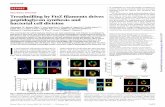
Polymorphism of FtsZ Filaments on Lipid Surfaces: Role of Monomer Orientation
Transcript of Polymorphism of FtsZ Filaments on Lipid Surfaces: Role of Monomer Orientation

Polymorphism of FtsZ Filaments on Lipid Surfaces: Role of MonomerOrientationMario Encinar,†,‡,§ Andrew V. Kralicek,§,∥ Ariadna Martos,⊥ Marcin Krupka,# Sandra Cid,#
Alvaro Alonso,† Ana, I. Rico,# Mercedes Jimenez,∇ and Marisela Velez*,†,○
†Instituto de Catalisis y Petroleoquímica, CSIC, Marie Curie, 2, Cantoblanco, 28049 Madrid, Spain∥The New Zealand Institute for Plant & Food Research Limited, Private Bag 92169, Auckland, New Zealand⊥Max Planck Institute of Biochemistry, Am Klopferspitz 18, D-82152 Martinsried, Munich, Germany#Centro Nacional de Biotecnología, CSIC, Darwin 3, 28049 Madrid, Spain∇Centro de Investigaciones Biologicas, CSIC, Ramiro de Maeztu 9, 28040 Madrid, Spain○Instituto Madrileno de Estudios Avanzados en Nanociencia, Ciudad Universitaria de Cantoblanco, Faraday, 9, 28049 Madrid, Spain
*S Supporting Information
ABSTRACT: FtsZ is a bacterial cytoskeletal protein involved in celldivision. It forms a ringlike structure that attaches to the membrane tocomplete bacterial division. It binds and hydrolyzes GTP, assembling intopolymers in a GTP-dependent manner. To test how the orientation of themonomers affects the curvature of the filaments on a surface, we performedsite-directed mutagenesis on the E. coli FtsZ protein to insert cysteineresidues at lateral locations to orient FtsZ on planar lipid bilayers. The E93Cand S255C mutants were overproduced, purified, and found to befunctionally active in solution, as well as being capable of sustaining celldivision in vivo in complementation assays. Atomic force microscopy wasused to observe the shape of the filament fibers formed on the surface. TheFtsZ mutants were covalently linked to the lipids and could be polymerizedon the bilayer surface in the presence of GTP. Unexpectedly, both mutants assembled into straight structures. E93C formed awell-defined lattice with monomers interacting at 60° and 120° angles, whereas S255C formed a more open array of straightthicker filament aggregates. These results indicate that filament curvature and bending are not fixed and that they can bemodulated by the orientation of the monomers with respect to the membrane surface. As filament curvature has been associatedwith the force generation mechanism, these results point to a possible role of filament membrane attachment in lateralassociation and curvature, elements currently identified as relevant for force generation.
■ INTRODUCTIONFtsZ is a bacterial cytoskeletal protein that plays an essentialrole in cell division. It is a soluble protein with GTPase activitythat attaches to the inner cytoplasmic membrane through itsinteraction with other proteins, such as FtsA or ZipA inEscherichia coli.1 FtsZ polymerizes into a ringlike structure andplays an important role in acting as a scaffold to recruit otherproteins forming the division ring2 and exerting a force to drivecell constriction.3,4 In a living cell, FtsZ attachment to themembrane and its interaction with other proteins is likely torestrict and modulate polymer formation and, consequently, theforce generated by the dynamic assembly of the protein. Invitro, the isolated protein forms linear polymers in the presenceof guanosine 5′-triphosphate (GTP) that can condense into alarge variety of higher-order structures depending on themedium conditions.5−7 Recent studies on reconstitutedmembrane systems have demonstrated that the structure ofthe polymers and the deformations induced in the membraneare indeed affected by the way they are anchored: The structureof FtsZ polymers anchored through ZipA to a planar lipid
membrane is sensitive to the type of underlying lipids,8 and theorientation of the protein on the membrane determines themembrane deformation observed. It has been described thatartificially membrane-bound FtsZ generated either convexbulges or concave depressions in the membrane of giantunilamellar vesicles depending on the position of themembrane anchor on FtsZ.4,9
To understand in more detail the effect of the proteinorientation on the curvature and shape of the filaments on alipid surface, we have developed a strategy based on covalentlyanchoring single cysteine mutants of E. coli (EcFtsZ) proteinsto maleimide-modified lipids included on a planar lipid bilayer.As EcFtsZ does not contain any cysteines,10 the location of theintroduced cysteine in the mutant will determine theorientation of the protein on the surface. The fluidity of theunderlying lipid membrane allows for rearrangement of the
Received: October 6, 2012Revised: July 1, 2013Published: July 9, 2013
Article
pubs.acs.org/Langmuir
© 2013 American Chemical Society 9436 dx.doi.org/10.1021/la401673z | Langmuir 2013, 29, 9436−9446

proteins upon GTP addition to form higher-order FtsZstructures. Each cysteine mutant is able to permit cell divisionin vivo in a complementation assay, showing they still retain thefunctional characteristics and interactions of EcFtsZ requiredfor cell division.Restricted FtsZ orientation on the lipid surface has strong
effects on the shape of the filaments formed. We conclude thatthe monomer orientation on the surface contributes signifi-cantly to the morphology of the functional polymer by exposingdifferent flexible domains of the protein and by makingavailable additional monomer interfaces that can contribute tothe shaping of the final structures.
