuals are particularly at high risk for development of pressure ulcers due to multiple factors (NPUAP et al., 2014; Pieper, 2012; Shepherd, Wipke-Tevis, & Alexander, 2015). Individuals in aged care settings have a higher rate of pressure ulcers than those in acute care settings (NPUAP et al., 2014).
• Multiple factors associated with aging contribute to an increased risk of developing pressure ulcers and delayed wound healing, including the following (Pieper, 2012; Wysocki, 2012):
o Gradual atrophy of blood and lymph vessels in the skin; increased vascular fragility; decreased vascular response for vasoconstriction and vasodilation.
o Decreased epidermal turnover; thinning of dermis and epidermis; loss of subcutaneous fat.
o Decreased surface barrier function; decreased sensory and pain perception; decreased hypersensitivity reactions.
o Impaired ability of the soft tissue to distribute a mechanical load without compromising blood flow.
o A decrease in vascular endothelial growth factor.
o Decreased endothelial cell permeability response.
• Lower extremity arterial disease (LEAD) can be an underlying cause of heel pressure ulcers (WOCN, 2014). LEAD occurs in 21% of individuals older than 65 years of age, in 30% of those older than 70 years of age, and in 40% of those 80 years of age and older (WOCN, 2014).

Wound, Ostomy, and Continence Nurses Society™ (WOCN®)
27
Considerations for Assessment of Pressure Ulcers in Special Patient Groups
Group Factors to Consider Comments
Surgical Patients
• Careful skin inspection should be performed before and after surgery (Bryant & Nix, 2012).
• Assess potential sites where excess pressure in the surgical environment may have occurred (Wadlund, 2010).
o Initial manifestation of tissue damage may present as skin discoloration (e.g., bruising) that evolves into a blister or necrosis (Bryant & Nix, 2012).
o Altered skin of patients with vascular compromise may present with a mottled, irregular pattern on the skin that might resolve, or evolve into a full-thickness skin breakdown (Bryant & Nix, 2012).
• Early assessment may prevent existing lesions from progressing to higher stages and facilitate early treatment.
• During surgery, pressure from an external source may compress the tissue between the source and the bone. The external force can be from the weight of equipment resting on/or against the patient, positioning devices such as stirrups, leg, or arm holders; the surgical team resting against the patient; or the patient’s own body weight (Wadlund, 2010).
• Tissue damage associated with surgery may become apparent within hours or it can be delayed for up to 3 days, and the process can continue to evolve over several days (e.g., 2 to 6 days), which makes it difficult to isolate the time of the original injury (Pieper, 2012).
• Multiple risks have been identified for development of pressure ulcers in intraoperative patients (Bryant & Nix, 2012):
o Vascular, cardiac, thoracic, or orthopedic surgeries.
o Impaired blood flow and hypotensive episodes.
o Surgeries longer than 4 hours.
o Thin stature; poor nutritional status.
o Diabetes or vascular disease.
o Specific positions (e.g., lithotomy or lateral supine).
o Having general anesthesia.
o Use of thermoregulatory devices.
o Use of a standard operating room mattress during surgery.
o Age greater than 70 years.
o Preoperative Braden score less than 20.
• According to a study by Aronovitch in 2007, the incidence of pressure ulcers in surgical patients was related to the presence of at least one comorbidity, use of a warming device, administration of three or more anesthetic agents, and a median operative time of 4.48 hours (Pieper, 2012).
• Recently, Scott (2015) reported that the following factors (called Scott Triggers) have been associated with pressure ulcer development in surgical patients, and that two or more triggers may increase the risk for perioperative pressure ulcers:
o Age (> 62 years).
o Serum albumin level (< 3.5 mg/dL).
o American Society of Anesthesiologists (ASA) physical status classification system score (≥ 3).
o Time on the table over 180 minutes.

