Placental insufficiency and fetal heart: Doppler...
Transcript of Placental insufficiency and fetal heart: Doppler...

PLACENTAL INSUFFICIENCY AND FETAL HEART: DOPPLER ULTRASONOGRAPHIC AND BIOCHEMICAL MARKERS OF FETAL CARDIAC DYSFUNCTION
KAARIN MÄKIKALLIO
Department of Obstetrics and Gynaecology, University of Oulu
Department of Physiology, University of Oulu
OULU 2002


KAARIN MÄKIKALLIO
PLACENTAL INSUFFICIENCY AND FETAL HEART: DOPPLER ULTRASONOGRAPHIC AND BIOCHEMICAL MARKERS OF FETAL CARDIAC DYSFUNCTION
Academic Dissertation to be presented with the assent of the Faculty of Medicine, University of Oulu, for public discussion in the Auditorium 4 of the University Hospital of Oulu, on June 28th, 2002, at 12 noon.
OULUN YLIOPISTO, OULU 2002

Copyright © 2002 University of Oulu, 2002
Reviewed by Professor Juriy Wladimiroff Professor Karel Mašrál
ISBN 951-42-6737-0 (URL: http://herkules.oulu.fi/isbn9514267370/)
ALSO AVAILABLE IN PRINTED FORMAT Acta Univ. Oul. D 681, 2002 ISBN 951-42-67362
ISSN 0355-3221 (URL: http://herkules.oulu.fi/issn03553221/)
OULU UNIVERSITY PRESS OULU 2002

Mäkikallio, Kaarin, Placental insufficiency and fetal heart: Doppler ultrasonographic and biochemical markers of fetal cardiac dysfunction Department of Obstetrics and Gynaecology, Department of Physiology, University of Oulu, P.O.Box 5000, FIN-90014 University of Oulu, Finland Oulu, Finland 2002
Abstract
The first aim of this study was to investigate the relationship between Doppler ultrasonographic parameters and biochemical markers of human fetal cardiac dysfunction and myocardial cell damage in pregnancies complicated by placental insufficiency and/or fetal growth restriction. Our second aim was to examine fetal central and peripheral hemodynamic characteristics associated with retrograde aortic isthmus net blood flow.
Fetuses with significant myocardial cell damage (cTnT > 0.10 ng/ml) had increased pulsatility in the blood velocity waveforms of ductus venosus, left hepatic vein and inferior vena cava, and had more often atrial pulsations in the umbilical vein. Their umbilical artery NT-proANP concentrations were higher than in fetuses without myocardial cell damage. The proportion of left ventricular cardiac output of the combined cardiac output was greater and the corresponding proportion of the right ventricle was less than in fetuses with only increased NT-proANP levels ( > 1145 pmol/l). Tricuspid regurgitation was present more often and the right ventricular fractional shortening was less in fetuses with myocardial cell damage than in fetuses with normal umbilical artery cTnT levels. In fetuses with placental insufficiency and/or growth restriction (n = 48), umbilical artery NT-proANP concentrations showed a significant positive correlation with ductus venosus, left hepatic vein and inferior vena cava pulsatility index values for veins. Fetuses with placental insufficiency and antegrade aortic isthmus net blood flow demonstrated a shift in their right ventricular cardiac output from the pulmonary to the systemic circulation, and foramen ovale volume blood flow made up the majority of the left ventricular cardiac output. Fetuses with retrograde aortic isthmus net blood flow failed to demonstrate these changes, and they had signs of increased left atrial pressure. In addition, right ventricular fractional shortening was decreased and the pulsatility in the ductus venosus blood velocity waveforms was increased.
In conclusion, human fetal myocardial cell damage was associated with a rise in systemic venous pressure, a change in the distribution of cardiac output towards the left ventricle and a rise in right ventricular afterload. Fetuses with retrograde aortic isthmus net blood flow failed to rearrange the distribution of the cardiac output and they had signs of increased left atrial pressure. In addition, right ventricular afterload and pulsatility in the ductus venosus blood velocity waveforms were increased.
Keywords: atrial natriuretic peptide, placenta, fetal echocardiography, aortic isthmus, cardiac troponin T, fetal growth restriction, physiology

L‘essential est invisible pour les yeux.
Antoine de Saint-Exupéry
To Tuomas, Johannes and Elias

Acknowledgements
The research for this thesis was carried out in the Departments of Obstetrics and Gynecology and Physiology at the University of Oulu, Finland during 1998–2002.
I warmly thank Professor Pentti Jouppila, MD, PhD, Head of the Department of Obstetrics and Gynecology, for his kind guidance throughout this research work. I have been honored to share his long experience in the field of perinatal ultrasonography and also his Ostrobothnian way of solving problems and enjoying life. I owe also my sincere thanks to Emeritus Professor Antti Kauppila, MD, PhD. His positive attitude towards younger clinicians and investigators shines clearly in my memories.
My deepest thanks belong to my teacher and supervisor, Docent Juha Räsänen, MD, PhD. Without his creativeness and patient way of sharing knowledge, this work would have been impossible. Our numerous discussions and daily routine work in ultrasound laboratory and laboratory animal center have given me the most interesting insights into maternal-fetal medicine. I am also thankful for his encouragement during the times when my own faith grew thin. I am deeply indebted to Professor Olli Vuolteenaho, MD, PhD, and his excellent laboratory in the Department of Physiology. Very early on, he brought up his interest in analyzing additional biochemical markers describing cardiac dysfunction, which were of great value during these studies. He also guaranteed absolutely prompt laboratory service and provided essential feedback.
Professor Karel Marsal, MD, PhD and Professor Juriy Wladimiroff, MD, PhD who kindly agreed to be the official examiners of this thesis deserve my sincerest gratitude. Their critically constructive comments were appreciatively received and treasured. I also feel more than honored that Professor Jean-Claude Fouron, MD, whom I consider a father of the concept of aortic isthmus net blood flow, has agreed to be my opponent.
The mothers and their neonates who participated these studies are warmly thanked for having an understanding of research work. Without the midwives, who unselfishly took the blood samples in the delivery rooms, this work would have been impracticle. It has also been a great pleasure to work with the personnel in the Research Unit of the Department of Obstetrics and Gynecology, especially with Ritva Vasala, RN and Pirjo Ylimartimo, RN. I have gained much positive strength from the special humorous attitude

in their unit. My sincere gratitude is also expressed to Nick Bolton, PhD, Daniel Page, MBA and Michael Spalding, MD, PhD for revising the English language of the papers and this thesis.
Leo Mäkäräinen, MD, PhD has always shown a great understanding in making a convenient schedule for the human and laboratory animal research work. My gratitude to him, and several other colleaques in our clinic is also due for their technical instructions in data processing. I owe particular thanks to Professor Juha Tapanainen, MD, PhD: his knowledge of scientific and clinical work is breathtaking, but he also provides the kind of emotional support and feedback all young researchers and clinicians need. I have been very lucky to work with dedicated and thoughtful colleagues. Riitta Koivunen, MD, PhD with her encouraging attitude and fabulously persistent nature, has lightened my day numerous times. I am also very happy to have Laure Morin-Papunen, MD, PhD, Heli Spalding, MD, PhD and Eila Suvanto-Luukkonen, MD, PhD in my corner. The time with them, Merja Kurkinen-Räty, MD, PhD, Marja Simojoki, MD, Eija Karjalainen, MD, Liisa Karinen, MD, Antti Perheentupa, MD, PhD, Päivi Vuolo-Merilä, MD, Marja Vääräsmäki, MD, PhD, Kirsti Paajanen, MD, and all the members of ”Ultrasound sisters and bros” is unforgettable.
My special acknowledgements are expressed to Mrs. Maire Syväri and Ms. Anneli Tiittanen for their friendly and skillful secretarial assistance. I also want to thank Mrs. Liisa Kärki and the personnel at the Photography Laboratory and Medical Library for their excellent services.
For a long friendship and enjoyable refreshing moments during all these years, I thank Liisa and Ari Räisänen, Hannele and Ilpo Typpö, Kirsi and Seppo Pönkänen, Sari and Petri Oksman, Kirsi Savusalo and Daniel Page with their families. They have been frequently at the ready with sharing a thought bigger than life. My new Bostonian friends softened the difficulties faced during the last year of this thesis, probably even without their knowing it – special thanks to Richie and Peter Vanderwarker, Maryam Bowen and Mary Schneider Enriquez. I cannot overstate the tender care and support given by my godparents, Rauni and Mauri Salminen, and my parents-in-law, Elma and Tero Anttila. Countless times they have helped me out in my childcare problems and had a three-kid-gang in their houses. No wonder that my sons say that they are so lucky to have six grandparents!
I feel incredibly grateful for my parents Raija and Heikki Mäkikallio, whose liberal attitude, support, strength and generosity are unmatched by anyone I have ever met. In addition, my two brothers, Eero and Timo with his family, have always been very special to me. Finally, I thank those closest to my endless questions, tears – and joy. A huge hug and kiss belong to my husband Vesa for patiently standing beside me during all these years. My heart full of love, I thank our sons Tuomas, Johannes and Elias. They have been the driving force in my life, given me new eyes through which to see the world – and they have always shown me the way out to the sunlight.
This research project was generously supported by grants provided by the University of Oulu, the Research Foundation of Orion Corporation, the Paolo Research Foundation, the Finnish Gynaecological Association, the Duodecim Society of Oulu, the Finnish Perinatal Society, and the Academy of Finland, which are gratefully acknowledged.
West Newton, May 16th, 2002, Kaarin Mäkikallio

Abbreviations
AEDV Absent end diastolic velocityANP Atrial natriuretic peptideAoV Aortic valveA-wave Atrial contraction waveBNP B-type natriuretic peptideCC Cardiac circumferenceCCO Combined cardiac output
Confidence interval CK-MB Creatine kinase myocardial band isoenzyme CNP C-type natriuretic peptide CSA Cross-sectional area cTnT Cardiac troponin T DA Ductus arteriosus DAo Descending aorta DNA Deoxyribonucleic acid DV Ductus venosus E-wave Early passive filling wave FGR Fetal growth restriction FHR Fetal heart rate FO Foramen ovale IMP Index of myocardial performance IRT Isovolumetric relaxation time IVC Inferior vena cava LHV Left hepatic vein LVCO Left ventricular cardiac output LVeFo Left ventricular ejection force LVFS Left ventricular fractional shortening MCA Middle cerebral artery MR Mitral valve regurgitation MV Mitral valve NT-proANP N-terminal peptide of proatrial natriuretic peptide
CI

PI Pulsatility indexPIV Pulsatility index for veinsPPA Proximal pulmonary arteryPV Pulmonary valveQ Volume blood flow (ml/min)QDA Ductus arteriosus volume blood flowQFO Foramen ovale volume blood flowQP Pulmonary volume blood flowREDV Retrograde diastolic velocityRI Resistance index RNA Ribonucleic acidRVCO Right ventricular cardiac outputRVeFo Right ventricular ejection forceRVFS Right ventricular fractional shorteningSD Standard deviationS/D- ratio Systolic to diastolic velocity- ratioTC Thoracic circumferenceTR Tricuspid valve regurgitationTV Tricuspid valveTVI Time-velocity- integralUA Umbilical arteryUtA Uterine artery

List of original publications
This thesis is based on the following articles, which are referred to in the text by their Roman numerals:
I Mäkikallio K, Vuolteenaho O, Jouppila P & Räsänen J (2000) Association of severe placental insufficiency and systemic venous pressure rise in the fetus with increased neonatal cardiac troponin T levels. Am J Obstet Gynecol 183: 717–725.
II Mäkikallio K, Vuolteenaho O, Jouppila P & Räsänen J (2001) Umbilical artery N-terminal peptide of proatrial natriuretic peptide in hypertensive pregnancies and fetal acidemia during labor. Obstet Gynecol 97: 23–28.
III Mäkikallio K, Vuolteenaho O, Jouppila P & Räsänen J (2002) Ultrasonographic and biochemical markers of human fetal cardiac dysfunction in placental insufficiency. Circulation 105: 2058–2063.
IV Mäkikallio K, Jouppila P & Räsänen J: Retrograde aortic isthmus net blood flow and human fetal cardiac function in placental insufficiency. Submitted.
V Mäkikallio K, Jouppila P & Räsänen J (2002) Retrograde net blood flow in the aortic isthmus in relation to human fetal arterial and venous circulations. Ultrasound Obstet Gynecol 19: 147–152.