■ MATERIALS AND METHODSStructural Analysis to Identify Surface Residues of EcFtsZ
Protein for Mutation to Cysteines. A conservative approach wasused to identify potential sites for cysteine mutagenesis to minimizeproblems with the expression, folding, and function of EcFtsZ both invivo and when purified. The CLUSTAL W1.6 program11 was used toalign 43 FtsZ protein sequences, and then three criteria were appliedto select sites for cysteine mutagenesis. First, 37 possible sites inEcFtsZ were identified because at least one of the other FtsZsequences in the alignment had a naturally occurring cysteine at theequivalent position. Second, a site was approved if it was found on thelateral surface of the structure of FtsZ but not near the two facesinvolved in polymerization. This was assessed by using the programRasMol to examine the equivalent sites in the M. jannaschii FtsZ(MjFtsZ) crystal structure12 and the structurally homologous tubulinα/β dimer crystal structure,13 as well as in a homology model ofEcFtsZ (provided by Biomol-Informatics). Bacterial FtsZ sequencesare 40−50% identical in sequence.14 Finally, a site was rejected if it waslocated in or near a region in FtsZ where a naturally occurring orpreviously introduced mutation had affected the functional propertiesof published FtsZ mutants. As a result of this selection process, onlytwo residues, Glu93 and Ser255 (Figure 1), were chosen. Finalconfirmation of the viability of these sites was made using the WHATIF program15 to mutate the selected residues to cysteines in theEcFtsZ homology model, confirming that the introduced cysteines hadexposed and free side chains available for chemical modification.Site-Directed Mutagenesis of the EcFtsZ Gene. Site-directed
mutagenesis was performed using a modified version of the inversepolymerase chain reaction (PCR) protocol of Weiner et al.16 ThepET28a derivative plasmid pMFV56,17 which has the EcFtsZ geneunder the control of a T7 promoter, was used as the PCR template.Primers were designed to introduce the cysteine mutationaccompanied by silent mutations that encode the marker restrictionsite for NarI. The primers used to generate E93C-EcFtsZ wereprimE93C (GCCGCACAGCGCCGCACGCAATGCATC) andprimNarIa (GCCGACATGGTCTTTATTGCTGC). The primersused to generate S255C-EcFtsZ were primS255C (GCCACA-CAGGTCGATATCTTCCAGCAGAG) and p r imNar Ib(GCCCGCGGCGTGCTGGTTAACATCACGGC). Primers werephosphorylated using E. coli E.D. pFDX polynucleotide kinase(Roche Molecular Biochemicals). PCR products for each mutantwere amplified using the Excite High Fidelity PCR system (Roche)and the following program: 5 min at 95 °C, followed by 15 cycles of 15s at 94 °C, 30 s at 55 °C, and 4 min at 68 °C, and terminated with 20min at 68 °C. PCR fragments were purified with the Concert RapidPCR Purification System (Life Technologies, Inc.) and polished withPwo DNA polymerase (Roche) to remove any additional nucleotidesadded to the 3′ end of the PCR product. Each polished PCR productwas purified and ligated by T4 DNA ligase (Roche). The ligated PCRproducts were transformed into E. coli DH5α cells and grown on Luriabroth (LB) plates containing 50 μg/mL kanamycin. Plasmid DNA waspurified from individual transformants, and the mutant plasmidspAKV3 (pET28a-E93C-EcFtsZ) and pAKV4 (pET28a-S255C-EcFtsZ)were identified by digestion with NarI. The presence of the mutationin the EcFtsZ gene was then confirmed by sequencing with the
primers ak1 (GTTTGTCGTTCGGGATAGTG), ak2 (CTGGAAGA-TATCGACCTCTCTGGC), and mf3 (GCACCAGTCGTCGCT-GAAGTGGCA).
Overexpression and Purification of E93C-EcFtsZ and S255C-EcFtsZ. Overexpression of the two mutants was initially tested bytransforming pAVK3 and pAVK4 into the E. coli strains BL21(DE3)and C41(DE3).18 Induction of protein expression with 1 mMisopropyl-1-thio-β-D-galactopyranoside resulted in only very low levelsof each mutant being produced. To achieve high levels of production,the E93C-EcFtsZ and S255C-EcFtsZ mutant genes were cloned into thelambda-promoter expression vector pND706.19 Both genes were PCRamplified using the primers EcFtsZnde (GGAGAGAACATATGTTT-GAACCAATGGAAC) and EcFtsZXhoI (TCCAGTCTCGAGT-TAATCAGCTTGCTTACGC) to incorporate an NdeI site beforethe start codon and an XhoI site after the stop codon. Digestion of theresulting PCR products with NdeI and XhoI enabled cloning intopND706 to create pAVK5 (pND706-E93C-EcFtsZ) and pAVK6(pND706-S255C-EcFtsZ). These constructions were checked byDNA sequencing as before.
E. coli C43(DE3) cells18 transformed with either pAVK5 or pAVK6were grown at 30 °C in LB broth supplemented with 50 μg/mLampicillin to an absorbance (at 595 wavelength) value of 0.5. Synthesis
Figure 1. Structural model of EcFtsZ in solution and oriented on aplanar lipid bilayer. (a) Mutated residues (spheres) were C93 andC255, located at the end of the H3 helix and at a long loop connectingH9 and S8, respectively. The N-terminal domain is shown in green/blue, the core helix H7 in yellow, and the C-termial domain in red/orange. The N- and C-terminal ends are the T8 and R316 residues,respectively. The polymerization direction is indicated by the grayarrow. (Homology model for E. coli FtsZ protein provided by Biomol-Informatics.) The introduced cysteines act as linkers to the lipid headsof the lipid bilayer substrate. (b,c) Possible orientations of the twomutant proteins with respect to the membrane: (b) for E93C and (c)for S255C.
Langmuir Article
dx.doi.org/10.1021/la401673z | Langmuir 2013, 29, 9436−94469437

was induced by the rapid shift of each culture to 42 °C by immersingthe culture in a 70 °C water bath for approximately 2 min. Synthesiswas then maintained for 3 h in a shaking water bath at 42 °C. Theproduced proteins were purified as described in ref 17, except with theaddition of 1 mM dithiothreitol (DTT) in buffers to keep theircysteine residues in a reduced state. Protein purities were checked bysodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and were found to be 95% for the two purified proteins (seeFigure S1 of the Supporting Information). Protein concentrationswere measured using the bicinchoninic acid (BCA) assay (Pierce). TheGTPase turnover rate for each mutant was determined by measuringreleased inorganic phosphate using the malachite green−molybdatereagent.20,21
Analytical Ultracentrifugation. Experiments were carried out ina Beckman Optima XL-I ultracentrifuge (Beckman-Coulter) equippedwith interference optics that allow monitoring of FtsZ sedimentationin the presence of different nucleotides. Each FtsZ mutant (0.5 g/L)was equilibrated in working buffer [20 mM tris(hydroxymethyl)-aminomethane hydrochloride (Tris-HCl) (pH 7.5), 500 mM KCl, 5mM Mg2Cl, and 1 mM tris(2-carboxyethyl)phosphine (TCEP)supplemented with 1 mM guanosine diphosphate (GDP)]. FtsZsamples were centrifuged at 30000 rpm and 25 °C using an An50Tieight-hole rotor and double-sector Epon-charcoal centerpieces.Differential sedimentation coefficient distributions, c(s), were calcu-lated by least-squares boundary modeling of the experimental datausing SEDFIT 12.52.22
Transmission Electron Microscopy. The mutant FtsZ polymers(protein concentration of 0.5 g/L) were prepared in the presence of 1mM GTP and 1 mM DTT and visualized by transmission electronmicroscopy (TEM) after negative staining with 2% uranyl acetate,using a JEOL-1200 electron microscope.Complementation Assays. The E93C-EcFtsZ, S255C-EcFtsZ, and
wild-type EcFtsZ genes were cloned under an isopropyl-β-D-thiogalactopyranoside- (IPTG-) inducible promoter in pJF119EH23