Wound, Ostomy, and Continence Nurses Society™ (WOCN®)
28
Glossary Bioburden: Refers to the diversity, virulence, and interaction of organisms with each other and the body. Biofilm: A polysaccharide matrix in which organisms attach, live and multiply on the wound surface, and can affect wound healing by creating chronic inflammation or infection. Blanchable: A vascular response showing a white or pale area that is created when pressure is applied to the tissue, and when the pressure is released, a normal color returns. Bulla: Vesicle greater than 1 centimeter in diameter. Callus: A common, usually painless thickening of the skin at locations of pressure or friction; a natural protective response to repetitive stress. Cellulitis: A diffuse, acute infection of the skin and subcutaneous tissue characterized most commonly by local heat, redness, pain, and swelling. Clean, non-granulating: Wound base that is smooth and red, but lacks the cobblestone-or berry-like appearance of granulation tissue. Denudation: The process of stripping bare; a condition of losing the outer layer of the skin such as the epithelium. Desiccated: Tissue that is dehydrated; dried-up. Erosion: Loss of part of the epidermis; depressed, moist, glistening tissue; follows the rupture of a vesicle or bulla. Erythema: Redness of the skin surface produced by vasodilatation. Eschar: Brown or black necrotic, devitalized tissue; tissue can be loose or firmly adherent and hard, soft or boggy. Exudate: Accumulation of fluids in a wound that may contain serum, and cellular debris. Fluctuance: Erythema with a soft, boggy feel. Intertriginous dermatitis: Inflammation resulting from moisture trapped in skin folds subjected to friction. Common areas involved in ITD are the groin and under the breasts where opposing skin surfaces are in prolonged contact and friction and moisture entrapment occur (Gray et al., 2011; Yates, 2012). Ischemia: Deficiency of blood caused by functional constriction or obstruction of a blood vessel. Kennedy Terminal Ulcer: A Kennedy Terminal Ulcer is characterized by location on the sacrococcygeal area; appears as a purple, red, blue, or black discoloration of the skin with a butterfly or pear shape that has irregular borders; has a sudden onset and develops rapidly into a full-thickness wound, despite appropriate preventive care; and may precede death in days to weeks (Emmons & Dale, 2016).

Wound, Ostomy, and Continence Nurses Society™ (WOCN®)
29
Maceration: Softening and breakdown of the skin from prolonged exposure to moisture. Marjolin’s ulcer: Malignancy that develops in an existing, chronic wound such as a pressure ulcer (Goldberg & Bryant, 2012). Moisture-associated skin damage (MASD): Inflammation and erosion of the skin due to prolonged exposure to moisture such as urine or stool, perspiration, wound exudate, mucus or saliva. It is an umbrella term for four different types of injury to the skin: Incontinence-associated dermatitis, intertriginous dermatitis, peristomal moisture-associated dermatitis, and periwound moisture-associated dermatitis (Gray et al., 2011; Yates, 2012). Necrotic: Nonviable or devitalized tissue that has died, and lost its physical properties and biologic activity. Non-blanchable: Redness that does not fade when the skin is touched/pressed and released. Non-healing wound: A wound that fails to progress in healing in a defined time frame, despite, comprehensive, appropriate wound management; wound might be clean but non-granulating; lack of healing can be due to infection, closed/hyperkeratotic wound edges, or avascular/necrotic tissue. Non-pitting edema: Skin is shiny and taut, and does not indent with pressure. Open/attached wound edges: Edges are even or flush with the wound base so that epithelial cells can migrate across the surface of the wound. Pus: Thick fluid containing leukocytes, bacteria, and cellular debris; indicates infection. Papule: An elevated, firm, circumscribed area; less than 1 centimeter in diameter. Pitting edema: Identified by firmly pressing a finger down into the tissues and waiting 5 seconds; on release of pressure, tissues fail to resume their previous position and an indentation appears. Purulent: Producing or containing pus. Pustule: Elevated, superficial lesion; similar to a vesicle but filled with purulent fluid. Sinus tracts/tunneling: Course or path of tissue destruction occurring in any direction from the surface or edge of the wound; results in dead space with potential for abscess formation. In contrast to undermining, a sinus tract/tunnel involves a small portion of the wound edge, whereas undermining involves a significant portion of the wound edge. Skin stripping/medical adhesive-related skin injury (MARSI): Superficial layers of skin are removed along with the adhesive product. MARSI is an injury in which erythema, and/or other signs of skin injury occur such as a vesicle, bulla, erosion, or tear that persists 30 minutes or longer after removal of an adhesive (McNichol et al., 2013). Slough: Soft, moist, avascular (necrotic/devitalized) tissue; may be white, yellow, tan, or green; may be loose or firmly adherent.

Wound, Ostomy, and Continence Nurses Society™ (WOCN®)
30
Undermining: Area of tissue destruction extending under intact skin along the periphery of a wound; commonly seen in shear injuries. In contrast to sinus tract/tunneling, undermining involves a significant portion of the wound edge, whereas a sinus tract/tunnel involves only a small portion of the wound edge. Vesicle: Elevated, circumscribed, superficial lesion that is not into the dermis; filled with serous fluid; less than 1 centimeter in diameter.