Contents
Abstract Acknowledgements Abbreviations List of original publications Contents 1 Introduction ................................................................................................................. 152 Review of the literature ............................................................................................... 17
2.1 Characteristics of fetal cardiovascular physiology ............................................ 172.1.1 Cardiac function ........................................................................................ 17
2.1.1.1 Preload ......................................................................................... 192.1.1.2 Afterload ...................................................................................... 192.1.1.3 Myocardial blood flow ................................................................ 20
2.1.2 Cardiac output and blood pressure ............................................................ 212.1.3 Peripheral circulation ................................................................................ 222.1.4 Placental circulation .................................................................................. 222.1.5 Fetal oxygen content ................................................................................. 232.1.6 Hypoxemia and hyperoxemia ................................................................... 23
2.2 Ultrasonographic methodology .......................................................................... 242.2.1 Doppler ultrasonography ........................................................................... 24
2.2.1.1 Continuous wave Doppler ultrasonography ................................ 242.2.1.2 Pulsed wave Doppler ultrasonography ........................................ 252.2.1.3 Color Doppler ultrasonography ................................................... 252.2.1.4 Analysis of Doppler spectra ........................................................ 252.2.1.5 Methodological problems ............................................................ 26
2.2.2 M-mode echocardiography ....................................................................... 262.2.3 Safety aspects ............................................................................................ 27
2.3 Doppler ultrasonographic assessment of fetal circulation ................................. 282.3.1 Uterine hemodynamics .............................................................................. 282.3.2 Umbilicoplacental hemodynamics ............................................................ 282.3.3 Fetal heart .................................................................................................. 29
2.3.3.1 Cardiac output and its distribution .............................................. 292.3.3.2 Systolic function .......................................................................... 302.3.3.3 Diastolic function ........................................................................ 322.3.3.4 Afterload ...................................................................................... 33

123456
123456
2.3.4 Fetal arterial circulation ............................................................................ 342.3.5 Fetal venous circulation ............................................................................ 36
2.4 Cardiac troponin T (cTnT) ................................................................................. 382.4.1 Release and elimination of cTnT .............................................................. 382.4.2 Physiologic effects of cTnT ...................................................................... 392.4.3 Cardiac troponin T in myocardial lesions ................................................. 392.4.4 Pregnancy and cTnT ................................................................................. 40
2.5 Atrial natriuretic peptide (ANP) ......................................................................... 402.5. Synthesis and release of ANP ................................................................... 402.5. Regulation of ANP release ........................................................................ 412.5. Physiologic effects of ANP ....................................................................... 422.5. Elimination of ANP ................................................................................... 422.5. N-terminal peptide of proatrial natriuretic peptide (NT-proANP) ............ 422.5. Pregnancy and ANP .................................................................................. 43
2.6 Validation of cTnT and NT-proANP assays ...................................................... 433 Hypothesis of the study ............................................................................................... 454 Material and Methods ................................................................................................. 46
4.1 Study populations ............................................................................................... 464.2 Ultrasonographic measurements ........................................................................ 48
4.2.1 Uterine and umbilicoplacental hemodynamics ......................................... 484.2.2 Cardiac measurements .............................................................................. 48
4.2.2. Volume blood flows .................................................................... 484.2.2. Time-intervals .............................................................................. 494.2.2. Ventricular ejection forces .......................................................... 494.2.2. Inflow waveforms ........................................................................ 494.2.2. Valvular regurgitation ................................................................. 494.2.2. M-mode measurements ............................................................... 50
4.2.3 Fetal arterial circulation ............................................................................ 504.2.4 Fetal venous circulation ............................................................................ 504.2.5 Ultrasonographic parameters of the fetal heart ......................................... 504.2.6 Analysis of cardiac biochemical markers ................................................. 514.2.7 Cardiac troponin T .................................................................................... 514.2.8 N-terminal peptide of proatrial natriuretic peptide ................................... 51
4.3 Statistical analysis .............................................................................................. 515 Results ......................................................................................................................... 53
5.1 Validation of methodology ................................................................................ 535.1.1 Intra-observer variability of Doppler ultrasonographic measurements .... 535.1.2 Assays of NT-proANP and cTnT .............................................................. 54
5.2 Increased NT-proANP production with normal cTnT concentration ................ 545.2.1 Clinical outcome ....................................................................................... 545.2.2 Fetal heart .................................................................................................. 56
5.2.2.1 Volumetric blood flows ............................................................... 565.2.2.2 Systolic and diastolic function of fetal heart ............................... 565.2.2.3 Afterload ...................................................................................... 56
5.2.3 Fetal arterial circulation ............................................................................ 575.2.4 Fetal venous circulation ............................................................................ 58

5.2.5 NT-proANP and acidemia during the labor .............................................. 595.3 Increased fetal cTnT concentration .................................................................... 59
5.3.1 Clinical outcome ....................................................................................... 595.3.2 Fetal heart .................................................................................................. 59
5.3.2.1 Volumetric blood flows ............................................................... 595.3.2.2 Systolic and diastolic function of fetal heart ............................... 605.3.2.3 Afterload ...................................................................................... 60
5.3.3 Fetal arterial circulation ............................................................................ 605.3.4 Fetal venous circulation ............................................................................ 60
5.4 Retrograde net blood flow in the aortic isthmus ................................................ 625.4.1 Clinical outcome ....................................................................................... 625.4.2 Fetal heart .................................................................................................. 62
5.4.2.1 Volumetric blood flows ............................................................... 625.4.2.2 Systolic and diastolic function of fetal heart .............................. 655.4.2.3 Afterload ...................................................................................... 65
5.4.3 Fetal arterial circulation ............................................................................ 655.4.4 Fetal venous circulation ............................................................................ 66
6 Discussion ................................................................................................................... 676.1 Validation of methodology ................................................................................ 67
6.1.1 Ultrasonographic parameters .................................................................... 676.1.2 Cardiac biochemical markers .................................................................... 68
6.2 Increased fetal NT-proANP levels without myocardial cell damage (II, III) ............................................................................................... 69
6.3 Fetal myocardial cell damage (I, III) .................................................................. 706.4 Retrograde aortic isthmus net blood flow (IV, V) ............................................. 726.5 Clinical implications .......................................................................................... 75
7 Conclusions ................................................................................................................. 778 References ................................................................................................................... 78


1 Introduction
Placental insufficiency and fetal growth restriction (FGR) are common obstetrical problems which may have long-term consequences. Intrauterine malnutrition may increase the risk of the development of diabetes, stroke, chronic hypertension, and death from coronary artery disease in adults (Barker et al. 1993, Eriksson et al. 2001). It can also permanently change lipid metabolism and the hemostatic factors leading to the increased risk of cardiovascular diseases (Barker et al. 1993).
Noninvasive Doppler ultrasonography enables the examination of placental, fetal central and peripheral hemodynamics (Jouppila & Kirkinen 1984, Giles et al. 1985a Wladimiroff et al. 1986, Moise et al. 1988). Increased placental vascular impedance as determined by abnormal umbilical artery Doppler findings has been connected with decreased number of arterioles in the placental tertiary villae (Giles et al. 1985a). A rise in placental vascular impedance has been associated with increased perinatal morbidity and mortality (Rochelson et al. 1987a, Rochelson et al. 1987b). The presence of pathologic umbilical artery blood velocity waveforms indicates the need for closer fetal surveillance. In these cases, the timing of the delivery has often been based on pathologic fetal heart rate tracings (Arduini et al. 1993). In pregnancies complicated by chronic fetal hypoxia, an end-diastolic block in the blood velocity waveforms of thoracic aorta preceded pathologic cardiotocographic changes (Jouppila & Kirkinen 1984). Placental insufficiency may lead to cardiac dysfunction (Rasanen et al. 1989). Increased pulsatility in the blood velocity waveforms of fetal systemic veins has been associated with biochemical evidence of a rise in fetal systemic venous pressure (Capponi et al. 1997). A rise in the pulsatility in the human fetal inferior vena cava and ductus venosus blood velocity waveforms was closely related to abnormal biophysical assessment findings and other signs of fetal distress (Hecher et al. 1995a, Hecher et al. 1995b). In fetal circulation, the aortic isthmus has a dynamic role in connecting parallel circulations of the fetal ventricles. An increase in placental vascular resistance can change the fetal aortic isthmus blood flow profile before any change in umbilical artery Doppler velocimetry (Bonnin et al. 1993). In addition, oxygen delivery to the brain is diminished in fetal lambs with retrograde net blood flow in the aortic isthmus (Fouron et al. 1999).
Atrial natriuretic peptide (ANP) is predominantly expressed in the atria of the heart. Conditions with cardiac pressure and volume overload are associated with increased

16
plasma levels of ANP (Lang et al. 1985, Brenner et al. 1990). Cardiac troponin T (cTnT) is released rapidly after myocardial injury in direct proportion to the extent of injury (Gerhardt et al. 1991).
The purpose of this clinical study was to correlate Doppler ultrasonographic parameters of fetal central and peripheral hemodynamics with biochemical markers of fetal cardiac dysfunction and myocardial cell damage in human pregnancies complicated by placental insufficiency. In addition, the association between retrograde aortic isthmus net blood flow and fetal central and peripheral hemodynamics was investigated to understand the mechanisms leading to diminished oxygen delivery in cerebral circulation.

2 Review of the literature
2.1 Characteristics of fetal cardiovascular physiology
Some of the anatomic differences between the fetal and adult circulations were described by Harvey in 1628 (Harvey 1628). Fetal circulation is characterized by three shunts: ductus venosus (DV), foramen ovale (FO) and ductus arteriosus (DA) that permit the blood to bypass the liver and lungs, and shunt the most oxygenated blood from the right to the left side of the heart (Fig. 1).
2.1.1 Cardiac function
Contractility of the heart is markedly affected by contractile state, preload and afterload of the heart. The contractile or inotropic state of the muscle is defined in terms of its ability to shorten and generate tension. It also involves the rate at which shortening and tension develop and decay. The contractile state depends on the number of cross-bridges formed between actin and myosin, which in turn is related to the calcium concentration released from the sarcoplasmic reticulum by the action potential. Substances that alter the inotropic state may act by increasing the availability of calcium at contractile sites. They also appear to change the ability of troponin to bind calcium (Fig. 2). These mechanisms are responsible for the enhanced contractility. The catecholamines epinephrine and norepinephrine are potent inotropic agents. They also increase the heart rate (positive chronotropic effect). Both catecholamines shorten the refractory period of the myocardium and enhance conduction through the heart. Acetylcholine increases potassium and chloride permeability, and thus reduces heart rate and lengthens the conduction time and the refractory period (negative inotropic effect). (Pickoff 1998).

18
Fig. 1. Characteristics of fetal circulation. (Modified from Moore KL, Persaud TVN: Before We Are Born: Essentials of Embryology and Birth Defects, 5th ed. Philadelphia, W.B. Saunders, 1998). RA and LA=right and left atria, RV and LV=right and left ventricles.

19
Fig. 2. Construction of actin-myosin complex. M=myosin fiber, A=actin fiber, I=troponin I, C=troponin C, T=troponin T and Tm=tropomyosin, Ca2+=calcium-ion.
2.1.1.1 Preload
Cardiac muscle responds to the stretching of its fibers (preload) by an increase in the strength of the subsequent contraction. This is called Frank-Starling’s law. Premature atrial contraction is followed by a longer refractory period, allowing enhanced filling of the ventricle. This, in turn, leads to increased stroke volume in the subsequent ventricular contraction, demonstrating that Frank-Starling’s law is effective in human fetal heart (Kirkpatrick et al. 1976, Lingman & Marsal 1987). Furthermore, the inverse relationship between umbilical artery flow velocity and fetal heart rate suggests that the Frank-Starling mechanism regulates cardiovascular control as early as 10–15 weeks of human gestation (Ursem et al. 1998). The heart is protected from being overstretched by several factors: elasticity of the muscle fibers, noncompliant pericardium and functional regulation of the heart.
2.1.1.2 Afterload
Fetal ventricles must eject blood against their own inertia and that of blood, the impedance of central blood vessels, and the resistance of peripheral vessels (Teitel 1998). Parameters describing afterload are complicated because they should take into account all the forces against which the ventricle is contracting. More commonly, arterial mean pressure or systemic vascular resistance is used to approximate afterload. Fetal ventricles, especially the right ventricle, are more sensitive to afterload than adult ventricles (Gilbert 1982, Thornburg & Morton 1983) This may be a consequence of inefficient arterial elasticity rather than an actual sensitivity of the immature myocyte to afterload (Teitel 1998). On the other hand, aortic pressure has a strongly negative effect on fetal left

20
ventricular stroke volume when filling pressures remain constant. It seems that the fetal left ventricle has a limited ability to increase stroke volume above left atrial filling pressures of 5–7 mmHg with volume infusion. However, when aortic pressure is held relatively constant, left ventricular stroke volume continues to rise, even above left atrial pressures of 8–10 mmHg (Hawkins et al. 1989).
2.1.1.3 Myocardial blood flow
Myocardial blood flow is regulated by hydrostatic forces, anatomic factors, metabolic status and autoregulation. Intracardiac pressure is the most important determinant of the pressure gradient from the aorta across the coronary beds during diastole (Bellamy 1978, Dole & Bishop 1982, Pantely et al. 1984, Spaan 1985). Experiments performed in dogs and human adults show that in normal conditions, 70–85% of the left ventricular myocardial blood flow occurs during diastole (Klocke 1976, Hess & Bache 1976). An increase in the strength of heart contraction immediately increases coronary blood flow, while a decrease in the force will have an opposite effect. Autoregulation is due to the fact that the coronary arteries arise from the sinus in the base of the aorta. When the stroke volume increases, more blood is automatically forced into the coronary arteries. During diastole, part of the increased blood volume ejected to the aorta flows rapidly into the coronary vessels. However, there is experimental evidence that the autoregulatory reserve in subendocardial arteries is less than in subepicardial arteries (Sestier et al. 1978, Boatwright et al. 1980, Vatner 1984). Furthermore, several substances including oxygen, carbon dioxide, potassium and adenosine, as well as the autonomic nervous system have been proposed to have a role in vasoregulation of the myocardium (Reller et al. 1992a, Reller et al. 1992b, Reller et al. 1995).
Animal studies have demonstrated that increased wall stress, especially during the diastole, leads to increased ventricular myocardial oxygen consumption (Sarnoff 1958, Sonnenblick et al. 1965, Pool et al. 1968, Sonnenblick & Skelton 1971, Rooke & Feigl 1982). Due to the elliptical structure of the ventricles, regional wall stress and myocardial oxygen consumption are not homogeneous throughout the ventricle. The wall tension is greater in the subendocardial region than in the subepicardial region (Berman & Gamble 1975, Holtz et al. 1977). In a lamb model, an increase in right ventricular pressure was associated with a marked accumulation of products of reactive O2 species generation in the right ventricular myocardium (Fouron et al. 2001a). The wall stress and myocardial oxygen consumption per gram of tissue will be restored to normal values when hypertrophy of the cardiac muscle occurs. However, hypertrophy compromises coronary vascular reserve.