to study the effect of their expression in vivo. The mutated genes wereobtained by PCR amplification from the plasmids pAVK3 and pAVK4,and the EcFtsZ+ gene was obtained from pZAQ.24 We used theupstream primer AR58 (5′-CGGGATCCCATATGTTTGAAC-CAATGGAAC-3′), which introduces the restriction sites BamHI/NdeI (underlined) containing the start codon (bold), and thedownstream primer AR54 (5′-CCCAAGCTTAATCAGCTTGCT-TACG-3′), which introduce a HindIII restriction site (underlined)immediately downstream of the stop codon (bold) of the f tsZ genes.The resulting plasmids were named pSCV2 (for E93C-EcFtsZ), pSCV3(for S255C-EcFtsZ), and pARV66 (for EcFtsZ+). These constructionswere checked by DNA sequencing as before.For complementation assays, the E. coli thermosensitive conditional
FtsZ strain VIP2 [MC1061 f tsZ:kan/pLAR10 rep(Ts) f tsZ+]25
transformed with pSCV2, pSCV3, or pSCV4 was replica-plated withBertani plates and grown overnight at the permissive (30 °C) orrestrictive (42 °C) temperature in LB agar plates supplemented withthe required antibiotics (50 μg mL−1 kanamycin, 20 μg mL−1
chloramphenicol, and 50 μg mL−1 ampicillin) and with 0, 50, 100,or 200 μM IPTG.Preparation of E93C-EcFtsZ and S255C-EcFtsZ Anchored to
Planar Lipid Bilayers. Two separate 0.1 M stock solutions of thelipids dioleoyl phosphatidylcholine (DOPC, 786 Da, Avanti PolarLipids) and distearoyl N-(3-maleimido-1-oxopropyl)-L-α-phosphatidy-lethanolamine (DSPE-MAL, 921 Da, NOF Corporation) wereprepared in CHCl3/CH3OH 1/1 (v/v) solvent. A mixture of 90%DOPC/10% DSPE-MAL (mol/mol) was evaporated under nitrogenand resuspended in buffer L [50 mM Tris-HCl (pH 7.4), 200 mMNaCl, 5 mM CaCl2] at a final lipid concentration of 2.5 mM. Toobtain large unilamellar vesicles (LUVs), the suspension was extruded31 times through a 200-nm-pore membrane. To fuse the lipid bilayeron the substrate, a dilute 0.1 mM solution of the LUVs was placed incontact with freshly cleaved mica for 45 min at 30 °C. Then, thesamples were rinsed with buffer Z [50 mM Tris-HCl (pH 7.4), 500mM KCl, 5 mM MgCl2] to remove excess LUVs.
To anchor each FtsZ mutant to the lipid bilayer surface, a 2 μMsolution of the protein in buffer Z was incubated on the formed bilayerfor several hours to ensure complete coverage of the active surface,under conditions similar to those used to attach cysteine-containingproteins to maleimide-containing surfaces.26 The amount of protein ina 2 μM solution is in large excess with respect to the amount of lipidlinker head on the surface. Buffer Z is an appropriate environment forFtsZ, as the high ionic strength prevents self-association andmagnesium and potassium are important ions for nucleotide-dependent polymerization and GTP hydrolysis.17,27−29 In addition,100 μM TCEP was added to reduce eventual disulfide bonds formedbetween proteins. After the incubation period, the sample was rinsedwith buffer Z to remove excess protein. Protein attachment to thelipids was confirmed with a density-gradient floating assay30
Atomic Force Microscopy. Atomic force microscopy (AFM)imaging was performed on the bilayer-anchored FtsZ mutants in bufferZ in the absence and presence of 5 mM GTP to study the polymerizedstate. AFM images were recorded with a microscope from NanotecElectronica (Madrid, Spain) operated in jump mode31 in a liquidenvironment. The scanning piezo was calibrated using siliconcalibrating gratings (NT-MDT, Moscow, Russia). Silicon nitride tips(Veeco) with a force constant of 0.05 N/m and a 20-nm tip radiuswere used.
■ RESULTS AND DISCUSSION
The strategy used to orient proteins on a lipid membrane isbased on the introduction of a cysteine group within theprotein that can be covalently anchored to a maleimide-containing lipid included in the planar lipid bilayer. EcFtsZ hasno cysteines,10 so introducing such an amino acid places areactive site at a known location on the protein surface. The useof maleimide−poly(ethylene glycol)-derivatized phospholipidsto bind proteins through sulfur-containing cysteines toliposomes has been previously described.32−34 We adaptedthis strategy to attach the monomer form of the self-assemblingbacterial cytoskeletal protein directly to a planar lipid bilayer.The maleimide anchoring group on the lipid polar head, withno poly(ethylene glycol) (PEG) spacer, imposes strongorientational restrictions on each individual monomer.Two different cysteine mutants were prepared: E93C-
EcFtsZ, in which the cysteine substitutes a glutamic acidresidue on the N-terminal GTP binding domain, located on thelateral region of the monomer, near the H3 α-helix, and S255C-EcFtsZ, in which the substituted amino acid is a serine in the C-terminal domain, located in the loop between α-helix 9 and β-S8 (see Figure 1). Both mutations are located outside themonomer−monomer interface region involved in the protofila-ment formation12,35 and at opposite sides with respect to thepolymerization direction. Therefore, both polymerization andGTP hydrolysis are possible, even with the protein orientedwith residue C93 or C255 facing the surface.Given the external position of the cysteine in both mutants,
no change in the secondary structure of the protein isexpected.36,37 Panels b and c in Figure 1 present cartoons ofthe possible orientations of the proteins with respect to themembrane. The precise orientations, however, determined bythe details of the stereochemistry and flexibility of the proteinsand steric effects due to surface proximity, are unknown.
Similarity of the Functional Properties of E93C-EcFtsZand S255C-EcFtsZ with Those of E. coli FtsZ. Biochemicalcharacterization of the mutant proteins was performed to assesstheir GTPase activity and their aggregation state. S255C-EcFtsZ was found to retain 98% of the GTPase activityobserved for the native protein; in contrast, E93C-EcFtsZdisplayed only 20% of this activity (Figure S2, Supporting
Langmuir Article
dx.doi.org/10.1021/la401673z | Langmuir 2013, 29, 9436−94469438

Information). The assays were carried out in a buffer containing1 mM GTP, so it is possible that the GTP affinity is perturbedin E93C-EcFtsZ even though the negative charge of theremoved glutamic acid is distant from the GTP binding pocket.Analytical ultracentrifugation experiments were carried out toidentify the level of aggregation of the monomers in theabsence of GTP. E93C-EcFtsZ showed a greater tendency todimerize, as 85% of this mutant was present as a dimer (Figure2a) under conditions in which S255C-EcFtsZ was more than70% present as a monomer (Figure 2b). Native FtsZ, undersimilar conditions, is also present mostly as a monomer.17
Decreased substrate accessibility due to this partial dimerizationcould also be the cause of the observed reduction in GTPaseactivity. Transmission electron microscopy images of thepolymers produced in the presence of 1 mM GTP show thatboth mutants produce the expected filaments in solution(Figure 2c,d).Complementation assays were carried out in the E. coli strain
VIP2 to explore the impact of the amino acid substitutions onthe biological function in vivo. VIP2 is a thermosensitive
conditional FtsZ strain with the chromosomal f tsZ geneinterrupted by a kanamycin cassette and an additional f tsZcopy in a plasmid with a thermosensitive replication origin.25
By varying the amount of IPTG added, the level of each mutantcould be controlled. In the presence of 50 μM IPTG, FtsZ wild-type and the two mutants could each rescue the absence ofFtsZ in VIP2 at 42 °C (Figure 3). However, only FtsZ wild-type and S255C-EcFtsZ were able to rescue VIP2 at 100 μMIPTG. This indicates that S255C-EcFtsZ is completelyfunctional, whereas the E93C-EcFtsZ protein, although activeat moderate levels, is toxic at higher levels.