Wound, Ostomy, and Continence Nurses Society™ (WOCN®)
31
References Bates-Jensen, B. M. (2016). Assessment of the patient with a wound. In D. B. Doughty & L. L. McNichol (Eds.), Wound, Ostomy and Continence
Nurses Society Core curriculum: Wound management (pp. 38-68). Philadelphia, PA: Wolters Kluwer. Beeckman, D., Campbell, J., Campbell, K., Chimentao, D., Coyer, F., Domansky, R.,…Wang, L. (2015). Proceedings of the Global IAD Expert Panel.
Incontinence associated dermatitis: Moving prevention forward. Wounds International 2015. Retrieved from http://www.woundsinternational.com/other- resources/view/incontinence-associated-dermatitis-moving-prevention-forward
Berke, C. T. (2015). Pathology and clinical presentation of friction injuries. Case series and literature review. Journal of Wound, Ostomy and Continence
Nursing, 42(1), 47-61. http://dx.doi.org/10.1097/WON.0000000000000087 Bernabe, K. Q. (2012). Pressure ulcers in the pediatric patient. Current Opinion in Pediatrics, 24(3), 352-356.
http://dx.doi.org/10.1097/MOP.0b013e32835334a0 Black, J., Baharestani, M., & the National Pressure Ulcer Advisory Panel. (2012). Pressure ulcers with exposed cartilage are Stage IV pressure ulcers.
Retrieved from http://www.npuap.org Black, J., Baharestani, M., Black, S., Cavazos, J., Conner-Kerr, T., Edsberg, L.,…Schultz, G. (2010). An overview of tissue types in pressure ulcers: A
consensus panel recommendation. Ostomy Wound Management, 56(4), 28-44. Black, J. M., Gray, M., Bliss, D. Z., Kennedy-Evans, K. L., Logan, S., Baharestani, M. M.,…Ratliff, C. R. (2011). MASD Part 2: Incontinence-associated
dermatitis and intertriginous dermatitis: A consensus. Journal of Wound, Ostomy and Continence Nursing, 38(4), 359-370. http://dx.doi.org/10.1097/WON.0b013e31822272d9
Bryant, R. A. (2012). Types of skin damage and differential diagnosis. In R. A. Bryant & D. P. Nix (Eds.), Acute and chronic wounds: Current
management concepts (4th ed., pp. 83-107). St. Louis, MO: Elsevier-Mosby. Bryant, R. A., & Nix, D. P. (2012). Developing and maintaining a pressure ulcer prevention program. In R. A. Bryant & D. P. Nix (Eds.), Acute and
chronic wounds: Current management concepts (4th ed., pp. 137-153). St. Louis, MO: Elsevier-Mosby. Camden, S. G. (2012). Skin care needs of the obese patient. In R. A. Bryant & D. P. Nix (Eds.), Acute and chronic wounds: Current management
concepts (4th ed., pp. 477-484). St. Louis, MO: Elsevier-Mosby. Centers for Medicare and Medicaid Services. (2013). Chapter 3, Section M: Skin Conditions. Centers for Medicaid and Medicare Services Long-Term
Hospital Quality Reporting Program Manual (version 2.0, pp. M-3). Retrieved July 23, 2015 from download section http://www.cms.gov/Medicare/Quality-Initiatives- Patient-Assessment-Instruments/LTCH-Quality-Reporting/LTCH-Quality-Reporting-Training.html
Coha, T., Wysocki, A. B., Bryant, R. A., & Nix, D. P. (2012). Skin care needs of the pediatric and neonatal patient. In R. A. Bryant & D. P. Nix (Eds.),

Wound, Ostomy, and Continence Nurses Society™ (WOCN®)
32
Acute and chronic wounds: Current management concepts (4th ed., pp. 485-504). St. Louis, MO: Elsevier-Mosby