21
2.1.2 Cardiac output and blood pressure
The cardiac output (stroke volume x fetal heart rate (FHR)) is affected by contractility, peripheral resistance, blood volume and viscosity of the blood. Venous blood return (preload) controls the cardiac output. The cardiac response depends on the sympathetic stimulation and on the contractile characteristics of the myocardium. Heart rate is influenced by the balance between sympathetic and parasympathetic impulses, ionic concentration, pH and secretion of several hormones. Stroke volume is affected by the same factors that affect myocardial contractility, preload and afterload of the heart, and heart rate. The dynamics of blood flow have a considerable effect on cardiac output and the work done by the heart. The law of Laplace states that the tension in the wall increases when the distending intraventricular pressure and/or the intraventricular volume increase. When intraventricular volume is increased, a greater tension must be developed in the myocardium to produce any given pressure. The amount of energy needed increases as pressure increases (Romero et al. 1972).
With advancing gestation fetal stroke volume, cardiac output and weight-indexed systemic vascular resistance increase (Rasanen et al. 1996a). Between 14 and 28 gestational weeks, left and right ventricular systolic and end diastolic pressures increased significantly in a linear fashion (Johnson et al. 2000). Ventricular systolic pressure increased from 13 mmHg to 37 mmHg and end diastolic pressure from 3 mmHg to 10 mmHg. No difference between left and right ventricular pressures was observed. In addition, atrial pressures were equal and remained constant between 14 and 28 gestational weeks (Johnson et al. 2000). Studies on fetal lambs have shown that the right ventricle ejects a larger stroke volume than the left ventricle, and represents a greater proportion of the combined cardiac output (CCO) (Rudolph 1985). According to studies on middle to late gestation lamb fetuses, right ventricular stroke volume (55%–67% of CCO) exceeds left ventricular stroke volume for any given physiologic filling pressure (Rudolph 1985, Reller et al. 1987). The weight-indexed fetal cardiac output is significantly higher than that of an adult person (Severi et al. 2000). In fetal lambs, CCO in term gestation was about 500 ml/min/kg and about 2.5% of CCO was directed to the heart (Itskovitz et al. 1987).
Mean (SD) umbilical venous oxygen saturation has been shown to be 63% (16%) and the corresponding umbilical artery value 24% (15%) (Richardson et al. 1998). Highly oxygenated blood from the placenta is directed via DV through FO to the left ventricle (Fig. 1) (Kiserud et al. 1991). In a study on near-term fetal lambs, about 44% of the umbilical venous blood was distributed to DV. With a 50% reduction in umbilical flow, this proportion increased to 72%, resulting a marked decrease in umbilical blood flow distributed to the fetal liver (Itskovitz et al. 1987). In fetal lambs, the left ventricle delivers the majority of its output to the heart, brain and the upper parts of the body, with only less than 10% crossing through the aortic isthmus to the descending aorta (DAo) (Rudolph 1985). The fetal right ventricle receives mainly poorly saturated blood from the superior vena cava, coronary sinus and inferior vena cava (IVC) (Rudolph 1985). The majority of the right ventricular cardiac output (RVCO) is delivered across the DA to the DAo.

22
2.1.3 Peripheral circulation
Poiseuille described in 1864 the relationship between the factors that govern the nonpulsatile flow of homogeneous liquids through rigid tubes. The driving force that causes the fluid to flow from one end to the other is the pressure gradient (dP) between the two ends. Several factors provide the resistance (R) to volume flow (Q) including the viscosity of the fluid (η), the radius of the tube (r), and the length of the tube (L). Poiseuille´s law expresses this relationship in the equation: Q = dPr4π / ηL8. In clinical practice, the relationship among volume flow, pressure and resistance is expressed as Q = P/R. Most of the vascular resistances in humans are in parallel. Even minor changes in the diameter of the blood vessel have significant effects on the conductance of blood through the vessel. A change in the blood vessel caliber has the most important effect on vascular resistance. In vessels connected in series, the resistances are additive. In parallel circulations, the pressure is maintained in each vessel and the rate of blood flow can be affected by selective opening or closing of the individual vessels.
Viscosity, which is the inner friction between the molecules in the adjacent layers or laminae, affects the resistance to flow. In laminar flow, the laminae move parallel to each other in longitudinally-oriented concentric sleeves, each moving at a different rate. The viscosity of the blood depends, for example, on the number of circulating blood cells and the bore of the vessels.
Blood pressure is almost directly proportional to volume distribution and vascular resistance. The arterioles have the ability to regulate the resistance of systemic circulation. Arteriolar resistance is affected by sympathetic stimulation. Blood vessel diameter increases as the internal pressure rises. The degree of distensibility varies considerably according to the thickness and composition of the vessel wall, and the degree of filling. The veins are referred to as the volume storers or capacitance vessels of the circulation, while the arteries, due to their ability to expand and recoil, are the pressure storers, storing the pressure during contraction of the heart and releasing it during cardiac relaxation.
2.1.4 Placental circulation
Maternal nutrient delivery depends upon placental mass, villous surface, and fetoplacental vasculature. Uteroplacental vascular lesions associated with FGR include incomplete uteroplacental vascular adaptation, fibrinoid necrosis or atherosis of the vessel wall, persistence of endovascular trophoblasts in the basal plate vasculature, uteroplacental thrombosis and chronic uteroplacental vasculitis (Salafia 1997). These lesions predispose to placental infarcts. It has been suggested that FGR may not be due to any particular placental lesions but to underperfusion of the placenta (Naeye 1989, Salafia et al. 1995). The presence of placental infarct more likely indicates overall poor perfusion with failed collateral flow. Poor uteroplacental perfusion may compromise terminal villous arborization due to effects of intervillous pressure on capillary net or by actual destruction of villous capillaries (Giles et al. 1985a).

23
2.1.5 Fetal oxygen content
The oxygen partial pressure (pO2) of the fetal arterial blood, which normally ranges from25 to 30 mmHg, is considerably lower than that of maternal arterial blood (Soothill et al.1986). Even though oxygen tension in fetal blood is only one-fifth to one-fourth that ofadult blood, fetal arterial blood oxygen content and oxyhemoglobin saturation are notmuch lower than those of an adult. Fetal hemoglobin (α2, δ2) is structurally differentfrom adult hemoglobin (α2, β2). It has a greater affinity for oxygen than has adulthemoglobin (Delivoria-Papadopoulos & McGowan 1998). Consequently, fetalhemoglobin combines more rapidly with oxygen at low tension than does adulthemoglobin. With advancing gestation, progressive decrease in umbilical artery pO2 isassociated with an increase in fetal hemoglobin concentration, thus maintaining fetaloxygen content constant (Soothill et al. 1986). Studies on moderately and severelyanemic fetal lambs show that a high hemoglobin-oxygen affinity is critical for normalmetabolism in fetuses subjected to a hypoxic stress (Edelstone et al. 1989). In fetal lambs,mean (SD) oxygen extraction increased from 33.6% (4.8%) to 67.7% (11.3%) during a75% reduction of umbilical blood flow (Itskovitz et al. 1983). Similarly in fetal lambs,diminished oxygen delivery due to restriction of uterine artery blood flow increased mean(SD) oxygen extraction significantly from 0.33 (0.04) to 0.43 (0.04) and 0.54 (0.05) at 1and 24 hours, respectively. Overall fetal oxygen consumption remained unchanged fromcontrol values (Bocking et al. 1992). Oxygen consumption in normal human fetusesbetween 28 and 40 weeks of gestation varied between 5.4–6.8 ml/kg/min (Bonds et al.1986), while adult oxygen consumption is estimated to be about 3.0–4.0 ml/kg/min inresting state. Thus, oxygen consumption per kilogram in a normal fetus is almost doublethe consumption of the adult.
2.1.6 Hypoxemia and hyperoxemia
Inadequate fetal oxygen supply may result from inadequate maternal blood oxygencontent, a fall in uterine blood flow or from disturbed placental circulation. A reductionin either uterine or umbilical blood flow will also interfere with carbon dioxide removalfrom the fetus resulting in fetal respiratory acidemia. In studies on fetal lambs, theproportion of cardiac output directed to the placenta rose from 41% to 48% in hypoxemicfetuses and from 41% to 57% in acidemic fetuses, respectively (Cohn et al. 1974). Inhypoxemic fetuses, cerebral and coronary circulations and blood flow to the adrenalsincreased two- to three-fold, while pulmonary, renal, splenic, gut and carcass blood flowsdecreased. These changes were of a greater magnitude in fetuses with acidemia (Cohn etal. 1974). After initial fetal tachycardia during hypoxemia, the fetal arterial pressureincreases and FHR decreases. Combined cardiac output is maintained (Cohn et al. 1980,Reuss et al. 1982, Court et al. 1984) unless the fetus becomes acidemic (Cohn et al.1974). Thus, it seems that a moderate reduction in oxygen delivery to the fetus stimulateschemoreceptor activity that results in vasoconstriction in certain peripheral vascular beds,hypertension, and a transient or persistent bradycardia in the fetus (Peeters et al. 1979,Itskovitz & Rudolph 1982).

24
Maternal hyperoxygenation increases fetal oxygen partial pressure (Morin & Egan1992). During maternal hyperoxygenation in the second half of the pregnancy, fetalmiddle cerebral artery (MCA) PI values increased and PI values of the aortic isthmusdecreased with no change in the umbilical artery PI values (Almstrom & Sonesson 1996).In addition, increased absolute velocities in DV have been noted during maternalhyperoxygenation (Soregaroli et al. 1993). In experiments on fetal sheep in earlypregnancy, maternal hyperoxygenation did not cause these changes (Kiserud et al. 2001).In studies on fetal lambs, increasing maternal oxygen tension increased pulmonary bloodflow 10-fold in the third trimester fetuses but only 0.2-fold in late second trimester fetuses(Morin & Egan 1992). At the normal low oxygen tension of the fetus, pulmonary bloodflow does not increase between these two points of gestation in the fetal lamb despite theincrease in vessel density in the lungs.
2.2 Ultrasonographic methodology
2.2.1 Doppler ultrasonography
Doppler phenomenon states that the pitch of the sound waves of a moving object isaltered when the distance between the observer and the source of sound changes. Thechange in relative motion between the observer and the object is known as the Dopplershift. The Doppler shift (Fd) can be calculated with the following formula: Fd = v2fcosθ/c, where v is the speed of the moving target, f is the frequency of the emitted pulse (i.e.the frequency of the transducer), θ is the angle between the direction of emitted soundwave and the direction of the moving target, and c is the average speed of sound withinthe tissue (Kremkau 1990, Kremkau 1992). Doppler shift is dependent on the speed ofblood flow, the angle between the transducer and the blood vessel, and the operatingfrequency of the Doppler transducer (Kremkau 1990, Kremkau 1992).
2.2.1.1 Continuous wave Doppler ultrasonography
In a continuous wave Doppler system, a sound wave is continuously transmitted andreceived with two different transducers. The transmitted and reflected beams begin tooverlap a short distance from the surface of the probe, and the overlap extends until thebeams attenuate. When several vessels are focused within the sensitive volume, theDoppler signals are superimposed and detected simultaneously (Gill 1987). This explainswhy the investigations of certain locations are not possible. On the other hand, continuousDoppler ultrasonography is not dependent on the depth of the location and speed of theblood flow.