Covalent Attachment of E93C-EcFtsZ and S255C-EcFtsZ to Lipid Bilayers in the Absence of GTP. Weused atomic force microscopy (AFM) to investigate whetherthe FtsZ mutants could anchor to a lipid surface in the absenceof GTP.A layer of protein was observed on the lipid surface after
incubation of the maleimide-containing bilayer with the mutantproteins. Given that there was only one attachment site permonomer and that excess protein was removed from the
Figure 2. Analytical ultracentrifugation and electron microscopy analysis of E93C-EcFtsZ and S255C-EcFtsZ in solution. Derived distributions ofsedimentation coefficients obtained from experiments conducted in the presence of 1 mM GDP for the (a) E93C and (b) S255C mutants. The 2−2.4S peak corresponds to the monomeric form, and the 5S peak corresponds to the dimer. Larger aggregates appear at even higher S values.Transmission electron microscopy analysis of the (c) E93C and (d) S255C mutants and (e) wild-type FtsZ polymers formed in the presence of 1mM GTP (bars, 100 nm).
Langmuir Article
dx.doi.org/10.1021/la401673z | Langmuir 2013, 29, 9436−94469439

solution before imaging, the presence of varying amounts ofreversible dimers or trimers formed in the presence of GDP didnot seem to affect the formation of a full dense layer of protein,one monomer thick, permitted by the 10% molar ratio ofDSPE-MAL lipid linker present. Figure 4a shows images of thelipid surfaces after incubation of the E93C mutant protein,revealing a densely packed protein surface with occasionaldefects having a depth of 2−3 nm (Figure 4b).Incubation of S255C-EcFtsZ on the maleimide-containing
lipid surface also gave full surface coverage, as expected fromthe density of linker lipid used (Figure 5a,b). The distribution
of the monomers, however, differed significantly from thatobserved for the E93C mutant. The protein layer was looselypacked, and the proteins were concentrated around emptyregions. The average height of the protein layer field was 1.7nm, probably reflecting that the attachment of the protein tothe surface was different than for the E93C mutant and, mostlikely, less rigid.We did not observe lipid phase separation on the bilayer,
either before or after protein addition, probably due to thesmall amount of DSPE present in the bilayer. Ongoingexperiments at variable sample temperatures using membranes
Figure 3. Suppression of cell division defects by expression of Ecf tsZ mutants under Ptac promoter control. At least five isolated colonies weresuspended in LB and replica-plated onto LB plates, supplemented with the corresponding antibiotics for vector selection, in addition to increasingconcentrations of IPTG (0, 50, 100, or 200 μM) for pSCV2 (pJF119/E93C-Ecf tsZ) and pSCV3 (pJF119/S255C-Ecf tsZ). pARV66 (pJF119/Ecf tsZ+)and the empty plasmid (pJF119/) were used as positive and negative controls, respectively. Growth was assessed after overnight incubation ofduplicate plates at 30 or 42 °C.
Figure 4. Atomic force microscopy analysis of E93C-EcFtsZ on DOPC/DSPE-Mal (9:1) lipid bilayer in the absence of GTP: (a) 2 × 2 μmmicrograph of the protein surface, (b) 500 × 500 nm image, with the profile marked as I given in panel bI. Both images show a compact protein layerwith occasional defects with a depth of 2−3 nm.
Langmuir Article
dx.doi.org/10.1021/la401673z | Langmuir 2013, 29, 9436−94469440

with different lipid compositions will address this point andexplore the role of lipids in the protein organization on thesurface.In the absence of GTP, the distribution of the proteins on
the surface remained stable for days for both mutants, reflectingthe stability of the maleimide−cysteine covalent bond.GTP-Induced Formation of Distinctive Filamentous
Structures by E93C-EcFtsZ and S255C-EcFtsZ. Thepresence of GTP in the imaging buffer induced thereorganization of both proteins on the lipid bilayer surface.As all of the excess protein was removed from solution prior toAFM imaging, the structures observed must have been formedby reorganization of previously covalently attached proteins.Both mutants were able to form longitudinal bonds to assembleinto filaments (Figures 6 and 7). The underlying lipid matrix,composed of 90% DOPC, had a transition temperature −22°C38 and was therefore fluid at room temperature, allowing theproteins to redistribute and align their monomer−monomerinteractions due to the presence of GTP. The times needed forthe proteins to reorient on the surface, however, were quitedifferent for the two mutants. S255C-EcFtsZ formedlongitudinal polymers immediately after GTP addition to thesolution (Figure 6), whereas E93C-EcFtsZ required severalminutes before forming visible filaments (Figure 7). The slowresponse of the E93C mutant could reflect the low GTPasehydrolysis rate described earlier, but steric restrictions tomonomer reorientation are also likely to be present in such adensely packed protein monolayer.The structures of the polymers formed were also found to be
strongly dependent on the orientation of the monomers on thesurface. Addition of GTP to the S255C mutant resulted in theformation of straight filament aggregates. In this case, shortfilaments (few hundred nanometers long) with differentorientations formed (Figure 6a). When the monomers aligned
to form filaments, they rose higher above the membrane surface(Figure 6b). Both the rapid polymerization after GTP additionand the increase in height upon polymerization could reflectthat the attachment point was far from the polymerization axis(see Figure 1) and located in a loop on the C-terminalsubdomain. This location would give a loose bonding to thesurface with enough degrees of freedom to allow the monomersto stand higher above the membrane surface once they formedthe longitudinal bonds required for polymerization (Figure 6b).This interpretation is compatible with previous resultsindicating that the C-terminal subdomain region is moreflexible than the N-terminal subdomain region39 and thatpolymerization induces a conformational change withinin themonomer, affecting the orientation between these subdo-mains.40
In the case of E93C-EcFtsZ, polymerization occurred severalminutes after GTP addition, and the filaments extended to forma network structured at 60° and 120° angles (Figure 7a). Thesurface attachment at residue 93 was located near the H3 loopin the more rigid N-terminal domain,39 placing the polymer-ization axis closer to the surface (Figure 1b). The filamentheight determined at regions with lower filament density was 2nm on average, which was lower than the 4 nm measured forthe structures formed from the S255C mutant. This heightdifference could reflect the different orientations of thefilaments and the fact that their interaction with the membranewas through the more rigid N-terminal domain of the protein,which had fewer degrees of freedom to accommodatereorientations with respect to the membrane plane.Previous observations of filaments on mica and lipid surfaces
showed that polymers adopt curved shapes.8,41 However, thefilaments observed here, in which the monomers werecovalently linked to the lipids with defined orientations,showed no indication of curvature. Because lipid membranes
Figure 5. Atomic force microscopy analysis of S255C-FtsZ on DOPC/DSPE-Mal (9:1) lipid bilayer in the absence of GTP: (a) 12 × 12 μm AFMimage of the protein surface, (b) 4 × 4 μm micrograph, with the profile marked as I given in panel bI. A dense layer of protein concentrated aroundempty depressions is shown.
