Pre-Quiz What is a valence electron?. Chapter 2: The Chemical Context of Life Objectives of...
-
Upload
clinton-fitzgerald -
Category
Documents
-
view
224 -
download
4
Transcript of Pre-Quiz What is a valence electron?. Chapter 2: The Chemical Context of Life Objectives of...
Chapter 2: The Chemical Context of Life
Objectives of Learning:
1.Atomic structure determines the behavior of an element
2.Life requires elements and compounds
3.Atoms combine by chemical bonding
4.Weak bonds play an important role in the chemistry of life
5.A molecule’s biological function is related to its shape
6.Chemical reactions make and break bonds.
Themes: Emergent Properties, Structure and Function
CHAPTER 2 THE CHEMICAL CONTEXT CHAPTER 2 THE CHEMICAL CONTEXT OF LIFEOF LIFE
What you do NOT need to know:
1. Exact atomic numbers, atomic mass, or Electron orbitals.
The TestThe Test will give you atomic number & mass. You will use them to explain atomic structure & bonding properties.
2. Table 2.1; Fig. 2.9; & Methods Box pg. 30. Table 2-1 - you should become familiar with the major elements in the human body not the atomic #’s & % column. Also Fig. 2.15; 2.16 & 2:18.
You should know:You should know:
1. Atomic number = # of protons & electrons. Each has atomic mass of approx. 1 dalton (1.7 x 10-24 grams.). Thus all elements are electrically neutral. (protons are +1 charge & electrons are -1 charge.)
2. Atomic mass (wt) = sum of protons & neutrons.3. Energy levels (shells; clouds) outside the nucleus (holds the protons & neutrons) have maximum of 2 in the first shell, 8 in the second, and 8 in the third).
4. Life made of about 25 elements, 4 major ones (96%) are: O (1st); C (2nd); H (3rd); N (4th).
Example: Neon has Atomic # of 10; Atomic weight of 20 so there are:
10 protons; 10 neutrons (20-10) and 10 electrons.Shells = 2 in the first (fill first shell first); 8 in
the second.
Note: what happens to PE (potential energy) as electrons move from lower to outer shells & vice versa..
Chemical properties of an atom - is determined by the # of outer ( or valence) electrons (e-). 1. An atom with a completed valence shell (8 e-) is un-reactive & will not combine with other elements to make a compound. They are INERT. These are atoms in the last column of the periodic table. The “happy” atom has a full valence shell.2. All other atoms are chemically reactive because their valence shell does not have 8 e-.
3. Valence 1 electron in outer shell = +1 valence; 2 in outer shell = +2; 3 in outer shell = +3; 5 in outer shell = -3; 6 in outer shell = -2; 7 in outer shell = -1.Think of Valence as the charge the atom will have when it gains or losses electrons in a chemical reaction.
Chemical Bonds – Atoms are held together to make compounds either by sharing or transferring their valence electrons.
1. Strongest bonds are Covalent (sharing valence e-) & ionic (transfer of valence e- from one element to another).
Covalent bonding - shared
NOTE: a = single bond; b = double bond; c = 2 single bonds; d = simplest organic compound. Sharing is also in number but not force. These are structural formulas.
Carbon & Covalent Bonding - Always 4 bonds. Either 4 singles; 2 doubles; a double & 2 singles; or a triple & one single.
Molecular Formula - (like H20) shows only what the compound consists of.
Electronegativity - Attraction of an atom for e-. The more electronegative an atom is the more it pulls shared e- toward it.
Nonpolar covalent bond – e- are shared equally (like H2 & 02). These are diatomic molecules, because they are 2 of the same atom.
Polar covalent bonds - one atom is more electronegative than another. This means electrons in a bond are not shared equally.
Example: H20
NOTE: O (the most electronegative of all “life” elements) attracts the shared e- more strongly than H (low electronegativity). H-0 bonds are always
very polar.
Chart of Electronegativities in Elements: Note that O is much more electronegative than any other element except F (which is not found in most biological systems).
Ionic & Covalent Bond ComparisonsIonic & Covalent Bond Comparisons
NOTE: The difference between ionic & covalent bonds is not always a clear cut line.
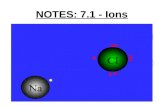
IONIC BONDS: Atoms are so unequal in their attraction for valence e- that the more electronegative atom strips an e- completely away from its partner.
Atoms then become ions. If the atom gains electrons it becomes a negative (anion) ion. If the atom loses electrons it becomes a positive (cation) ion.
Ionic Bond - is the attraction between atoms, or ions due to the fact that they are unlike charged.
Ionic Compounds - are called salts. (ex. NaCl). They have stronger bonds as solids & weaker in solution.
IMPORTANCE OF WEAK CHEMICAL BONDS IN CHEMISTRY OF LIFE:
The connection between molecules always them to come in contact, respond to each other & then separate.
Why would this be important in the body or other organisms?EX. OF WEAK BONDS - H BONDS
1. Occurs when a hydrogen atom covalently bonds to an electronegative atom that is attracted to another electronegative atom.
VERY IMPORTANT IN BIOLOGY!!!!………
A molecules biological function is related to its shape!!
The shape of molecules determine how most molecules of life recognize & respond to each other.Example of a natural & man-made substance that have areas of the molecule that have similar shapes, thus they will have similar reactions in the body.