25
2.2.1.2 Pulsed wave Doppler ultrasonography
A Doppler system with range resolution allows the selection of location where theDoppler signals are obtained. This is possible by sending the ultrasound waves in pulses,and thus only waves from certain areas return back before the next pulse is transmitted. Inorder to analyse reflected waves during a certain time period after pulse transmission, it ispossible to set a sample volume located in predetermined range (Gill 1987). The axiallength of the sample volume is determined by the time period the gate is open. Changingfrequency of waves reflected from a moving target limits the use of pulsed Doppler. Themaximum measurable Doppler shift frequency is related to half of the pulse repetitionfrequency (Nyqvist limit). The pulse repetition frequency must be increased with highvelocities, and thus the depth reached with the ultrasound decreases. The velocity of theblood flow and the depth of the object are limitations of pulsed Doppler ultrasonography.
2.2.1.3 Color Doppler ultrasonography
Phase quadrature detection in the demodulators enable the observer to distinguish higheror lower received signals from the transmitted frequency, corresponding to Doppler shiftstoward or away from the transducer. Flow toward the transducer is visually demonstratedas red and flow away from the transducer as blue, and non-moving targets remain grey.The saturation of the color is related to the velocity of the flow (Burns 1993). Thelimitations of color flow imaging are similar to pulsed Doppler ultrasonography.
2.2.1.4 Analysis of Doppler spectra
The presence or absence of blood flow, and the shape of the blood velocity waveform areanalysed qualitatively. As a result of different physical, anatomic and morphologicfactors, blood velocity waveform profiles differ from one vessel to another. The presenceof a diastolic blood velocity waveform component is detected in arteries supplying low-resistance vascular systems. As the peripheral impedance increases, the diastoliccomponent disappears. The presence of a diastolic notch in uterine arteries has been usedto predict adverse pregnancy outcome (Harrington et al. 1996). In the umbilical vein,blood flow is normally nonpulsatile and the appearance of atrial pulsations has beenthought to indicate the deterioration of fetal well-being (Reed 1997).
Semiquantitative analysis of Doppler spectra involves the evaluation of the relationbetween systolic and diastolic blood velocity waveform components. The most commonindices are the pulsatility index (PI = (peak systolic velocity – end diastolic velocity) /time-averaged maximum velocity over the cardiac cycle) and the resistance index (RI =(peak systolic velocity – end diastolic velocity) / peak systolic velocity). The correlationbetween these indices is highly significant as long as the diastolic blood flow velocity ispresent (Thompson 1987). These angle independent indices are thought to describe

26
downstream impedance, which is resistance to pulsatile flow (O'Rourke 1982). However,they are only indirect estimations of true blood flow (Dickey 1997). No correction forheart rate, if it is within normal physiologic range, or blood pressure is required (Kofinaset al. 1989, Burns 1993, Dickey 1997).
Volume flow analysis is the most difficult to perform. It is dependent on the angle ofinsonation, accurate measurement of the vessel diameter, tortuousity of the vessel andanalytical power of the ultrasonographic equipment. Due to these limitations, it isrecommended for larger vessels (Dickey 1997). However, Kiserud et al. (Kiserud et al.1999) have demonstrated by in vitro and in vivo measurements that ultrasonographicdiameter assessment has a high reproducibility even for vessels of small dimensions whenmeasurements are taken with a high-frequency ultrasound. The flow volume (Q (ml/min))of the vessel can be calculated if the mean velocity (V mean = TVI x HR) and the cross-sectional area (CSA) of the vessel are known according to following formula: Q = TVI xHR x CSA, where CSA is derived from the diameter (r) of the vessel with the formula:CSA= π(r/2)2, time-velocity- integral (TVI) is calculated by planimetering the areaunderneath the Doppler spectrum and HR is heart rate (Rizzo et al. 1995a).
2.2.1.5 Methodological problems
When measuring absolute velocity and volume, the angle of insonation is of greatconcern. For angles less than 20 degrees, the maximum error in calculating velocity andvolume caused by 5% error in estimating the angle of insonation is equal or less than1.5% (Dickey 1997). If the angle of insonation is kept at less than 30 degrees, the effectof the angle on the absolute velocities and volume is minimal (Tessler et al. 1990). In PIand RI calculations, an angle of less than 60 degrees is considered nonsignificant(Gudmundsson et al. 1990, Davies et al. 1990).
In volume flow calculations, the main source of error is the vessel CSA. Vesseldiameter changes during the cardiac pulsations and this error is magnified when squared.Error in vessel diameter measurements becomes less as vessel diameter increases.However, at least three separate measurements of the diameter from sequential cardiaccycles are recommended (Dickey 1997).
2.2.2 M-mode echocardiography
M-mode imaging shows the instantaneous position of one interface with respect to otherinterfaces along the selected transmission line. Monitored interfaces will change eachtime the selected transmission penetrates through the area. As each transmission collectsand displays its interface data with its own time reference, variations in the position ofechoes received are recognized as motion displacements. This method is possible withoutany physical changes of the transducer when the transducer is set to collect data from onedirection and from only one line of transmission. In fetal cardiology, it is used to estimate

27
the diameters of ventricular cavities and walls and the fractional shortenings of theventricles. It is also useful in the diagnosis of fetal arrhythmia (DeVore et al. 1984, Reed1997).
2.2.3 Safety aspects
Mechanical index indicates cavitation potential in the tissues, and thus the amplitude ofultrasonographic pulses at any time. Increased pulse amplitudes result in proportionallyhigher mechanical index values. It is estimated that cavitation potential can be of majorconcern when intensities exceed 3300 W/cm3. Thermal index is an indicator for tissueheating. Thermal index is an estimate of the tissue temperature rise in ºC, which might bepossible in “reasonable worst case conditions". It is divided into soft tissue, bone andcranial thermal indices. Ultrasonographic equipment has to display the emitted energy insettings in which thermal and/or mechanical indices might be over or equal to 0.4(European Committee for Medical Ultrasound Safety 1999a, European Committee forMedical Ultrasound Safety 1999b). Diagnostic exposure that produces a maximum in-situ temperature rise of no more than 1.5ºC above normal physiologic level (37ºC) maybe used clinically without reservations on thermal grounds. Fetal temperature elevationabove 41ºC for 5 minutes or more is considered hazardous (Barnett et al. 1997).
In animal ultrasonographic studies during organogenesis and later pregnancy, no long-term ramifications, abortions, gross malformations, or stillbirths have been observed inthe exposed animals (Tarantal & Hendrickx 1989a, Tarantal & Hendrickx 1989b, Tarantalet al. 1993, Tarantal et al. 1995). A follow-up study after human fetal ultrasonographicexposure revealed no association between ultrasound exposure in early fetal life andgrowth or impaired vision or hearing during childhood, at least until the age of sevenyears (Kieler et al. 1997). However, the results concerning the relationship betweenimpaired fetal growth and repeated fetal ultrasound exposures are controversial(Newnham et al. 1993, Kieler et al. 1997). No association between diagnostic ultrasoundexposure during pregnancy and childhood malignancies or neurological maldevelopmenthas been found (Salvesen & Eik-Nes 1999). Kieler et al. (Kieler et al. 1998) found nodifference in non-right handedness between exposed and non-exposed children at the ageof 8–9 years, but, separately, the boys exposed to ultrasound had higher prevalence ofnon-right handedness. There exists a consensus that frequently repeated ultrasonographicexposures should be of a clinical benefit (Kossoff 1997).

28
2.3 Doppler ultrasonographic assessment of fetal circulation
2.3.1 Uterine hemodynamics
The major arterial blood supply to the uterus is derived from the uterine arteries. Bloodvelocity waveform indices in the uterine artery remain high and quite stable during thefirst trimester of pregnancy (Dickey & Hower 1995). In the beginning of the secondtrimester, Doppler indices decrease sharply and early diastolic notching of uterine arterydisappears. However, a diastolic notch can be a normal finding until 26 weeks ofgestation (Schulman et al. 1986). Little or no change is observed in either the uterine orarcuate artery indices after 26 weeks of gestation. The Doppler indices of the uterineartery on the placental side are significantly lower than on the nonplacental side. Acentrally located placenta demonstrates similar Doppler indices on both sides (Bewley etal. 1989). Abnormal uterine artery blood velocity waveforms are identified by apersistent abnormal index, a persistent diastolic notch or an abnormal difference betweenthe indices in the left and right uterine arteries (Fleischer et al. 1986, Thaler et al. 1992).
2.3.2 Umbilicoplacental hemodynamics
The umbilical circulation has been studied by Doppler ultrasonography from 7 weeks ofgestation onwards (den Ouden et al. 1990, Wladimiroff et al. 1991). After 12 gestationalweeks, the diastolic blood flow component becomes progressively present and should bepresent in all pregnancies after 16 weeks. From 20 gestational weeks onwards, the growthof the placental unit with an increase in the number of functioning vascular villaedecreases its vascular impedance (Trudinger et al. 1985). In humans, umbilical bloodflow increases in direct proportion to the increase in human fetal body weight, so thatweight-indexed flow remains approximately constant at 110 ml/min/kg to 125 ml/min/kgover the last third of the pregnancy (Gill et al. 1981, Sutton et al. 1990). In near-termfetal sheep, it has been demonstrated that about 45% of fetal CCO is directed to theplacenta (Itskovitz et al. 1987). In human studies, about 30% of CCO is estimated to bedirected to the placenta (Sutton et al. 1991a).
Abnormal umbilical artery blood velocity waveforms in humans are associated with adecrease in the number of small muscular arteries in the placental tertiary stem villi (Gileset al. 1985a, Giles et al. 1985b). These morphologic changes may deteriorate theplacental oxygen and other nutritional transport, and lead to FGR. Abnormal umbilicalartery findings are demonstrated to correlate better with fetal acidosis than a non-stresstest (Arduini et al. 1991). In addition, abnormal umbilical artery blood velocitywaveforms are shown to precede pathologic non-stress tests (Donner et al. 1995). On theother hand, even profound fetal acidemia does not change the umbilical arterywaveforms, unless the fetus becomes hypotensive (Muijsers et al. 1990, Morrow et al.1990). Occlusion of placental arteriolar bed by plastic microspheres leads to increasedplacental vascular resistance and to a diminished umbilical artery diastolic blood flow

29
component (Trudinger et al. 1987). It has been estimated that over 60% of theintraplacental fetal vasculature must be occluded before significant changes in umbilicalartery blood velocity waveform pattern occur (Thompson & Trudinger 1990).
The diastolic blood flow component in umbilical artery increases with advancinggestation. In late second and third trimesters, the systolic to diastolic velocity- ratio (S/D-ratio) is considered to be increased if it is ≥ 3.5 (Forouzan et al. 1991). Absent diastolicvelocity (AEDV) or retrograde diastolic velocity (REDV) in the umbilical artery indicateextremely increased placental vascular impedance. Usually, AEDV persists andoccasionally deteriorates into REDV. The median interval between the appearance ofAEDV and abnormal fetal heart rate tracings is about one week with very wide individualvariability (Arduini et al. 1993). Fetal condition cannot be inferred from AEDV alonebecause AEDV itself is not diagnostic for hypoxemia and acidemia at the delivery(Nicolini et al. 1990). By the time REDV is detected, severe fetal compromise withacidemia and high perinatal mortality is evident (Brar & Platt 1988, Kurkinen-Raty et al.1997). In animal studies, REDV immediately preceded fetal death (Morrow et al. 1989).In humans, structurally normal fetuses with umbilical artery AEDV or REDV comprised19% of all deaths occurring before 30 weeks of gestation. The perinatal mortality rate wassignificantly higher for fetuses with REDV (35.7%) than for fetuses with AEDV (8.9%)(Kurkinen-Raty et al. 1997). The incidence of FGR with AEDV has been reported to be83% and over 50% of the pregnancies with AEDV are complicated by maternalhypertensive disorders. There is also an increased incidence of fetal abnormalities inpregnancies complicated by AEDV (Farine et al. 1994). In a meta-analysis, umbilicalartery blood flow assessment in high-risk pregnancies reduced the odds of perinatal deathby 38% without an increase in the rate of unnecessary obstetric interventions (Alfirevic &Neilson 1995, Divon 1996).
2.3.3 Fetal heart
2.3.3.1 Cardiac output and its distribution
In appropriately grown human fetuses, stroke volume increased exponentially from 0.7ml at 20 weeks to 7.6 ml at 40 weeks for the right ventricle (r = 0.87) and from 0.7 ml at20 weeks to 5.2 ml at 40 weeks for the left ventricle (r = 0.91). Right (RVCO) and left(LVCO) ventricular cardiac outputs increased respectively (Kenny et al. 1986). Duringthe second half of human gestation, RVCO was reported to increase from 100 ml/min to1000 ml/min, while LVCO increased from 100 ml/min to 800 ml/min (Rasanen et al.1996a). Right ventricular dominance persisted during the second half of gestation, and iteven increased towards the term of gestation in appropriately grown human fetuses(Rasanen et al. 1996a). In FGR, a relative increase in LVCO associated with decreasedRVCO has been demonstrated (al-Ghazali et al. 1989). Studies of progressivelydeteriorating growth-restricted fetuses have shown gradually decreasing cardiac output