Langmuir Article
dx.doi.org/10.1021/la401673z | Langmuir 2013, 29, 9436−94469441

on hydrophilic solid supports remain fluid, we interpret that theshapes observed were mainly due to the polymerizationproperties of the proteins. In the case of S255C-EcFtsZ,filament aggregates formed in different orientations followingthe previous ordering of the monomers on the surface.Aggregates were around 10 filaments thick on average andone monomer thick, as measured from the AFM images.Although other experimental conditions facilitate the formationof round filament bundles,7 filament aggregates are verysensitive to the presence of crowding agents, ions, and pH,and it is not surprising that sample preparation protocols usedto negatively stain and dry the samples for observation bytransmission electron microscopy might give different aggregateshapes from the ones described here.The filament aggregates observed here did not curve
following the initial monomer distribution. They assembledas short, straight aggregates oriented at different angles andgrew mainly by modifications at their ends, probably indicatingthat monomers closer to the ends of the filaments exchangeed
at a higher rate than monomers within the central region of thefilaments42,43 (Figure 6d). This allowed for reorganization ofthe filaments on the surface to give longer and more alignedstructures. Although the filaments formed from the E93Cmutant were also straight, the mesh mainly consisted of singlefilaments or aggregates only a few filaments thick. Because themeasured width of 15 nm was close to the nominal width of theAFM tip of 10−20 nm, we cannot provide reliable informationof structures smaller than this size.Individual filaments formed at lower protein concentrations
were also straight, indicating that it was probably theorientation of the monomers, not the aggregation of thefilaments, that forced the straight conformation (see Figure S3,Supporting Information).Another striking feature is the well-defined angle of
interaction between filaments formed from E93C-EcFtsZ,which is possible only through the presence of a contact sitebetween monomers that is different from the head-to-tailinteraction that governs the formation of longitudinal filaments
Figure 6. Atomic force microscopy analysis of S255C-EcFtsZ on DOPC/DSPE-Mal (9:1) lipid bilayer in the presence of 5 mM GTP: (a) 10 × 10μm AFM image of the protein surface on the bilayer immediately after addition of GTP to the sample. Short and straight filaments begin to lift overthe surrounding monomeric layer. (b) This image shows the two height levels as measured in profile I (panel bI). (c) 9 × 9 μm AFM image obtained1 h after addition of GTP. (d) White arrows point to filament junctions. Filaments are thinner at their ends, where the monomers incorporate.Profile I (panel dI) gives a value of 60 nm for the width at half-maximum of two filaments, which is near the average width (70 nm). Their height is4.5 nm (diameter of one protein).
Langmuir Article
dx.doi.org/10.1021/la401673z | Langmuir 2013, 29, 9436−94469442

(Figure 7a). The crystal structure of FtsZ from Methanococcusjannaschii (MjFtsZ)12 was previously described as indicating thepresence of a protein trimer, although there is no directevidence of its biological relevance. However, there areindications that the formation of trimers is feasible. When theasymmetric unit of MjFtsZ (PDB entry 1FSZ) was entered intoeither the Protein Quaternary Server (PQS)44 or, alternatively,the ProtBUD program,45 the trimer shown in Figure 8a wasproduced. Both methods apply the crystallographic symmetryoperations from the contents of the deposited coordinates toderive potential oligomeric assemblies. PQS analysis, based onassigning an empirical weighted score to different energeticcontributions (size and number of buried residues in thesolvent-accessible area, difference in solvation energy of folding,and number of interchain and disulfide bridges formed)suggests that the formation of trimers is energetically feasible.The presence of a preferential lateral interaction can explain
the formation of some of the structures observed under similarexperimental conditions.46 The restricted orientation of themonomers would expose protein surfaces involved in theformation of the trimer found in the crystal packingarrangement (Figure 8a), particularly if the protein wereattached through residue 93. Experiments carried out with
analytical ultracentrifugation also indicated an increasedtendency of the E93C mutant monomers to interact witheach other in solution. The oriented surface attachment of themonomers would then only amplify a tendency already presentin solution. Figure 8b,c illustrate how monomers preoorganizedas trimers could seed the formation of filaments growingprecisely at the 60°/120° observed experimentally.The fact that the E93C-EcFtsZ filaments were thinner than
those formed by S255C-EcFtsZ could reflect the fact that H3helix region, known to be relevant for lateral interactions,47 wasfacing the membrane surface, preventing it from formingthicker filament aggregates. The S255C mutation, located in themore flexible C-terminal subdomain,39 could make the H3region more available to promote the formation of filamentaggregates.
■ CONCLUSIONS
The large polymorphism of FtsZ polymers extensivelydocumented for polymers formed in solution also holds forpolymers oriented on a surface. Previously describedmodulation of polymer curvature on surfaces was attributedto the presence of the flexible protein ZipA as a membraneanchor.8 The results presented here show that the protein
Figure 7. Atomic force microscopy analysis of E93C-EcFtsZ on DOPC/DSPE-Mal (9:1) lipid bilayer in the presence of 5 mM GTP: (a) 3.5 × 3.5μm AFM micrograph of the protein surface after GTP addition. A network of filaments crossing at 60°/120° angles is formed. The profiles of thepolymers growing in the two preferential directions are marked as I and II. The measured widths (panel aI) are close to 15 nm, measured eitherpeak-to-peak or at half-maximum. Isolated filaments in the other direction have widths of around 30 nm (panel aII). (b) Profile of the contact pointbetween filaments.
