Powerpoint Jeopardy
description
Transcript of Powerpoint Jeopardy

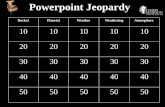
Powerpoint JeopardyWater Acid Base Stoichiometry Gas Laws Redox
10 10 10 10 10
20 20 20 20 20
30 30 30 30 30
40 40 40 40 40
50 50 50 50 50

What is the name of the intermolecular force that exists between water molecules?

What are the units on the vertical axis on a solubility curve?

What is the minimum temperature required to completely dissolve 350 g of sodium nitrate in 250 mL of water?

10.0g of sodium hydroxide, NaOH, was completely dissolved in water so that the final volume of solution was 200mL. The
concentration of this solution is?

NaF will dissolve in water.Name the following:1.The type of bond broken in the NaF crystal2.The type of bond formed between Na+ and Water

What is the Bronsted Lowry definition of an acid

What is the conjugate base of HSO3-?

The pH of a 0.01M HNO3 solution at 25C is?

Write two equations showing the two ionisation stages of H2PO4
-

The pH of a 0.100M Ba(OH)2 solution at 25C is

What do the coefficients in a reaction represent?

What are the 4 steps to solving a stoic question?

HCl(g) + NH3(g) NH4Cl(s)If 1 mole of HCl was reacted with 2
moles of NH3, what gases would be present at the end of the reaction?

What is the name of the instrument that delivers the aliquot in a titration?

2HCl(aq) + Na2CO3(aq) 2NaCl(aq) + CO2(g) + H2O(l)
If 2 moles of HCl and 4 moles of Na2CO3 were reacted. Which is in excess, and by how many moles?

What temperature is 0K in celcius?

Convert 2atm to kPa

State all units for each part of the general gas equation

A gas has a volume of X L at 10°C. If the pressure is doubled, at what temperature will the volume still be X L?

Calculate the mass of O2 in 1.0L of air at 17°C and 100kPa, given that oxygen forms 21% by volume of air

What does OIL RIG stand for?

In a galvanic cell, do the electrons flow from the anode to the cathode,
or the other way round?

What is the oxidation number of N in NO3
-?

Is the following reaction a Redox Reaction?Ag+(aq) + Cl-(aq) AgCl(s)

Complete the following half reactionCr2O7
2- (aq) Cr3+(aq)

• Hydrogen Bonding

g(solute)/100g(solvent)

75°C

1.25M

1. Ionic Bond2. Ion-Dipole Bond

A substance the donates a proton to a base

H2SO3

2

1. H2PO4- + H2O HPO4
2- + H3O+
2. HPO42- + H2O PO4
3- + H3O+

13.3

Mole Ratio

1. Start with a balanced equation2. Find n
3. Apply mole ratio! (divide by what you got, multiply by what you
want)4. Answer the question

NH4Cl and NH3

Pipette

Na2CO3 by 3 moles

-273

202.6 kPa

P – kPaV – L
n – MolR – J/K/molT - Kelvin

293K (20°C)

0.28g

Oxidation is LossReduction is Gain

Anode to cathode

+5

No.

Cr2O72- (aq) Cr3+(aq)
Cr2O72- (aq) Cr3+(aq) + 7H2O(l)
Cr2O72- (aq) + 14H+(aq) Cr3+(aq) + 7H2O(l)
Cr2O72- (aq) + 14H+(aq) +6e- 2Cr3+(aq) + 7H2O(l)