Wound, Ostomy, and Continence Nurses Society™ (WOCN®)
33
DeLaune, S. C., & Ladner, P. K. (2010). Fundamentals of nursing: Standards & practice (4th ed.). Clifton Park, NY: Delmar Cengage Learning. Doughty, D., Junkin, J., Kurz, P., Selekof, J., Gray, M., Fader, M.,…Logan, S. (2012). Incontinence-associated dermatitis. Consensus statements,
evidence-based guidelines for prevention and treatment, and current challenges. Journal of Wound, Ostomy and Continence Nursing, 39(3), 303-315. http://dx.doi.org/10.1097/WON.0b013e3182549118
Emmons, K. R., & Dale, B. A. (2016). Palliative wound care. In D. B. Doughty & L. L. McNichol (Eds.), Wound, Ostomy and Continence Nurses Society Core curriculum: Wound management (pp. 690-703). Philadelphia, PA: Wolters Kluwer. Everett, J. S., Budescu, M., & Sommers, M. S. (2012). Making sense of skin color in clinical care. Clinical Nursing Research, 21(4), 495-516.
http://dx.doi.org/10.1177/1054773812446510 Goldberg, M. T., & Bryant, R. A. (2012). Managing wounds in palliative care. In R. A. Byrant & D. P. Nix (Eds.), Acute & chronic wounds. Current
management concepts (4th ed., pp. 505-513). St. Louis, MO: Elsevier Mosby. Gray, M., Beeckman, D., Bliss, D. Z., Fader, M., Logan, S., Junkin, J.,…Kurz, P. (2012). Incontinence–associated dermatitis: A comprehensive review
and update. Journal of Wound, Ostomy and Continence Nursing, 39(1), 61-74. http://dx.doi.org/10.1097/WON.0b013e31823fe246 Gray, M., Black, J. M., Baharestani, M. M., Bliss, D. Z., Colwell, J. C., Goldberg, M.,…Ratliff, C. (2011). Moisture-associated skin damage: Overview
and pathology. Journal of Wound, Ostomy and Continence Nursing, 38(3), 233-241. http://dx.doi.org/10.1097/WON.0b013e318215f798 Ham, W., Schoonhoven, L., Schuurmans, M. J., & Leenen, L. P. (2014). Pressure ulcers from spinal immobilization in trauma patients: A systematic
review. Journal of Trauma and Acute Care Surgery, 76(4), 1131-1141. http://dx.doi.org/10.1097/TA.0000000000000153 Krasner, D. L. (2012). Wound pain: Impact and assessment. In R. A. Bryant & D. P. Nix (Eds.), Acute and chronic wounds: Current management
concepts (4th ed., pp. 368-378). St. Louis, MO: Elsevier-Mosby. Langemo, D., Anderson, J., Hanson, D., Hunter, S., & Thompson, P. (2008). Measuring wound length, width, and area: Which technique? Advances in
Skin & Wound Care, 21(1), 42-45. http://dx.doi.org/10.1097/01.ASW.0000284967.69863.2f Langemo, D., Spahn, J., & Snodgrass, L. (2015). Accuracy and reproducibility of the wound shape measuring and monitoring system. Advances in Skin & Wound Care, 28(7), 317-23. http://dx.doi.org/10.1097/01.ASW.0000465900.04721.18 Langemo, D., Spahn, J., Spahn, T., & Pinnamaneni, V. C. (2015). Comparison of standardized clinical evaluation of wounds using ruler length by width
and Scout length by width measure and Scout perimeter trace. Advances in Skin & Wound Care, 28(3), 116-21. http://dx.doi.org/10.1097/01.ASW.0000461117.90346.0d
Larson, D. L., Gilstrap, J., Simonelic, K., & Carrera, G. F. (2011). Is there a simple, definitive, and cost-effective way to diagnose osteomyelitis in the
pressure ulcer patient? Plastic and Reconstructive Surgery, 127(2), 670-676. http://dx.doi.org/10.1097/PRS.0b013e3181fed66e

Wound, Ostomy, and Continence Nurses Society™ (WOCN®)
34
LeBlanc, K., Baranoski, S., Christensen, D., Langemo, D., Sammon, M. A., Edwards, K.,...Regan, M. (2013). International skin tear advisory panel: A tool kit to aid in the prevention, assessment, and treatment of skin tears using a simplified classification system©. Advances in Skin & Wound Care, 26(10), 459-476. http://dx.doi.org/10.1097/01.ASW.0000434056.04071.68
McNichol, L., Lund, C., Rosen, T., & Gray, M. (2013). Medical adhesives and patient safety: State of the science. Consensus statements for the
assessment, prevention, and treatment of adhesive-related skin injuries. Journal of Wound, Ostomy and Continence Nursing, 40(4), 365-380. http://dx.doi.org/10.1097/WON.0b013e3182995516
National Pressure Ulcer Advisory Panel, European Pressure Ulcer Advisory Panel, & Pan Pacific Pressure Injury Alliance. (2014). Prevention and
treatment of pressure ulcers: Clinical practice guideline. Perth, Australia: Cambridge Media. Neonatal Infant Pain Scale. (n.d.). Retrieved January 11, 2016 from http://www.cincinnatichildrens.org/assets/0/78/176/4711/4717/4213d844-3558 4c76-a342- 84a9f377420c.pdf Nix, D. D. (2012). Skin and wound inspection and assessment. In R. A. Bryant & D. P. Nix (Eds.), Acute and chronic wounds: Current management
concepts (4th ed., pp. 108-121). St. Louis, MO: Elsevier-Mosby. Perry, D., Borchert, K., Burke, S., Chick, K., Johnson, K., Kraft, W.,…Thompson S. (2014). Institute for Clinical Systems Improvement (ICSI) health care
protocol: Pressure ulcer prevention and treatment protocol (3rd ed.). Retrieved from https://www.icsi.org/_asset/6t7kxy/PressureUlcer.pdf Pieper, B. (2012). Pressure ulcers: Impact, etiology, and classification. In R. A. Bryant & D. P. Nix (Eds.), Acute and chronic wounds: Current
management concepts (4th ed., pp. 123-136). St. Louis, MO: Elsevier-Mosby. Pittman, J., Beeson, T., Kitterman, J., Lancaster, S., & Shelly, A. (2015). Medical device-related hospital-acquired pressure ulcers: Development of an
evidence- based position statement. Journal of Wound, Ostomy and Continence Nursing, 42(2), 151-154. http://dx.doi.org/10.1097/WON.0000000000000113
Scott, S. M. (2015). Progress and challenges in perioperative pressure ulcer prevention. Journal of Wound, Ostomy and Continence Nursing, 42(5),
480-485. http://dx.doi.org/10.1097/WON.0000000000000161 Shepherd, M. M., Wipke-Tevis, D. D., & Alexander, G. L. (2015). Analysis of qualitative interviews about the impact of information technology on
pressure ulcer prevention programs: Implications for the wound, ostomy and continence nurse. Journal of Wound, Ostomy and Continence Nursing, 42(3), 235-241. http://dx.doi.org/10.1097/WON.0000000000000136
Sibbald, R. G., Kelley, J., Kennedy-Evans, K. L., Labrecque, C., & Waters. N. (2013). A practical approach to the prevention and management of
intertrigo or moisture-associated skin damage, due to perspiration: Expert consensus on best practice. Wound Care Canada, Supplement, 11(2). Retrieved from http://www.woundcarecanada.ca/wp-content/uploads/WCCv11n2SUPPLEMENT-Intertrigonc.pdf
Sibbald, R. G., Krasner, D. L., & Woo, K. Y. (2011). Pressure ulcer staging revisited: Superficial skin changes & deep pressure ulcer framework©.
Advances in Skin & Wound Care, 24(12), 571-580. http://dx.doi.org/10.1097/01.ASW.0000408467.26999.6d