Langmuir Article
dx.doi.org/10.1021/la401673z | Langmuir 2013, 29, 9436−94469443

monomer itself has an inbuilt capacity to modulate its curvatureand aggregate shape depending on its orientation with respectto a nearby surface. The localization of the anchoring elementon the protein surface can affect the extent of lateralaggregation and the degree of curvature, two traits that havebeen repeatedly pointed out as relevant in determining theforce generation mechanism.48−56 Some models have assumedthat filament curvature is fixed and well-defined and that itguides the sense of membrane deformation to be either concaveor convex depending on whether the polymers are anchoredthrough the N- or C-terminal end of the protein.9 Our results
indicate, however, that the polymer curvature is sensitive to theway in which the protein is attached to and oriented on thesurface. These results are more consistent with a picture of theFtsZ monomer containing regions of different flexibility whoserelative motions could modulate filament curvature.39
It is difficult to extrapolate the significance of the behavior ofFtsZ filaments in vitro to their role in vivo. However, it hasgenerally been accepted that the polymerization behavior andassembly capacity described in reconstituted systems containinga few of the elements present in the cell reveal important traitsthat support the behavior in the more complex living cell.9,57
Although the results described in this work belong to thiscategory of artificially reconstituted systems, it is likely that themain observation indicating the importance of surface attach-ment in determining filament curvature and aggregation couldpoint to the biological relevance of modulating these twoelements to control the function of FtsZ on the cell membrane.Interestingly, most of the FtsZ-binding proteins that are
known to play an important role in its membrane attachment,mainly FtsA and ZipA, bind to the conserved segment of the C-terminal domain that follows a flexible variable spacerregion.58,59 It could be that this flexible attachment opens thepossibility of using the orientation of surface attachment tofurther tune filament curvature and aggregation state. Theexistence of other nonessential proteins that bind FtsZpolymers such as ZapA, ZapB, ZapC, and ZapD has beendescribed recently.60−62 They are thought to play a role instabilizing the formation of the functional polymeric structure.The cross-linking and guided stabilization of the filamentscould contribute to the promotion of the right orientation onthe surface to facilitate their function. These proteins, as well asthe localized membrane lipid charges,63 could influence thelateral aggregation, curvature, and stiffness of the polymers, allissues with a strong impact on its force generating function.Surface attachment also facilitates filament branching at well-
defined orientations, indicating that monomer regions differentfrom those involved in head-to-tail interactions favored by thepresence of GTP are strong enough to affect the shape of thefilament aggregates. This effect is stronger on filaments formedby the E93C mutant. Biochemical characterization indicatedthat this mutant has decreased GTPase activity and anincreased monomer−monomer interaction that stabilizesdimers and trimers formed in the presence of GDP. In vivoexperiments also indicated that its overexpression was toxic tothe cell. This higher tendency to branch would then reflect notonly the orientation on the surface but also some additional andnot well-defined effect of the cysteine mutant. The picture thatemerges is that monomer flexibility and oriented membraneanchoring could be subtle ways to further modulate the shapeand possibly the force exerting mechanism of the FtsZ filamentson the surface.
■ ASSOCIATED CONTENT
*S Supporting InformationAdditional information as noted in text. This material isavailable free of charge via the Internet at http://pubs.acs.org.
■ AUTHOR INFORMATION
Corresponding Author*E-mail: [email protected].
Figure 8. Models of possible filament formations formed by E93C-EcFtsZ and S255C-EcFtsZ on the suface of a planar lipid bilayer. (a)Trimeric oligomeric state derived from the MjFtsZ monomer (PDBentry 1FSZ) using the Protein Quaternary Server. The residues labeledas 93 and 255 represent the cysteines of the EcFtsZ mutants. The H0and H3 helices and GDP nucleotides (in spherical representation) arealso highlighted. (b,c) Three-way junctions of filaments (crossing at a60°/120° angle) growing from (b) all monomers of the trimeric entityor (c) two of them. In the latter case, the magenta monomer is insteric conflict with the blue monomer and does not have free surfacesto polymerize. Three MjFtsZ dimers (PDB entry 1W5A) have beenused to show the polymer formation. The polymerization directionsare indicated by the appropriately colored arrows.
Langmuir Article
dx.doi.org/10.1021/la401673z | Langmuir 2013, 29, 9436−94469444

Present Address‡Instituto de Microelectronica de Madrid, CSIC, Isaac Newton8 (PTM), Tres Cantos 28760 Madrid, Spain.
Author Contributions§Both M.E. and A.V.K. contributed equally to this work.
NotesThe authors declare no competing financial interest.
■ ACKNOWLEDGMENTS
The authors acknowledge Miguel Vicente, Jesus Mingorance,and German Rivas for useful discussions and the followingsources of funding: COMBACT S-BIO-0260/2006 (Comuni-dad de Madrid to M.V.), NOBIMAT-M S2OO9/MAT·1507(Comunidad de Madrid to M.V.), DIVINOCELL FP7HEALTH-F3-2009-223431 (European Commission to M.V.),Plan Nacional BIO2008-04478-C03-00 (Ministerio de Cienciae Innovacion, Madrid, Spain, to M.V.), and CONSOLIDERINGENIO 2010 CSD2007-00010 (Ministerio de Ciencia eInnovacion to M.V.). A.V.K. was supported by a fellowshipfrom the Spanish Government (Ref SB97-BL 0332192), M.K.was a Ph.D. fellow of the La Caixa Foundation InternationalFellowship Programme (La Caixa/CNB), and S.C. acknowl-edges Consejo Superior de Investigaciones Cientificas (CSIC),Madrid, Spain, for JAE-intro grants.
■ REFERENCES(1) Rueda, S.; Vicente, M.; Mingorance, J. Concentration andassembly of the division ring proteins FtsZ, FtsA, and ZipA during theEscherichia coli cell cycle. J. Bacteriol. 2003, 185 (11), 3344−3351.(2) Vicente, M.; Rico, A. I.; Martínez-Arteaga, R.; Mingorance, J.Septum enlightenment: Assembly of bacterial division proteins. J.Bacteriol. 2006, 188 (No.1), 19−27.(3) Mingorance, J.; Rivas, G.; Velez, M.; Gomez-Puertas, P.; Vicente,M. Strong FtsZ is with the force: Mechanisms to constrict bacteria.Trends Microbiol. 2010, 18 (8), 348−356.(4) Osawa, M.; Anderson, D. E.; Erickson, H. P. Reconstitution ofcontractile FtsZ rings in liposomes. Science 2008, 320 (5877), 792−794.(5) Erickson, H. P.; Taylor, D. W.; Taylor, K. A.; Bramhill, D.Bacterial cell division protein FtsZ assembles into protofilament sheetsand minirings, structural homologs of tubulin polymers. Proc. Natl.Acad. Sci. U.S.A. 1996, 93, 519−523.(6) Popp, D.; Iwasa, M.; Erickson, H. P.; Narita, A.; Maeda, Y.;Robinson, R. C. Suprastructures and dynamic properties ofMycobacterium tuberculosis FtsZ. J. Biol. Chem. 2010, 285 (15),11281−11289.(7) Popp, D.; Iwasa, M.; Narita, A.; Erickson, H. P.; Maeda, Y. FtsZcondensates: An in vitro electron microscopy study. Biopolymers 2009,91 (5), 340−350.(8) Mateos-Gil, P.; Marquez, I.; Lopez-Navajas, P.; Jimenez, M.;Vicente, M.; Mingorance, J.; Rivas, G.; Velez, M. FtsZ polymers boundto lipid bilayers through ZipA form dynamic two dimensionalnetworks. Biochim. Biophys. Acta: Biomembr. 2012, 1818 (3), 806−813.(9) Osawa, M.; Anderson, D. E.; Erickson, H. P. Curved FtsZprotofilaments generate bending forces on liposome membranes.EMBO J. 2009, 28 (22), 3476−3484.(10) Qing-Ming, Y.; Lutkenhaus, J. The nucleotide sequence of theessential cell-division gene ftsZ of Escherichia coli. Gene 1985, 36 (3),241−247.(11) Aiyar, A. The use of CLUSTAL W and CLUSTAL X formultiple sequence alignment. Methods Mol. Biol. 2000, 132, 221−41.(12) Lowe, J.; Amos, L. A. Crystal structure of the bacterial cell-division protein FtsZ. Nature 1998, 391 (6663), 203−206.(13) Nogales, E.; Wolf, S. G.; Downing, K. H. Structure of the αβtubulin dimer by electron crystallography. Nature 1998, 393, 199−203.