30
(Rizzo & Arduini 1991). In addition, there is evidence of a temporal association betweena drop in cardiac output and the onset of late heart rate decelerations (Rizzo & Arduini1991).
The distribution of fetal CCO changes during gestation (Rudolph & Heymann 1970,Rasanen et al. 1996a). In studies on near-term fetal sheep, it has been demonstrated thatapproximately 2.5% of CCO is directed to the heart, 3.7% to the brain, 8.0% to the lungs,31% to the carcass, and 45% to the placenta, respectively (Itskovitz et al. 1987). In latethird trimester human fetuses, the proportion of right (RVCO%) ventricular cardiac output(60%) of CCO is significantly higher than the proportion of left (LVCO%) ventricularcardiac output (40%) of CCO (Rasanen et al. 1996a). The proportion of human fetalpulmonary volume blood flow (QP) of CCO increased from 13% to 25% between 20 and30 weeks of gestation, and after 30 weeks of gestation, it remained unchanged (Rasanenet al. 1996a). On the other hand, Sutton et al. found a four-fold increase in the absolutepulmonary volume blood flow, which comprised 22% of CCO between 18 and 38 weeksof gestation (Sutton et al. 1994). Maternal hyperoxygenation did not change the reactivityof the human fetal pulmonary circulation between 20 and 26 weeks. Between 31 and 36weeks, the PI values of right and left pulmonary arteries decreased, the PI of DAincreased. At the same time QP increased while ductus arteriosus (QDA) and foramenovale (QFO) volume blood flows decreased significantly with unchanged LVCO andRVCO (Rasanen et al. 1998). This suggests that fetal pulmonary circulation is underacquired vasoconstriction at least beyond 32 gestational weeks. The proportion of QDAremained stable during the second half of the gestation, varying between 32% and 40% ofCCO. Between 20 and 30 weeks of gestation, the proportion of QFO has beendemonstrated to decrease from 34% to 18% of CCO. After 30 weeks, QFO did not change(Rasanen et al. 1996a). Sutton et al. reported that blood flow through FO increasedthreefold between 18 and 38 weeks of gestation representing 31% to 17% of CCO (Suttonet al. 1994).
2.3.3.2 Systolic function
The right (RVeFo) and left (LVeFo) ventricular ejection forces are calculated by theformula: (1.055xCSAxTVIac)x(PSV/TTP), in which TVIac is the TVI during accelerationperiod in systole, PSV is the peak systolic velocity, and TTP is the time period from theonset of ejection to systolic peak velocity (Isaaz et al. 1989, Sutton et al. 1991b). BothRVeFo and LVeFo, which estimate the energy transferred from the ventricular myocardialshortening to work done by accelerating blood into the circulation, increase significantlyduring the second half of pregnancy with no difference between the ventricles (Sutton etal. 1991b). Ventricular ejection force calculation does not require estimation ofventricular volumes and is independent of ventricular configuration. Animal studies havesuggested that early systolic flow is less affected by changes in afterload and preload thanflow during late systole (Noble 1968). In addition, blood acceleration seems to be closelyrelated to the force developed by the ventricle in early systole (Noble et al. 1966). Ingrowth-restricted fetuses, ventricular ejection forces were reduced compared to normalfetuses. The degree of impairment seemed to be related to the severity of fetal

31
compromise (Rizzo et al. 1995a). In human fetuses with severe ductal constriction orocclusion, RVeFo was decreased, showing that a marked increase in the right ventricularafterload affects RVeFo development (Rasanen et al. 1997).
In the presence of mitral (MR) or tricuspid regurgitation (TR), ventricular contractilitycan be assessed from the upstroke of the regurgitation jet (dP/dT) (Fig. 3) (Tulzer et al.1991b). It may have prognostic value in pregnancies complicated by nonimmune hydropsfetalis (Tulzer et al. 1991b).
The index of myocardial performance (IMP) describes the combined systolic anddiastolic performance of the heart with the formula: IMP=(ICT+IRT)/ET, in which IRTand ICT are isovolumetric relaxation and contraction times, and ET is ejection time (Fig.4) (Tei et al. 1995, Tsutsumi et al. 1999). The IMP of the fetal left and right ventriclesdecreases during the second half of the gestation (Tsutsumi et al. 1999). Tsutsumi et al.(Tsutsumi et al. 1999) found no difference in IMP values between 18 and 26 weeks ofgestation in fetuses with FGR. However, from 27 to 40 weeks of gestation, IMP wassignificantly greater in fetuses with FGR than in the controls (Tsutsumi et al. 1999).
Fig. 3. Holosystolic tricuspid valve regurgitation.

32
Fig. 4. Schematic and ultrasonographic presentation of left ventricular time-intervals. ICT =isovolumetric contraction time, ET = ejection time, IRT = isovolumetric relaxation time, FT =filling time, early passive (E-wave) and atrial contraction (A-wave) waves of inflow waveform.
2.3.3.3 Diastolic function
The IRT is the time interval between closure of the semilunar valve and opening of theatrioventricular valve (Fig. 4). It represents the time period needed for the ventricle toactively decrease its pressure to the atrial level. The proportion (%) of IRT of the totalcardiac cycle can be used to describe diastolic function of the heart. No change in fetalIRT% has been found in normal pregnancies during the second half of gestation (Tulzeret al. 1994). In growth-restricted fetuses, an increase in left ventricular IRT may reflectcardiac diastolic dysfunction (Tsyvian et al. 1995)
At the level of the atrioventricular valves, monophasic inflow pattern changes tobiphasic after the 8th gestational week (van Splunder et al. 1995, van Splunder et al.1996, Leiva et al. 1999). The biphasic inflow waveform contains two peaks representingearly passive filling (E-wave) and atrial contraction (A-wave) during atrial systole (Fig.4). Both E- and A-wave maximum velocities as well as total- and A-wave- TVI of bothvalves increased significantly with advancing gestation, and the A/E velocity- ratiodecreased significantly (Tulzer et al. 1994, Veille et al. 1999). Normally, the maximumvelocity of A-wave is higher than that of E-wave in human fetuses (Tulzer et al. 1994),and E- and A-wave velocities are higher in the tricuspid valve (TV) than in the mitralvalve (MV), with no difference in A/E velocity ratio between the valves (Tulzer et al.1994). The A-TVI/total TVI- ratio did not change significantly with advancing gestation,but this ratio was significantly higher at TV than at MV (Tulzer et al. 1994). The inflowwaveforms are affected by preload of the heart and ventricular compliance. During the

33
first year of life, inflow velocities remain unchanged, but the ventricular filling shiftstowards an early passive filling period (Veille et al. 1999). However, the E/A velocity-and TVI- ratios can also reflect atrial pressure. In growth-restricted fetuses, the TV andMV E/A velocity- ratio was significantly lower than in normal fetuses (Rizzo et al. 1988).However, no consistent differences were found in E/A velocity- ratios betweencompromised and noncompromised fetuses (Hecher et al. 1995a).
2.3.3.4 Afterload
The ratio of cardiac circumference to thoracic circumference (CC/TC) and their area ratioare used to evaluate the heart size. The ratio between the fetal cardiac and thoracic areasremains constant during the second half of pregnancy at 0.30 (0.05) (mean (SD))(Respondek et al. 1992). In normal pregnancies, the CC/TC-ratio is found to be normalwhen less than 50% (Huhta et al. 2000). In cases with suspected cardiomegaly, includingfetal hydrops, arrhythmias and increased septal thickness due to maternal diabetes, theseratios have been demonstrated to be significantly greater than in the normal group(Respondek et al. 1992)
In fetuses with normal cardiac anatomy, the prevalence of TR is 6.8% (Respondek etal. 1994). Tricuspid regurgitation has been classified to be trivial (nonholosystolic≥ 72ms, maximum velocity <2m/s) or significant (holosystolic, maximum velocity >2m/s) (Fig. 3). Fetal TR can be a sign of elevated right ventricular pressure related to theconditions causing increased afterload, including the constriction of the DA and severeplacental insufficiency. In addition, it can be related to the conditions causing increasedpreload of the heart, such as increased volume overload (Respondek et al. 1994). Achange in the compliance of the right ventricle, for example, due to hypertrophy of theventricular wall may lead to the development of TR. It has been speculated that TR ismore uncommon in the fetus than in the newborn, because the fetal right ventriclefunctioning at systemic blood pressure level optimally supports the competent TV. Thelower pressure in the right ventricle after birth results in nonsynchronous leaflet closure(Respondek et al. 1994).
Right (RVFS) and left (LVFS) ventricular fractional shortenings are calculated by theformula (ventricular fractional shortening (%) = [(inner diastolic diameter – inner systolicdiameter)/inner diastolic diameter] x 100) (DeVore et al. 1984). They are obtained fromM-mode recordings by placing the M-mode cursor perpendicularly towards theinterventricular septum at the level of atrioventricular valves in the four chamber-view ofthe heart (DeVore et al. 1984). In normal fetuses, the diameter of the right and leftventricles correlated with the biparietal diameter, and the right/left ratio of the ventriculardiameters remained constant (1:1) throughout gestation. In addition, RVFS and LVFSwere independent of gestational age, being between 28–40% during the second half ofgestation (DeVore et al. 1984). An abnormal ventricular fractional shortening can reflectmyocardial compromise or an increase in the ventricular workload (Huhta et al. 2000). Inacute fetal ductal occlusion in lambs, the right ventricular systolic dimension increasedand RVFS decreased due to an increase in the right ventricular afterload (Tulzer et al.1991a). In fetuses with intrauterine distress, RVFS was found to be decreased and the

34
ratio of the right and left ventricular end-diastolic diameters was increased, while nodifference in the left ventricular size and myocardial contractility was observed (Rasanenet al. 1989).
2.3.4 Fetal arterial circulation
Placental insufficiency may lead to inadequate tissue perfusion which can activate fetalcompensatory mechanisms including increased oxygen extraction, redistribution of bloodflow and blood volume augmentation.
Throughout the normal pregnancy, there is a continuous diastolic component in theblood velocity waveform patterns of cerebral arteries. In late gestational fetuses, the PI ofMCA decreases (Woo et al. 1987, van den Wijngaard et al. 1989, Mari & Deter 1992).Animal studies have shown that during chronic hypoxemia, the fetus redistributes theblood flow to the brain and the myocardium at the expense of the lower body (Peeters etal. 1979). Accordingly, Doppler studies of growth-restricted and hypoxemic humanfetuses have demonstrated reduced impedance and increased blood velocities in theinternal carotid and middle cerebral arteries, and opposite changes in the DAo, the so-called brain-sparing effect (Jouppila & Kirkinen 1984, Wladimiroff et al. 1986, Vyas etal. 1990, Bilardo et al. 1990). The ratio between umbilical artery and fetal MCA PIvalues has been demonstrated to be the best predictor of adverse perinatal outcome(Wladimiroff et al. 1987). In addition, blood flow velocities in renal, adrenal andabdominal arteries have been investigated, but their clinical significance is unclear (Mariet al. 1995, Rizzo et al. 1995b, Tekay & Jouppila 2000).
During the second half of gestation, the blood velocity waveform pattern in DAo isnormally continuous throughout the cardiac cycle due to low vascular resistance inplacental circulation. It has been demonstrated that weight-indexed blood flow of DAoremains stable until 37 weeks of gestation, after which it slightly decreases (Marsal et al.1987). In normal pregnancies, the total blood flow in the aorta has been demonstrated tocorrelate well with heart size and left ventricular output (Rasanen et al. 1988). Inpregnancies complicated by hypertensive disorders, fetal aortic velocities were lower andthe waveform indices were higher than in normal pregnancies, especially in FGR(Jouppila & Kirkinen 1984, Rasanen et al. 1988). In addition, fetuses in diabeticpregnancies had smaller volume blood flow in DAo than in the normal pregnancies(Rasanen et al. 1988). The ratio between DAo and MCA PI values has been used todescribe redistribution of arterial circulation (Harrington et al. 1995).
The myocardium receives approximately 8% of the LVCO (Baschat et al. 1998).Technical development has enabled the visualization of the fetal coronary arteries. Theright and left coronary arteries originate from the aortic sinuses. They supply themyocardium with highly oxygenated blood returning from the placenta via DV and FO toleft ventricle. In appropriately grown fetuses with normal perinatal outcome, coronaryartery blood flow was visualised in 10% of the fetuses after 31 gestational weeks with amedian of 37 gestational weeks (Baschat et al. 1997). In FGR, the coronary artery bloodflow was detected already at 27 gestational weeks in 20% of cases (heart-sparing effect).Baschat et al. (Baschat et al. 1997) also showed that visualization of coronary artery

35
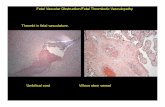
blood flow was associated with a 50% mortality rate in FGR. Hypoxemia may causevasodilatation of the coronaries and increase their blood flow to a demonstrablemagnitude (Fig. 5) (Gembruch & Baschat 1996).
Fig. 5. Coronary artery blood velocity waveform of a growth-restricted 32 week fetus (heartsparing effect).
Two parallel circulatory systems (right and left ventricles) in the fetus are connected toeach other by the aortic isthmus. Aortic isthmus blood velocity waveforms are recordedby placing the Doppler gate along the aortic arterial bridge between its junction with leftsubclavian arteria and DA (Fig. 6) (Fouron et al. 1994). Experimental studies havedemonstrated that an increase in placental resistance causes changes in aortic isthmusblood flow profile before any significant change in umbilical artery Doppler bloodvelocity waveforms occurs (Bonnin et al. 1993). In normal human pregnancies, fetalaortic isthmus may reveal a short retrograde blood velocity waveform component duringthe last trimester which may indicate normal physiologic increase in the placentalvascular resistance or in the fetal systemic vascular resistance (Fig. 6) (Fouron et al.1994, Rasanen et al. 1996b). In addition, fetal lamb studies have suggested that thedelivery of oxygen to the brain is preserved as long as the net blood flow in the fetalaortic isthmus is antegrade. In cases with retrograde aortic isthmus net blood flow, thedelivery of oxygen has been shown to be diminished despite slightly increased volumeblood flow to the cerebral arteries (Fouron et al. 1999).