Wound, Ostomy, and Continence Nurses Society™ (WOCN®)
35
Stites, M. (2013). Observational pain scales in critically ill adults. Critical Care Nurse, 33(3). http://dx.doi.org/10.4037/ccn2013804 Stotts, N. A. (1990). Seeing red, yellow and black: The three-color concept of wound care. Nursing, 20(2), 59-61 (Historical reference). Stotts, N. A. (2012). Wound infection: Diagnosis and management. In R. A. Bryant & D. P. Nix (Eds.), Acute and chronic wounds: Current management
concepts (4th ed., pp. 270-278). St. Louis, MO: Elsevier-Mosby. Wadlund, D. L. (2010). Maintaining skin integrity in the OR. OR Nurse, 4(2), 26-32. Wong-Baker FACES Foundation. (n.d.). Wong-Baker FACES pain rating scale. Retrieved January 11, 2016 from http://wongbakerfaces.org/ Wound, Ostomy and Continence Nurses Society. (2008). Pressure ulcer evaluation: Best practice for clinicians. Mt. Laurel, NJ: Author. Wound, Ostomy and Continence Nurses Society. (2010). Guideline for prevention and management of patients with pressure ulcers. WOCN clinical
practice guideline series 2. Mt. Laurel, NJ: Author. Wound, Ostomy and Continence Nurses Society. (2011a). Incontinence associated dermatitis (IAD): Best practice for clinicians. Mt. Laurel, NJ: Author. Wound, Ostomy and Continence Nurses Society. (2011b). Guideline for management of wounds in patients with lower-extremity venous disease.
WOCN clinical practice guideline series 4. Mt. Laurel, NJ: Author. Wound, Ostomy and Continence Nurses Society. (2012). Guideline for management of wounds in patients with lower-extremity neuropathic disease.
WOCN clinical practice guideline series 3. Mt. Laurel, NJ: Author. Wound, Ostomy and Continence Nurses Society. (2014). Guideline for management of wounds in patients with lower-extremity arterial disease. WOCN
clinical practice guideline series 1. Mt. Laurel, NJ: Author. Wysocki, A. B. (2012). Anatomy and physiology of skin and soft tissue. In R. A. Bryant & D. P. Nix (Eds.), Acute and chronic wounds: Current
management concepts (4th ed., pp. 40-62). St. Louis, MO: Elsevier-Mosby. Yates, S. (2012). Differentiating between pressure ulcers and moisture lesions. Wounds Essentials 2012, 7(2), 16-22. Retrieved September 23, 2015 \
from http://www.wounds-uk.com/pdf/content_10714.pdf