(14) Erickson, H. P. Evolution of the cytoskeleton. BioEssays 2007,29 (7), 668−677.(15) Vriend, G. WHAT IF: A molecular modeling and drug designprogram. J. Mol. Graph. 1990, 8, 52−56.(16) Weiner, M. P.; Costa, G. L.; Schoettlin, W.; Cline, J.; Mathur, E.;Bauer, J. C. Site-directed mutagenesis of double-stranded DNA by thepolymerase chain reaction. Gene 1994, 151, 119−123.(17) Rivas, G.; Lopez, A.; Mingorance, J.; Ferrandiz, M. J.; Zorrilla,S.; Minton, A. P.; Vicente, M.; Andreu, J. M. Magnesium-inducedlinear self-association of the FtsZ bacterial cell division proteinmonomer. The primary steps for FtsZ assembly. J. Biol. Chem. 2000,275, 11740−11749.(18) Miroux, B.; Walker, J. E. Over-production of proteins inEscherichia coli: Mutant hosts that allow synthesis of some membraneproteins and globular proteins at high levels. J. Mol. Biol. 1996, 260,289−98.(19) Love, C. A.; Lilley, P. E.; Dixon, N. E. Stable high-copy-numberbacteriophage lambda promoter vectors for overproduction of proteinsin Escherichia coli. Gene 1996, 176, 49−53.(20) Hoenig, M.; Lee, R. J.; Ferguson, D. C. A microtiter plate assayfor inorganic phosphate. J. Biochem. Biophys. Methods 1989, 19, 249−252.(21) Lanzetta, P. A.; Alvarez, L. J.; Reinach, P. S.; Candia, O. A. Animproved assay for nanomole amounts of inorganic phosphate. Anal.Biochem. 1979, 100, 95−97.(22) Lebowitz, J.; Lewis, M. S.; Schuck, P. Modern analyticalultracentrifugation in protein science: A tutorial review. Protein Sci.2002, 11 (9), 2067−2079.(23) Furste, J. P.; Pansegrau, W.; Frank, R.; Blocker, H.; Scholz, P.;Bagdasarian, M.; Lanka, E. Molecular cloning of the plasmid RP4primase region in a multi-host-range tacP expression vector. Gene1986, 48, 119−131.(24) Ward, J. E.; Lutkenhaus, J. Overproduction of FtsZ inducesminicell formation in E. coli. Cell 1985, 42, 941−949.(25) Pla, J.; Sanchez, M.; Palacios, P.; Vicente, M.; Aldea, M.Preferential cytoplasmic location of FtsZ, a protein essential forEscherichia coli septation. Mol. Microbiol. 1991, 5, 1681−1686.(26) Torrance, L.; Ziegler, A.; Pittman, H.; Paterson, M.; Toth, R.;Eggleston, I. Oriented immobilisation of engineered single-chainantibodies to develop biosensors for virus detection. J. Virol. Methods2006, 134, 164−170.(27) Mukherjee, A.; L, J. Analysis of FtsZ assembly by light scatteringand determination of the role of divalent metal cations. J. Bacteriol.1999, 181, 823−832.(28) Tadros, M.; Gonzalez, J. M.; Rivas, G.; Vicente, M.; Mingorance,J. Activation of the Escherichia coli cell division protein FtsZ by a low-affinity interaction with monovalent cations. FEBS Lett. 2006, 580(20), 4941−4946.(29) Mendieta, J.; Rico, A. I.; Lopez-Vinas, E.; Vicente, M.;Mingorance, J.; Gomez-Puertas, P. Structural and functional modelfor ionic (K+/Na+) and pH dependence of GTPase activity andpolymerization of FtsZ, the prokaryotic ortholog of tubulin. J. Mol.Biol. 2009, 390 (1), 17−25.(30) Nozawa, A.; N., H.; Miyata, T.; Linka, N.; Endo, Y.; Weber, A.P. M.; Tozawa, Y. A cell-free translation and proteoliposomereconstitution system for functional analysis of plant solute trans-porters. Plant Cell Physiol. 2007, 48 (12), 1815−1820.(31) Moreno-Herrero, F.; de Pablo, P. J.; Fernandez-Sanchez, R.;Colchero, J.; Go mez-Herrero, J.; Baro , A. M. Scanning forcemicroscopy jumping and tapping modes in liquids. Appl. Phys. Lett.2002, 81, 2620−2622.(32) Shahinian, S.; Silvius, J. R. A novel strategy affords high-yieldcoupling of antibody Fab′ fragments to liposomes. Biochim. Biophys.Acta: Biomembr. 1995, 1239 (2), 157−167.(33) Luan, N. M.; Teramura, Y.; Iwata, H. Layer-by-layer co-immobilization of soluble complement receptor 1 and heparin onislets. Biomaterials 2011, 32 (27), 6487−6492.