36
Fig. 6. In the sagittal view of the fetus, the aortic arch and the location of the aortic isthmus(white triangle) are shown. Aortic isthmus blood velocity waveform profile with a) normalblood flow pattern in an uncomplicated pregnancy, b) antegrade net blood flow (antegrade/retrograde ratio of 2.0), and c) retrograde net blood flow with a corresponding value of 0.54 inpregnancies complicated by placental insufficiency.
2.3.5 Fetal venous circulation
Studies on fetal lambs have shown that oxygenated blood returning from the placenta viaumbilical veins flows through the DV and left hepatic vein (LHV), and is mainly directedtowards FO and the left atrium (Edelstone & Rudolph 1979). This has been confirmedalso in human fetuses (Kiserud et al. 1991, Kiserud et al. 2000a). Compared to the 40–50% shunting of umbilical blood through DV found in animal experiments, the degree ofshunting in the human fetus under physiologic conditions is reported to be less: 30–40%at 20 weeks and decreasing to 18% at 32 weeks, and to 15% at 38 weeks of gestation(Kiserud 2000b, Bellotti et al. 2000). The decrease in weight-indexed DV volume bloodflow was from 60 ml/min/kg to 17 ml/min/kg (Bellotti et al. 2000). The right hepatic veinand IVC carry the lowest oxygen saturated blood mainly from the fetal lower body to theright atrium and across the tricuspid valve to the right ventricle (Rudolph 1985). Thedistribution of the venous return is designed to optimize adequate oxygen supply to themyocardium and the brain. It has been estimated in studies on fetal lamb that duringhypoxemia or reduced umbilical flow, the blood shunted across DV increases and couldreach as much as 70% of the umbilical blood flow (Behrman et al. 1970, Edelstone &Rudolph 1979). Active dilatation of DV and increased shunting has also been observed inhuman fetuses (Gennser 1992, Bellotti et al. 1998).

37
Pulsation in the umbilical vein is a normal finding during the first trimester ofpregnancy, and normally pulsations have completely disappeared after 13 gestationalweeks (Rizzo et al. 1992). Umbilical venous pulsations in ventricular systole aresometimes seen in oligohydramniotic fetuses and fetuses with an arteriovenous fistula.The systolic pulsation is thus transmitted directly to the local venous flow signal (Nakai etal. 1997). In severe placental insufficiency, pulsations in umbilical vein, increased reverseflow component in the fetal IVC and hepatic veins, and decreased or reversed flowcomponent in DV during the atrial contraction have been observed (Fig. 7) (Hecher et al.1995a). Umbilical venous pulsations during atrial systole are atrial contraction pressurewaves which are transmitted from the right atrium back to the venous circulation (Huhta1997). Elevated end-diastolic pressure in the right ventricle causes an increase in reversalof flow during atrial systole in IVC, hepatic veins, DV and umbilical vein. The changes inumbilical venous velocities originate in the fetal venous system and are transmitted to,rather than from, the placenta (Reed & Anderson 2000). The distance that these pressurewaves are transmitted is proportional to the central venous pressure, the venouscompliance and the force of atrial contraction (Reuss et al. 1983, Indik et al. 1991,Gudmundsson et al. 1991). Gudmunsson et al. (Gudmundsson et al. 1996) have alsodescribed umbilical venous pulsations in severe placental insufficiency (AEDV) withnormal IVC and DV Doppler findings. They speculated that atrial pulsations transmittedthrough severely insufficient placenta could explain this finding. The volumetric bloodflow values between umbilical arteries and umbilical vein seem to correspond with eachother (Ferrazzi et al. 2000). Blood flow through the umbilical vein increases significantlybetween 20 and 38 weeks of gestation with no significant decrease (from 123 ml/min/kgto 109 ml/min/kg) in weight-indexed umbilical vein volume blood flow (Bellotti et al.2000). Umbilical vein volumetric blood flow in fetuses with abnormal umbilical arteryblood velocity waveforms (63–98 ml/min/kg) was significantly lower than in controlfetuses (117–124 ml/min/kg) at any gestational age between 25 and 38 weeks (Ferrazzi etal. 2000).
Growth-restricted fetuses with abnormal blood velocity waveforms in their IVC hadsignificantly increased ANP levels, indicating increased systemic venous pressure inthese fetuses (Capponi et al. 1997). In addition, it has been demonstrated that growth-restricted fetuses with pathological umbilical venous pulsations have significantly lowerpH and pO2 values and higher pCO2 values than those without pulsations (Rizzo et al.1995c). Gudmundsson et al. (Gudmundsson et al. 1991) reported only four survivers outof 14 fetuses with nonimmune hydrops and umbilical venous pulsations, while all thefetuses without umbilical venous pulsations survived. With further widening of the ductusvenosus and/or increased central venous pressure, the pulsating pattern in the umbilicalvein may become biphasic. Double umbilical venous pulsations are associated with worseperinatal outcome than single pulsations (Hofstaetter et al. 2001).

38
Fig. 7. Normal (left panel) and abnormal (right panel) blood velocity waveforms of ductusvenosus (top), left hepatic vein (middle) and umbilical vein (bottom).
2.4 Cardiac troponin T (cTnT)
2.4.1 Release and elimination of cTnT
Three troponin T genes have been described on the basis of molecular cloning in humans(Townsend et al. 1994). These are expressed in a tissue-specific manner and encode thetroponin T isoforms expressed in cardiac muscle, slow skeletal muscle, and fast skeletalmuscle, respectively. Multiple cTnT messenger RNAs have been shown to be present inthe human heart, and a single-copy gene locus is mapped to the long-arm of chromosome1 (Townsend et al. 1994). The human heart expresses four cTnT isoforms (cTnT1 throughcTnT4) (Townsend et al. 1994, Saba et al. 1996). Cardiac troponin T is a thin filamentprotein which takes part in muscle contraction. Cardiac myocytes contain contractileproteins, which mainly form longitudinally running myofibrils. Each myofibril containsabout 60 similar consecutive contractile sarcomeres. The sarcomeres are separated fromeach other by electron microscopically-detected Z-zones. Light fibers formed from actinfibers are linked to Z-zones. The light fibers contain the tropomyosin-troponin group. Thetroponin group consists of troponin C, troponin I and troponin T. The troponin complexon the actin filament regulates the force and the velocity of muscle contraction. Troponin

39
C functions as a calcium-receptor while troponin I prevents the adenosine triphosphataseactivity when bound to actin. Troponin T fixes the troponin group to tropomyosin. Duringthe relaxation period, the troponin group is bound to actin and tropomyosin, blocking theinteraction of myosin and actin. The contraction of the sarcomere starts when calciumwill be bound by troponin C, resulting in unlinkage of troponin I and actin. Troponin C isthus the protein which triggers heart muscle contraction. (Fig. 2). It has been shown inpatients with congenital cardiac defects that cTnT4 expression is modulated by heartfailure, and it is increased in hearts that are hemodynamically highly stressed (Saba et al.1996).
After myocardial cell damage, troponins are released from the myocytes. The cTnTlevels are detectable in 3–12 hours after the myocardial injury, and the concentration is indirect proportion to the extent of myocardial injury. Mean time to peak cTnT level isabout 12–48 hours. The concentration returns to normal range after 5–14 days, which isfour times longer than with creatine kinase myocardial band isoenzyme (CK-MB)fraction, probably due to sustained release of structurally bound protein fromdisintegrating myofibrils (Donnelly & Millar-Craig 1998). Cardiac troponin T is verycardiac specific, and it is not present in the serum following nonmyocardial muscle orother tissue damage (Gerhardt et al. 1991). In contrast to CK-MB-fraction, cTnT is morecardiospecific, and persists longer and shows higher elevations relative to normal ranges(Karim et al. 1995). For these reasons, troponins are routinely used to diagnose acutemyocardial ischemia.
2.4.2 Physiologic effects of cTnT
In a study on transgenic mice, the mutant human cTnT responsible for humanhypertrophic cardiomyopathy leads to disarray, increased collagen synthesis in the heart,as well as diastolic dysfunction (Oberst et al. 1998). It has been proposed that impairedcardiac myocyte function leads to synthesis of a number of autocrine and paracrinefactors, such as angiotensin II, that not only mediate the increased collagen synthesis butalso cardiac hypertrophy. With current assays, a significant diagnostic difference does notappear to exist between cardiac troponin I and cTnT in patients with acute coronaryartery syndromes (Adams 1999).
2.4.3 Cardiac troponin T in myocardial lesions
Analysis of cTnT has shown to be effective in detecting minor myocardial injury inhuman patients with acute coronary syndromes. The low cut-off concentration (0.10 ng/ml) is based on the fact that cTnT is not increased in patients with skeletal muscle diseaseor injury, resulting in low baseline levels of cTnT in the absence of active cardiac disease.In addition, cTnT has a higher myocardial tissue content relative to CK-MB fraction,thereby increasing its clinical sensitivity to irreversible injury. Cardiac troponin I andcTnT have been detected in adult patients with advanced congestive heart failure. Their

40
concentrations correlated with the severity of congestive heart failure and suggest anassociation with worse prognosis (Del Carlo & O'Connor 1999). In pediatric patients atrisk of myocardial injury due to cardiovascular surgery or dosing of cardiotoxicdoxorubicin for acute lymphoblastic leukemia, elevations in blood cTnT related to theseverity of myocardial damage and predicted subsequent subclinical and clinical cardiacmorbidity and mortality (Lipshultz et al. 1997).
2.4.4 Pregnancy and cTnT
The serum cTnT concentration in 15 neonates of pre-eclamptic mothers (0.70 ng/ml) wassignificantly higher than that in 17 control neonates (0.10 ng/ml). Neonates withincreased cTnT demonstrated lower mitral A-wave maximum velocity (39 cm/s) thancontrol fetuses (53 cm/s), and their mitral E/A peak velocity- ratio was higher (1.75) thanthat of control fetuses (1.23) (Narin et al. 1999). In addition, elevated umbilical arteryand vein cTnT levels have been demonstrated to correlate with maternal magnesiumsulfate exposure (Shelton et al. 1999). Elevated maternal cTnT levels have beenassociated with tocolytic therapy, while umbilical artery and vein cTnT concentrationsdid not correspond with maternal cTnT levels (Adamcova et al. 1999). Cord blood cTnTlevels are reported to be unaffected by gestation, birth weight, sex, or mode of delivery(Clark et al. 2001). Cardiac troponin I and cTnT have been demonstrated to be usefulbiochemical markers for monitoring pregnant women for myocardial injury (Shivvers etal. 1999).
2.5 Atrial natriuretic peptide (ANP)
2.5.1 Synthesis and release of ANP
In 1981, de Bold and co-workers found that granule-enriched atrial extracts contained asubstance which caused natriuresis and vasodilatation (de Bold et al. 1981). Atrialnatriuretic peptide molecule was purified and sequenced two years later (Flynn et al.1983). Soon after that B- (BNP) and C-type natriuretic peptides (CNP) were found. Themain source of BNP is the cardiac ventricle, although it was initially found in porcinebrain (Sudoh et al. 1988). C-type natriuretic peptide was first localised in the nervoussystem (Sudoh et al. 1990), but later found to be produced by the endothelial cells (Sugaet al. 1992). The gene of ANP is located on chromosome 1 in humans. Transcription ofthe ANP gene yields a messenger RNA species that encodes a 151-amino acid pre-proANP precursor containing a 25-amino acid signal sequence. This signal peptide isimportant for the translocation of pre-proANP from the ribosome into the sarcoplasmicreticulum. Pre-proANP is converted after cleavage of the signal peptide to a 126-aminoacid proatrial natriuretic peptide, proANP1–126 which is the principal storage form of