Wound, Ostomy, and Continence Nurses Society™ (WOCN®)
36
Appendix A Differential Assessment of Pressure Ulcers from other Common Lesions/Wounds
Typical Characteristics
Pressure Ulcer MASD: Incontinence- Associated Dermatitis (IAD)
MASD: Intertriginous Dermatitis (ITD)
Skin Tears Wounds due to Lower Extremity Arterial Disease
Wounds due to Lower Extremity Venous Disease
Wounds due to Lower Extremity Neuropathic Disease
Location Most often occurs over a bony prominence due to pressure or pressure and shear; can result from use of medical devices.
Usually occurs in skin folds, inner thighs or perineal/perianal area, and is associated with urinary or fecal incontinence; often occurs in a confined area where a containment garment is worn.
Can occur over a bony prominence, but most commonly occurs in folds of opposing skin surfaces (e.g., anal cleft, perineal/perianal area, inguinal and axilla areas, under breasts; appears as “kissing lesions” on buttocks); and is due to trapped moisture, perspiration and friction.
Can occur anywhere, but most often occur on the forearms/arms, dorsal aspects of hands, and legs/feet; injury associated with shear, friction, and/or blunt force.
Occur between toes, over phalangeal heads, over lateral malleolus, mid tibia (shin), and in areas exposed to pressure, repetitive trauma, or rubbing of footwear.
Typically occur above the ankle superior to the medial malleolus (gaiter/sock area), but might be present anywhere on the lower leg, including the posterior calf.
Usually occur on pressure points on the plantar surface of the foot; common sites include interphalangeal joint of the great toe, first metatarsal head, and the heel.
Shape Often round, but may be linear or irregularly shaped.
Diffuse; might be blotchy.
Diffuse; might have superficial spots.
Linear with/or without epidermal flap.
Round, punched out appearance.
Irregular Round or oblong.
Edges Well defined; smooth to rolled edges.
Poorly defined edges; diffuse without a discrete border.
Diffuse and/or irregular edges.
Well-defined edges.
Punched out; smooth; might have rolled edges.
Irregular but defined edges.
Well-defined and smooth edges; callus may be present.

Wound, Ostomy, and Continence Nurses Society™ (WOCN®)
37
Appendix A Differential Assessment of Pressure Ulcers from other Common Lesions/Wounds Continued
Typical Characteristics
Pressure Ulcer MASD: Incontinence- Associated Dermatitis (IAD)
MASD: Intertriginous Dermatitis (ITD)
Skin Tears Wounds due to Lower Extremity Arterial Disease
Wounds due to Lower Extremity Venous Disease
Wounds due to Lower Extremity Neuropathic Disease
Wound Base Characteristics vary based on stage of ulceration from non- blanchable erythema of intact skin to full thickness; may be deep red or purple color in suspected deep tissue injury; can be pink, red, yellow, tan, brown, or black; undermining and tunneling may be present.
Can have intact, erythematous skin, or might have denudation with a shiny, red, glistening wound base.
Red or pink; can have erythema and inflammation that may progress to erosion or denudation.
Varies from a linear break in the skin with separation of the skin layers, but without tissue loss, to a complete loss of the epidermis.
Dry, pale; minimal or absent granulation tissue.
Ruddy red, granulation tissue; might have yellow adherent or loose slough; undermining and tunnels are uncommon.
Pale pink; undermining can be present.
Periwound Skin Varies Can be red, irritated, or edematous with papular/vesicular formations; might have flaking or crusting of the skin or maceration; secondary infection can occur and candidiasis is common with satellite lesions.
May be red, pink, or white; maceration common; secondary fungal or bacterial infection may occur.
Varies Pallor on elevation and dependent rubor; atrophy of the skin; skin cool to touch; absent hair; edema uncommon.
Maceration, crusty, scaling skin, hyperpigmenta- tion, and edema are common.
Might appear normal, or callus might be present.