Langmuir Article
dx.doi.org/10.1021/la401673z | Langmuir 2013, 29, 9436−94469445

(34) Huwyler, J.; Wu, D.; Pardridge, W. M. Brain drug delivery ofsmall molecules using immunoliposomes. Proc. Natl. Acad. Sci. U.S.A.1996, 93 (24), 14164−14169.(35) Oliva, M. A.; Cordell, S. C.; Lowe, J. Structural insights intoFtsZ protofilament formation. Nat. Struct. Mol. Biol. 2004, 11 (12),1243−1250.(36) Díaz-Espinoza, R.; Garces, A. P.; Arbildua, J. J.; Montecinos, F.;Brunet, J. E.; Lagos, R.; Monasterio, O. Domain folding and flexibilityof Escherichia coli FtsZ determined by tryptophan site-directedmutagenesis. Protein Sci. 2007, 16 (8), 1543−1556.(37) Shin, J. Y.; Vollmer, W.; Lagos, R.; Monasterio, O. Glutamate 83and arginine 85 of helix H3 bend are key residues for FtsZpolymerization, GTPase activity and cellular viability of Escherichiacoli: Lateral mutations affect FtsZ polymerization and E. coli viability.BMC Microbiol. 2013, 13 (1), 26.(38) Tamm, L. K.; McConnell, H. M. Supported phospholipidbilayers. Biophys. J. 1985, 47 (1), 105−113.(39) Martín-Galiano, A. J.; Buey, R. M.; Cabezas, M.; Andreu, J. M.Mapping flexibility and the assembly switch of cell division proteinFtsZ by computational and mutational approaches. J. Biol. Chem. 2010,285 (29), 22554−22565.(40) Chen, Y.; Erickson, H. P. Conformational changes of FtsZreported by tryptophan mutants. Biochemistry 2011, 50 (21), 4675−4684.(41) Mingorance, J.; Tadros, M.; Vicente, M.; Gonzalez, J. M.; Rivas,G.; Velez, M. Visualization of single Escherichia coli FtsZ filamentdynamics with atomic force microscopy. J. Biol. Chem. 2005, 280 (21),20909−20914.(42) Mateos-Gil, P.; Paez, A.; Horger, I.; Rivas, G.; Vicente, M.;Tarazona, P.; Velez, M. Depolymerization dynamics of individualfilaments of bacterial cytoskeletal protein FtsZ. Proc. Natl. Acad. Sci.U.S.A. 2012, 109 (21), 8133−8138.(43) Martín-García, F.; Salvarelli, E.; Mendieta-Moreno, J. I.; Vicente,M.; Mingorance, J. M., J.; Gomez-Puertas, P. Molecular dynamicssimulation of GTPase activity in polymers of the cell division proteinFtsZ. FEBS Lett. 2012, 586, 1236−1239.(44) Henrick, K.; Thornton, J. M. PQS: A protein quaternarystructure file server. Trends Biochem. Sci. 1998, 23, 358−361.(45) Xu, Q.; Canutescu, A.; Obradovic, Z.; Dunbrack, R. L. ProtBuD:A database of biological unit structures of protein families andsuperfamilies. Bioinformatics 2006, 22, 2876−2882.(46) Gonzalez de Prado Salas, P.; Encinar, M.; Velez, M.; Tarazona,P. Structure of FtsZ protein filaments anchored on bilayer membranes.Soft Matter 2013, 9, 6072.(47) Lu, C.; Stricker, J.; Erickson, H. Site-specific mutations of FtsZ:Effects on GTPase and in vitro assembly. BMC Microbiol. 2001, 1 (1),7.(48) Shlomovitz, R.; Gov, N. S. Membrane-mediated interactionsdrive the condensation and coalescence of FtsZ rings. Phys. Biol. 2009,6 (4), 046017.(49) Paez, A.; Mateos-Gil, P.; Horger, I.; Mingorance, J.; Rivas, G.;Vicente, M.; Velez, M.; Tarazona, P. Simple modeling of FtsZpolymers on flat and curved surfaces: Correlation with experimental invitro observations. PMC Biophysics 2009, 2 (1), 8.(50) Erickson, H. P. Modeling the physics of FtsZ assembly and forcegeneration. Proc. Natl. Acad. Sci. U.S.A. 2009, 106 (23), 9238−9243.(51) Allard, J. F.; Cytrynbaum, E. N. Force generation by a dynamicZ-ring in Escherichia coli cell division. Proc. Natl. Acad. Sci. U.S.A. 2009,106 (1), 145−150.(52) Lan, G.; Daniels, B. R.; Dobrowsky, T. M.; Wirtz, D.; Sun, S. X.Condensation of FtsZ filaments can drive bacterial cell division. Proc.Natl. Acad. Sci. U.S.A. 2009, 106 (1), 121−126.(53) Fischer-Friedrich, E.; Gov, N. Modeling FtsZ ring formation inthe bacterial cellAnisotropic aggregation via mutual interactions ofpolymer rods. Phys. Biol. 2011, 8, 026007.(54) Fischer-Friedrich, E.; Friedrich, B. M.; Gov, N. S. FtsZ rings andhelices: Physical mechanisms for the dynamic alignment ofbiopolymers in rod-shaped bacteria. Phys. Biol. 2012, 9, 016009.
(55) Ghosh, B.; Sain, A. Origin of contractile force during celldivision of bacteria. Phys. Rev. Lett. 2008, 101 (17), 178101.(56) Ghosh, B.; Sain, A. Force generation in bacteria withoutnucleotide-dependent bending of cytoskeletal filaments. Phys. Rev. E2011, 83 (5), 051924.(57) Loose, M.; Fischer-Friedrich, E.; Ries, J.; Kruse, K.; Schwille, P.Spatial regulators for bacterial cell division self-organize into surfacewaves in vitro. Science 2008, 320 (5877), 789−792.(58) Ma, X.; Margolin, W. Genetic and functional analyses of theconserved C-terminal core domain of Escherichia coli FtsZ. J. Bacteriol.1999, 181 (24), 7531−7544.(59) Mosyak, L.; Zhang, Y.; Glasfeld, E.; Haney, S.; Stahl, M.; Seehra,J.; Somers, W. S. The bacterial cell-division protein ZipA and itsinteraction with an FtsZ fragment revealed by X-ray crystallography.EMBO J. 2000, 19 (13), 3179−3191.(60) Galli, E.; Gerdes, K. FtsZ-ZapA-ZapB interactome of Escherichiacoli. J. Bacteriol. 2012, 194 (2), 292−302.(61) Hale, C. A.; Shiomi, D.; Liu, B.; Bernhardt, T. G.; Margolin, W.;Niki, H.; de Boer, P. A. J. Identification of Escherichia coli ZapC(YcbW) as a component of the division apparatus that binds andbundles FtsZ polymers. J. Bacteriol. 2011, 193 (6), 1393−1404.(62) Durand-Heredia, J.; Rivkin, E.; Fan, G.; Morales, J.;Janakiraman, A. Identification of ZapD as a cell division factor thatpromotes the assembly of FtsZ in Escherichia coli. J. Bacteriol. 2012,194 (12), 3189−3198.(63) Mileykovskaya, E.; D, W. Role of membrane lipids in bacterialdivision-site selection. Curr. Opin. Microbiol. 2005, 8 (2), 135−42.
Langmuir Article
dx.doi.org/10.1021/la401673z | Langmuir 2013, 29, 9436−94469446