41
ANP. The ProANP1–126 is transported through the Golgi complex to secretory granulesof atrial cardiocytes, and finally released by exocytosis to the extracellular space(Ruskoaho 1992). It is cleaved to the N-terminal fragment (proANP1–98 = NT-proANP)and the major biologically active hormone (C-terminal peptide ANP99–126), which ismore commonly marked as ANP1–28 or ANP (Michener et al. 1986, Sundsfjord et al.1988, Thibault et al. 1988). Thus NT-proANP and ANP are produced in equimolaramounts. Cleavage of ProANP1–126 into ANP and NT-proANP occurs in connection withexocytosis, presumably by the membrane-bound endonuclease Corin (Yan et al. 2000).The circulating 28-amino acid human ANP is the biologically active form (Misono et al.1984).
The ANP gene is very actively expressed in fetal and neonatal ventricle (Sagnella1998). Soon after birth, the ANP expression in the ventricles decreases to very low levels,but it can be re-induced by increased ventricular load. Elevation of the activity of ANPgene represents the return to fetal phenotype in response to ventricular load, together withthe induction of genes of skeletal alpha actin, myosin light chain 1 and beta-tropomyosin(Nadal-Ginard & Mahdavi 1993). In addition, detectable levels of ANP messenger RNAhave been found in central nervous system, lung, adrenal gland, kidney and vasculartissue. However, these levels are less than 1% of those detected in the atria, and thereforeunlikely to have significant contribution to ANP plasma concentrations (Rosenzweig &Seidman 1991, Ruskoaho 1992).
2.5.2 Regulation of ANP release
The predominant signal for ANP release is atrial wall stretch or atrial distension due tovolume expansion (Lang et al. 1985). Hypoxia is also a potent stimulus to ANP release(Lew & Baertschi 1989). Atrial stretch, increased heart rate, sympathetic stimulus andmetabolic factors may mediate this effect (Ruskoaho 1992). Enhanced ANP releaseresulting from hyperosmolality with volume expansion has also been demonstrated(Arjamaa & Vuolteenaho 1985).
Endothelin-1, a potent vasoconstrictor of vascular smooth muscle, induces ANPsecretion directly from the heart (Mantymaa et al. 1990). Inhibition of endothelin-1receptors decreases the ANP release induced by the volume load (Leskinen et al. 1997).Endothelin-1 may also mediate atrial stretch-induced ANP release and effects of pressorhormones on the stress-activated release of ANP (Ruskoaho 1992). Endothelium- orendocardium-derived nitric-oxid may have an inhibitory effect on ANP secretion(Leskinen et al. 1995). In addition, cathecholamines (Ruskoaho 1992), acethylcoline(Ruskoaho et al. 1985), angiotensin, arginine vasopressin, prostaglandins (Ruskoaho1992) and both glucocorticoids and thyroid hormones increase circulating ANP levels(Rosenzweig & Seidman 1991).

42
2.5.3 Physiologic effects of ANP
Atrial natriuretic peptide exerts its effects by binding to specific membrane-boundreceptors. Three natriuretic peptide receptors have been identified. The ANPA and ANPBreceptors have guanylate cyclase activity and mediate the biological effects of thenatriuretic peptides. The ANPC receptor functions mainly as a clearance receptorremoving ANP from the circulation. All natriuretic peptides are bound by the ANPCreceptor. Atrial natriuretic peptide and BNP act through the ANPA receptor and CNPthrough the ANPB receptor (Yandle 1994)
The main targets of ANP are kidneys and vascular smooth muscle. It decreases bloodpressure due to a direct relaxation of vascular smooth muscle. In addition, it increases saltand water excretion, enhances capillary permeability, and inhibits the release or action ofseveral hormones, such as aldosterone, angiotensin II, endothelin, renin and vasopressin(Ruskoaho 1992). The natriuretic effect results from a direct inhibition of sodiumabsorption in the renal collecting duct, increased glomerular infiltration and inhibitedaldosterone production and secretion (Rosenzweig & Seidman 1991). Atrial natriureticpeptide therefore counteracts the renin-angiotensin-aldosterone system. Thus, increasedadult ANP levels are detected in adult congestive heart failure, chronic renal failure andin severe essential hypertension (Ruskoaho 1992, Yandle 1994).
2.5.4 Elimination of ANP
The half-life of ANP is 2 to 5 minutes in humans and its metabolic clearance rate is about14 to 25 ml/min/kg (Rosenzweig & Seidman 1991). The hormone is eliminated eitherenzymatically or through the clearance receptor. The ANPC receptor internalizes ANPand delivers it to lysosomes for degradation while the receptor itself is recycled. The mostimportant enzyme regulating elimination of ANP is neutral endopeptidase 24.11. It ispresent in the glomerulus, in kidney smooth muscle cells, and at high levels in brushborder membranes of the proximal tubule. It is also expressed in vascular tissue andvascular smooth muscle cells (Yandle 1994). Experimental and human studies haveshown that inhibition of this enzyme potentiates the action of ANP (Kukkonen et al.1992). Inhibitors that inhibit both angiotensin converting enzyme and neutralendopeptidase 24.11 are more potent antihypertensive agents than pure angiotensinconverting enzyme- inhibitors (Backlund et al. 2001).
2.5.5 N-terminal peptide of proatrial natriuretic peptide (NT-proANP)
N-terminal peptide of proatrial natriuretic peptide (NT-proANP) is secreted in equimolaramounts with ANP (Itoh et al. 1988). Its high plasma concentration relative to ANP isprobably due to its longer half-life in the circulation (Thibault et al. 1988). This isreflected in the proportionally larger increase in NT-proANP levels (15–20 fold)

43
compared with those of ANP (4–5 fold) in subjects with congestive heart failure andchronic renal failure (Yandle 1994). Elevated circulating NT-proANP levels in cases ofcongestive heart failure have been suggested to demonstrate symptomless left ventriculardysfunction (Lerman et al. 1993, Kettunen et al. 1994). The NT-proANP is eliminated bythe kidney. Demonstration of biological activity of NT-proANP has failed, probably dueto the absence of a specific receptor for the N-terminal fragment (Ruskoaho 1992). TheNT-proANP assay can be used to characterize endogenous ANP secretion (Itoh et al.1988).
2.5.6 Pregnancy and ANP
Conflicting data concerning the synthesis or storage of ANP in the placenta have beenpublished (Inglis et al. 1993, McQueen et al. 1993). In experimental studies on rats, it hasbeen shown that ANP does not cross the placenta (Mulay & Varma 1989). The lack ofcorrelation between maternal and neonatal ANP concentrations supports the view thatANP does not cross the human placenta either (Shilo et al. 1989).
The fetus appears to produce its own atrial natriuretic factor (Hatjis et al. 1989). ANPhas been detected as early as 16 gestational weeks in umbilical venous blood samplestaken by cordocentesis (Ville et al. 1994). Fetuses with Rhesus isoimmunisation,characterized by long-term cardiac overload, showed significantly higher ANP levelscompared to the controls (Kingdom et al. 1991, Ville et al. 1994, Walther et al. 2001).Fetal ANP levels correlated inversely with hematocrit, but not with gestational age. FetalANP levels showed a significant rise after transfusion, and this rise was related to thepercentage of fetoplacental blood volume transfused. The changes in fetal ANP levels dueto the volume expansion have been demonstrated as early as 21 weeks’ gestation (Panoset al. 1989). A weak, statistically non-significant, correlation was found between thechange in fetal ANP levels and transient reductions in umbilical artery S/D-ratio(Kingdom et al. 1991). Increased neonatal ANP-levels as well as increased fetal umbilicalvenous ANP-levels have been reported in cases with growth restriction (Kingdom et al.1992, Ville et al. 1994) and in fetuses born to patients with severe preeclampsia (Hatjis etal. 1989). In addition, studies on mice suggest that the maternal diabetes-induced increasein fetal ANP might be related to fetal myocardial hypertrophy (Mulay et al. 1995).
2.6 Validation of cTnT and NT-proANP assays
The performance of cTnT assay at low levels and the analytical limit of sensitivity havebeen established in children (Lipshultz et al. 1997). A precision study using serialdilutions of serum with elevated cTnT showed the linearity of the serum cTnT assay inthe low range of 0.01 to 0.10 ng/ml (Lipshultz et al. 1997).
The sensitivity of the NT-proANP assay is 30 pmol/l with good reproducibility. Theantiserum cross-reacts 100% with the purified human proatrial natriuretic peptide1–126,whereas it does not recognize ANP itself, BNP or CNP (cross-reactivity less than 0.01%).

44
In the radioimmunoassay, the intra- and interassay coefficients of variation were less than10% and 15%, respectively (Vuolteenaho et al. 1992). NT-proANP circulates in plasma inhigher concentration, and it is more stable ex vivo than ANP. NT-proANP is shown tocorrelate well with ANP (Boomsma et al. 1996).

3 Hypothesis of the study
In placental insufficiency, several abnormalities in the fetal cardiovascular hemodynamicshave been described by Doppler ultrasonography. The first hypothesis to be tested in thisstudy is that there exists a relationship between Doppler ultrasonographic parameters andbiochemical markers of fetal cardiac dysfunction and myocardial cell damage inpregnancies complicated by placental insufficiency and/or fetal growth restriction. Fetallamb studies have revealed that oxygen content of the blood entering the cerebralcirculation is maintained until aortic isthmus net blood flow becomes retrograde. Thesecond hypothesis of this thesis is that retrograde aortic isthmus net blood flow isassociated with changes in fetal cardiac function and peripheral arterial and venouscirculations.
The specific aims of the research project are:
1. To determine the significance of atrial pulsations in the umbilical vein as regards tobiochemical evidence of fetal cardiac dysfunction and cell damage (I–II).
2. To establish Doppler ultrasonographic parameters of human fetal central andperipheral circulations that are associated with biochemical evidence of cardiacdysfunction and myocardial cell damage (III).
3. To find the changes in fetal cardiac hemodynamics associated with diminishedoxygen delivery to cerebral circulation in fetuses with signs of placental insufficiency(IV).
4. To demonstrate the changes in fetal peripheral arterial and venous circulations whichare associated with retrograde net blood flow in the aortic isthmus in fetuses withplacental insufficiency (V).

4 Material and Methods
4.1 Study populations
The subjects were recruited to the study protocols from the Department of Obstetrics andGynecology at Oulu University Hospital in Oulu, Finland, during the years 1998–2001.All the study designs were prospective and cross-sectional. The gestational age wasconfirmed by ultrasonographic examination prior to 20 weeks of gestation in all cases.Cases with abnormal karyotype and major structural anomalies were excluded. The studyprotocols were approved by the local ethics committee and the subjects gave theirinformed consent before entering the study protocol. Table 1 describes the study groupsand the Doppler ultrasonographic parameters and biochemical analyses used. In study III,the decision to break the study population into three subgroups based on umbilical arterialconcentrations of fetal NT-proANP and cTnT was made prior to data analysis. Inaddition, in study III, the investigators obtaining and analyzing ultrasonographic datawere blinded to the biochemical data. In studies I, II, IV and V, the control groupconsisted of subjects with uncomplicated pregnancy and labor studied between the samegestational weeks as the study populations. The neonatal birth weights in control groupwere between the 10th and 90th percentile. Dianostic criteria of maternal hypertensivedisorder followed American College of Obstetricians and Gynecologists guidelines(Committee on Technical Bulletins of the American College of Obstetricians andGynecologists 1996). Placental insufficiency was defined as an abnormal umbilical arteryblood velocity waveform profile (umbilical artery S/D ratio>3.5) (Forouzan et al. 1991).Fetal growth restriction was defined as a birth weight below the 10th percentile growthcurve. In study II, the diagnostic criteria for fetal acidemia was umbilical artery pH< 7.10at birth.