Wound, Ostomy, and Continence Nurses Society™ (WOCN®)
38
Appendix A Differential Assessment of Pressure Ulcers from other Common Lesions/Wounds Continued
Typical Characteristics
Pressure Ulcer MASD: Incontinence- Associated Dermatitis (IAD)
MASD: Intertriginous Dermatitis (ITD)
Skin Tears Wounds due to Lower Extremity Arterial Disease
Wounds due to Lower Extremity Venous Disease
Wounds due to Lower Extremity Neuropathic Disease
Exudate Varies; can be high volume and purulent in some cases.
Varies from none to clear serous, to weepy and sticky.
Weepy; sticky. Minimal Minimal Varies from mild, moderate, to heavy (often heavy).
Small to moderate.
Pain Varies; can be absent to severe; might be increased by dressing changes.
Can have pain, burning, and itching.
Itching, burning. Variable depending on tissue loss.
Intermittent claudication with ambulation; resting pain; pain with elevation.
Minimal, unless infected.
Variable; foot can be insensate; might have neuropathic pain.
Depth Ranges from intact skin to full thickness tissue loss.
Usually superficial/ shallow with a partial thickness tissue loss.
Usually superficial, with partial thickness tissue loss.
Can be partial or full thickness.
Often full thickness; might be deep.
Usually shallow; can be partial or full thickness.
Varies partial to full thickness with bone involvement.
Necrotic Tissue Only full thickness pressure ulcers can have necrotic tissue.
No necrotic tissue.
No necrotic tissue.
Might have a necrotic epidermal flap.
Might have necrotic tissue; gangrene (wet or dry) might be present.
Might have non- viable tissue, or yellow, adherent fibrin, or loose slough.
Might have necrotic tissue.
Citations (Bryant, 2012; Doughty et al., 2012; Gray et al., 2012; NPUAP et al., 2014)
(Beeckman et al., 2015; Berke, 2015; Black et al., 2011; Doughty et al., 2012; Gray et al., 2012; Sibbald, Kelley, Kennedy- Evans, Labrecque, & Waters, 2013; WOCN, 2010; WOCN, 2011a)
(Berke, 2015; Black et al., 2011; Doughty et al., 2012; Gray et al., 2011; Sibbald et al., 2013; Yates, 2012)
(Bryant, 2012; LeBlanc et al., 2013; McNichol et al., 2013)
(WOCN, 2014) (WOCN, 2011b) (WOCN, 2012)

Wound, Ostomy, and Continence Nurses Society™ (WOCN®)
39
Appendix B Pressure Ulcer Classification Systems
International NPUAP/EPUAP Pressure Ulcer Classification System (NPUAP et al., 2014)
Pressure Ulcer Staging
Definition Further Description Comments
International Classification System
A pressure ulcer classification system in which pressure ulcers are classified according to the amount of visible tissue loss (NPUAP et al., 2014). Note: Terminology and definitions are subject to change. Please refer to the NPUAP’s website for verification of current definitions and images (www.npuap.org)
The NPUAP and EPUAP classification systems are the most commonly used, and in 2009 the two systems were combined to create the NPUAP/EPUAP system (NPUAP et al., 2014).
• Advantages of the International Classification System:
• Widely utilized in various settings and populations.
o Recognized by regulatory and reimbursement agencies.
o Facilitates communication between health professionals and allows for comparison of data between institutions (NPUAP et al., 2014).
• Disadvantages of the International Classification System:
o Numerical system misleadingly implies that pressure ulcers progress through defined stages (Sibbald et al., 2011).
o Reliability is poor; misinterpretation of staging is common (Sibbald et al., 2011).
Stage I: Non- blanchable Erythema (NPUAP et al., 2014)
Intact skin with non-blanchable redness of a localized area usually over a bony prominence. Darkly pigmented skin may not have visible blanching; its color may differ from the surrounding area.
The area may be painful, firm, soft, warmer or cooler as compared to adjacent tissue. Stage I may be difficult to detect in individuals with dark skin tones. May indicate “at risk” individuals (a heralding sign of risk).
• Use the finger or the transparent disk method to assess whether skin has blanchable or non-blanchable erythema.
o Blanchable erythema (normal reactive hyperemia) is visible skin redness that becomes white when pressure is applied, and reddens when pressure is relieved.
o Non-blanchable erythema is visible skin redness that persists with the application of pressure. It indicates structural damage to

Wound, Ostomy, and Continence Nurses Society™ (WOCN®)
40
the capillary bed/microcirculation.
Stage II: Partial Thickness Skin Loss (NPUAP et al., 2014)
Partial thickness loss of dermis presenting as a shallow open ulcer with a red pink wound bed, without slough. May also present as an intact or open/ruptured serum- filled blister.
Presents as a shiny or dry shallow ulcer without slough or bruising: Bruising indicates suspected deep tissue injury. This stage should not be used to describe skin tears, tape burns, perineal dermatitis, maceration or excoriation.
Stage II pressure ulcers do not have necrotic tissue and heal with epithelialization, rather than granulation tissue.

Wound, Ostomy, and Continence Nurses Society™ (WOCN®)
41
Appendix B Pressure Ulcer Classification Systems Continued
International NPUAP/EPUAP Pressure Ulcer Classification System (NPUAP et al., 2014)
Pressure Ulcer Staging
Definition Further Description Comments
Stage III: Full Thickness Skin Loss (NPUAP et al., 2014)
Full thickness tissue loss. Subcutaneous fat may be visible, but bone, tendon or muscle are not exposed. Slough may be present but does not obscure the depth of tissue loss. May include undermining and tunneling.
The depth of a Stage III pressure ulcer varies by anatomical location. The bridge of the nose, ear, occiput, and malleolus do not have subcutaneous tissue; and Stage III ulcers can be shallow. In contrast, areas of significant adiposity can develop extremely deep Stage III pressure ulcers. Bones or tendons are not visible or directly palpable.
Stage IV: Full Thickness Tissue Loss (NPUAP et al., 2014)
Full thickness tissue loss with exposed bone, tendon or muscle. Slough or eschar may be present on some parts of the wound bed. Often includes undermining and tunneling.
The depth of a Stage IV pressure ulcer varies by anatomical location. The bridge of the nose, ear, occiput and malleolus do not have subcutaneous tissue and these ulcers can be shallow. Stage IV ulcers can extend into muscle and/or supporting structures (e.g., fascia, tendon or joint capsule) making osteomyelitis possible. Exposed bones or tendons are visible or directly palpable.
Note: While not part of the original classification system, NPUAP has indicated that pressure ulcers with exposed cartilage should be classified as Stage IV pressure ulcers (Black, Baharestani, & NPUAP, 2012).
Unstageable: Depth Unknown (NPUAP et al., 2014)
Full thickness tissue loss in which the base of the ulcer is covered by slough (yellow, tan, gray, green or brown) and/or eschar (tan, brown or black) in the wound bed.
Until enough slough and/or eschar is removed to expose the base of the wound, the true depth, and therefore stage, cannot be determined. Stable (i.e., dry, adherent, intact without erythema or fluctuance) eschar on the heels serves as “the body’s natural (biological) cover” and should not be removed.
Suspected Deep Tissue Injury: Depth Unknown (NPUAP et al., 2014)
A purple or maroon, localized area of discolored intact skin; or a blood-filled blister due to damage of underlying soft tissue from pressure and/or shear. The area may be preceded by tissue that is painful, firm, mushy, boggy, warmer or cooler as compared to adjacent tissue.
Deep tissue injury may be difficult to detect in individuals with dark skin tones. Evolution may include a thin blister over a dark wound bed. The wound may continue to evolve and become covered by thin eschar. Evolution may be rapid exposing additional layers of tissue, even with optimal treatment.

Wound, Ostomy, and Continence Nurses Society™ (WOCN®)
42
Appendix B Pressure Ulcer Classification Systems Continued
Additional/Alternative Classification Systems
Classification System
Definition Further Description Comments
Red-Yellow-Black (RYB) Classification System (DeLaune & Ladner, 2010; Stotts, 1990)
• The RYB system was developed by Marion Laboratories in 1988 to simplify wound assessment and direct treatment options by classifying wounds according to color of the tissue:
o Red:
• Has normal granulation tissue.
• Keep the wound moist, clean, and protected.
o Yellow:
• Fibrinous slough and/or purulent exudate from bacteria present.
• Wound needs to be cleansed, and non- viable tissue removed.
o Black:
• Necrotic tissue/eschar present.
• Wound needs debridement.
• The RYB categorizes the wound based on the least desirable color of tissue that is present.
• Advantages of the RYB system:
o Simple system for staff not familiar with wound care.
o Still included in many nursing fundamental textbooks.
• Disadvantages of the RYB system:
o Assessment limited to the wound bed.
o Fails to account for foreign bodies and underlying exposed structures.
o Universal recommendations to debride eschar may not be consistent with current treatment guidelines (i.e., maintain stable black eschar on heels; assess perfusion status for ischemia before debride; WOCN, 2014).

Wound, Ostomy, and Continence Nurses Society™ (WOCN®)
43
Superficial Skin Changes & Deep Pressure Ulcer Framework (Sibbald et al., 2011)
• An alternative, conceptual framework to differentiate skin breakdown based on etiology.
• Superficial skin changes that occur from the outside in:
o Partial thickness.
o Primarily due to moisture and friction.
• Deep pressure ulcers that occur from the inside out:
o Full thickness.
o Primarily due to tissue deformation from compression, shear, and tension.
• Current terminology for superficial skin changes includes Stage II.
• Examples of superficial skin changes: Skin tears, IAD, contact dermatitis, and blisters due to friction.
• The etiology of superficial redness or discoloration on intact skin is poorly understood and should not be included in classification systems, but should be documented and described.
• Current terminology for deep pressure ulcers includes Stage III and IV, suspected deep tissue injury, and unstageable. Not all suspected deep tissue injury results in ulcers.
• Advantages of the framework:
• Improved delineation based on etiologic factors.
• Decreased likelihood of misinterpretation.
• Disadvantages of the framework:
• Recently proposed.
• Not widely disseminated or evaluated.











![Pressure ulcer prevention[2]](https://static.fdocuments.in/doc/165x107/55894026d8b42ab55b8b467a/pressure-ulcer-prevention2.jpg)