47
Table
1. C
hara
cter
istic
s of
the
mat
eria
l and
met
hods
use
d in
the
diffe
rent
stu
dies
of t
his
rese
arch
pro
toco
l. In
stu
dies
III
–V, a
ll ex
cept
cont
rol f
etus
es su
ffere
d fro
m fe
tal g
row
th re
stri
ctio
n an
d/or
pla
cent
al in
suffi
cien
cy.
Stud
y I
Stud
y II
Stud
y II
ISt
udy
IVSt
udy
V
Incl
uded
subj
ects
1) 6
6 co
ntro
ls2)
Mat
erna
l hyp
erte
nsiv
e di
sord
er a
nd n
o at
rial
puls
atio
ns in
UV,
n=3
23)
Mat
erna
l hyp
erte
nsiv
e di
sord
er w
ith a
trial
pu
lsat
ions
in U
V, n
=5
1) 5
0 co
ntro
ls2)
Mat
erna
l hyp
erte
nsiv
e di
sord
er an
d no
atria
l pul
satio
ns
in U
V, n
=22
3) M
ater
nal h
yper
tens
ive
diso
rder
with
atri
al p
ulsa
tions
in
UV,
n=5
4) F
etal
aci
dem
ia (p
H<7
.10)
du
ring
labo
r, n
=27
1) F
etus
es w
ith n
orm
al N
T-pr
oAN
P an
d cT
nT le
vels
, n=
122)
Fet
uses
with
ele
vate
d N
T-pr
oAN
P an
d no
rmal
cTn
T le
vels
, n=2
53)
Fet
uses
with
ele
vate
d N
T-pr
oAN
P an
d cT
nT le
vels
, n=
11
1) 4
3 co
ntro
ls2)
Fet
uses
with
an
tegr
ade a
ortic
isth
mus
ne
t blo
od fl
ow, n
=18
3) F
etus
es w
ith
retro
grad
e ao
rtic
isth
mus
net
blo
od fl
ow,
n=11
1) 3
1 co
ntro
ls2)
Fet
uses
with
ant
egra
de
aorti
c is
thm
us n
et b
lood
flo
w, n
=18
3) F
etus
es w
ith re
trogr
ade
aorti
c is
thm
us n
et b
lood
flo
w, n
=11
Bio
chem
ical
mar
kers
UA
cTn
TU
A N
T-pr
oAN
PU
A N
T-pr
oAN
P U
A c
TnT
Ultr
ason
ogra
phic
par
amet
rsU
tA P
IU
tA n
otch
UA
S/D
- rat
ioPu
lsat
ions
in IA
UV
UtA
PI
UtA
not
chU
A S
/D- r
atio
Puls
atio
ns in
IA U
V
UA
PI,
DA
o PI
, MC
A P
IA
OI T
VI
Hea
rt sp
arin
g ef
fect
CC
O, L
VC
O, R
VC
ORV
eFo,
LVe
FoIR
T, IM
PTV
and
MV
TV
ITR
and
MR
RVFS
, LV
FSC
C/T
C- r
atio
IVC
, LH
V a
nd D
V P
IVPu
lsat
ions
in IA
UV
UA
S/D
- rat
ioA
OI T
VI
CC
/TC
- rat
ioC
CO
, LV
CO
, RV
CO
QD
A, Q
P,QFO
RVeF
o, L
VeFo
TV a
nd M
V T
VI
IRT,
IMP
UA
S/D
– ra
tio a
nd P
IA
OI T
VI
DA
o, P
PA a
nd M
CA
PI
DA
PI
Hea
rt sp
arin
g ef
fect
RVFS
, LV
FSRV
D, L
VD
TR a
nd M
RIV
C a
nd D
V P
IVPu
lsat
ions
in IA
UV

48
4.2 Ultrasonographic measurements
Image-directed pulsed and color Doppler ultrasonographic equipment (Acuson Sequoia512, Mountain View, CA, USA) was used with a 4–8 MHz convex or a 5 MHz sectorprobe. The high pass filter was set at minimum. The acoustic output of the system wascontrolled according to ECMUS recommendations (European Committee for MedicalUltrasound Safety 1999a, European Committee for Medical Ultrasound Safety 1999b).All the ultrasonographic data were videotaped and analyzed afterwards, using theultrasound equipment’s own cardiac measurement package. A Doppler waveformobtained at an angle of less than 15 degrees between the vessel and the Doppler beamwas accepted for analysis. Three consecutive cardiac cycles were analyzed from Dopplervelocity waveforms and their mean values were used for further analysis. For the volumeblood flow calculations, the cross-sectional diameters of vessels were measured fromfrozen real-time images during systole by using the leading edge-to-leading edge method.Three separate measurements of vessel diameters were taken, and the mean value wasused for further analysis. Calculations of CSAs of the arteries were based on theassumption that the cross-sections of the vessels were circular.
4.2.1 Uterine and umbilicoplacental hemodynamics
The main uterine arterial Doppler recordings were obtained from the placental side. If theplacenta was centrally located, both uterine arteries were examined and the mean valuewas used in the analysis (I, II). Placental circulation was assessed by determining the PI-values of umbilical artery in free loops of the umbilical cord (I–V). Fetal heart rate wasobtained from umbilical artery Doppler tracings (I–V).
4.2.2 Cardiac measurements
4.2.2.1 Volume blood flows
Volumetric blood flows across aortic (AoV) and pulmonary (PV) valves and DA werecalculated directly (Q=CSAxTVIxFHR) (III–IV). The TVIs were obtained byplanimetering the area underneath the Doppler spectrum. The LVCO equals the QAoV, theRVCO equals the QPV, and their sum is CCO (III, IV). The pulmonary volume blood flow(QP) was calculated indirectly by the formula: RVCO-QDA (IV). Foramen ovale volumeblood flow (QFO) was estimated as LVCO-QP (IV). The distribution (%) of CCO andweight-indexed CCO, LVCO, RVCO, QDA, QP and QFO were calculated (III, IV). Theactual birth weight was used for indexing purposes when the time interval between thelast ultrasonographic examination and delivery was equal to or less than 4 days (III–V).In other cases, fetal weight estimation was based on the measurements of biparietal

49
diameter, head and abdominal circumferences and femur length, which is considered tobe the most reliable method with 7.5% standard deviation (SD) (Hadlock et al. 1984)(IV–V).
4.2.2.2 Time-intervals
Left ventricular time-intervals were assessed by placing the Doppler gate into the leftventricle and obtaining simultaneously MV and AoV blood velocity waveforms (III, IV)(Fig. 4). The IRT was measured as the time period between the end of ejection and theonset of filling (Tulzer et al. 1994). The proportion (%) of IRT of the total cardiac cyclewas calculated (III, IV). An index of myocardial performance (IMP) was calculated bythe formula: IMP=(ICT+IRT)/ET, in which ICT is isovolumetric contraction time and ETis ejection time (Tei et al. 1995, Tsutsumi et al. 1999) (III, IV).
4.2.2.3 Ventricular ejection forces
Right (RVeFo) and left (LVeFo) ventricular ejection forces were calculated by theformula: (1.055xCSAxTVIac)x(PSV/ TTP), in which TVIac is the TVI duringacceleration period in systole, PSV is the peak systolic velocity, and TTP is the timeperiod from the onset of ejection to systolic peak velocity (Isaaz et al. 1989, Sutton et al.1991b, Rizzo et al. 1995a) (III,IV). Both ventricular ejection forces were weight-indexed.
4.2.2.4 Inflow waveforms
Inflow blood velocity waveforms were recorded at the level of the tricuspid (TV) andmitral (MV) valves. The total TVI of TV and MV, as well as the TVI ratio of E- (earlypassive filling) and A- (atrial contraction) waves (E/A TVI), and A-wave TVI/total TVI-ratio of both valves were calculated (Tulzer et al. 1994) (III, IV).
4.2.2.5 Valvular regurgitation
The presence of tricuspid (TR) and mitral (MR) regurgitations were noted. Tricuspidregurgitation was classified as trivial (nonholosystolic ≥ 72ms) or holosystolic (Fig. 3)(Respondek et al. 1994)(III, V).

50
4.2.2.6 M-mode measurements
M-mode recordings were obtained by placing the M-mode cursor perpendicularlytowards the interventricular septum at the level of atrioventricular valves in a fourchamber-view of the heart. From M-mode recordings, right and left ventricular innerdiameters (RVD, LVD) were measured during diastole and systole (III, V). Right and leftventricular fractional shortenings (RVFS, LVFS) were calculated (ventricular fractionalshortening [%]=[(inner diastolic diameter-inner systolic diameter)/inner diastolicdiameter]x100) (DeVore et al. 1984) (III, V).
4.2.3 Fetal arterial circulation
Middle cerebral artery blood velocity waveforms were obtained by placing the Dopplergate along the course of MCA (III, V). Blood flow in the DAo was assessed at the levelof the diaphragm (III, V), and blood velocity waveforms of branch pulmonary artery(PPA) were recorded immediately after the bifurcation of the main pulmonary arterybefore the first branch of the right or left pulmonary artery (IV, V). Distribution of arterialcirculation was obtained by using MCA, DAo and PPA PI values which describe vascularimpedance in the arterial circulation (III–V). In addition, umbilical artery/MCA PI- andDAo/MCA PI- ratios were calculated (III, V). Aortic isthmus blood velocity waveformswere recorded (Fig. 6) (III–V). Time-velocity- integrals of antegrade and retrogradecomponents were measured and their ratio was calculated. Net blood flow was consideredantegrade if the ratio was equal to or more than 1, and retrograde when the ratio was lessthan 1 (Fouron et al. 1999). In addition, the visualization rate of coronary arterycirculation by color and pulsed Doppler ultrasonography demonstrating heart sparingeffect was noted (Baschat et al. 1997) (Fig. 5) (III, V).
4.2.4 Fetal venous circulation
From fetal venous circulation, blood velocity waveforms of DV, LHV and IVC wereassessed and their pulsatility index values for veins (PIV) were calculated (III, V).Pulsations occurring during atrial contraction in intra-abdominal umbilical vein and infree loops of umbilical vein were noted (Fig. 7.) (I–III, V).
4.2.5 Ultrasonographic parameters of the fetal heart
Absolute and weight-indexed cardiac outputs, QP, QDA and QFO were calculated and thedistribution of cardiac output was assessed. Systolic function of the heart was describedby weight-indexed RVeFo and LVeFo. Right ventricular contractility was assessed by

51
using dP/dT values in the presence of TR. Diastolic function was assessed by using IRT%and the E/A TVI- and A TVI/total TVI- ratios of atrioventricular valves. The Index ofmyocardial performance was used to describe the combined systolic and diastolicperformance of the heart. The CC/TC- ratio, RVFS, LVFS and both TR and MR wereused to describe the afterload of the heart.
4.2.6 Analysis of cardiac biochemical markers
Immediately after delivery, at least 1 ml of blood was collected from the umbilical arteryand centrifuged (I–III). In a subgroup (n=62) of subjects in studies I–II, blood samples ofat least 1 ml from the umbilical vein and 5 ml from the maternal cubital vein weresimultaneously taken. The serum samples were stored at –80ºC until analyzed.
4.2.7 Cardiac troponin T
Serum cTnT concentrations were measured with commercially available enzyme-linkedimmunosorbent assay kits (Enzymum-Test Troponin T; Boehringer Diagnostics,Mannheim, Germany) according to manufacturer’s instructions.
4.2.8 N-terminal peptide of proatrial natriuretic peptide
The NT-proANP radioimmunoassay was carried out directly on unextracted plasmasamples. Plasma samples in duplicates of 25 µL were incubated with rabbit antiserum and125I-labelled human Tyrosine0-proatrial natriuretic peptide79–98 overnight at 4ºC. Thebound and free fractions were separated with sheep antirabbit gammaglobulin antiserumin the presence of 8% polyethyleneglycol 6000. Synthetic human proatrial natriureticpeptide79–98 was used as a standard.
4.3 Statistical analysis
Statistical analysis was performed by using the analysis of variance when comparisonswere made between more than two groups and the data were normally distributed. Ifstatistical significance was shown, the Scheffe F-test was used for further analysis. If thedata were not normally distributed, the nonparametric Kruskal-Wallis test was chosen.Between two groups, comparisons were made by using Student t-test if the data werenormally distributed. Otherwise, the Mann Whitney U-test was chosen. In study II, linearregression analysis was used to show the relationship between umbilical vein and artery

52
and maternal cubital vein NT-proANP. The categorical data were compared using thechi-square test. Linear regression analysis was used to show the relationship of NT-proANP concentration to PIV values in venous circulation (III). A p value of 0.05 or lesswas selected as the level of statistical significance.

5 Results
5.1 Validation of methodology
5.1.1 Intra-observer variability of Doppler ultrasonographic measurements
The intra-observer variability in ultrasonographic measurements was tested in thedifferent study populations by performing two ultrasonographic examinations. Thesecond examination was performed 1–2 hours after the first examination. In study III, 17subjects with uncomplicated pregnancy and 6 subjects with placental insufficiency wereincluded in the analysis of the intra-observer variability. The mean intra-observervariability of RVCO and LVCO calculations varied from 5.4% to 6.3% (95% CI 4.7%–7.9%). The corresponding variability of ventricular ejection force calculations was 8.8%(95% CI 4.7%–12.8%). The mean intra-observer variabilities of MV and TV total TVIcalculations ranged from 5.0% to 5.2% (95% CI 2.4%–7.8%). In time-intervalcalculations, the corresponding variability was from 8.0% to 9.7% (95% CI 5.4%–13.2%), respectively. In the umbilical artery, DAo and MCA PI calculations, the meanintra-observer variability ranged from 3.9% to 6.0% (95% CI 2.5%–9.5%). The meanintra-observer variability of DV, LHV and IVC PIV calculations ranged from 3.8% to5.8% (95% CI 1.9% –7.5%).
Twelve fetuses in study IV showed that the mean intra-observer variability ofvolumetric blood flow measurements across AoV, PV and DA and direct QP calculationvaried from 5.0% to 8.5% with 95% CI of 2.9%–11.1% In addition, the correlationbetween indirect and direct QP in study IV was good (R= 0.93, p=0.0001). The meanintra-observer variability of MV and TV TVI calculations ranged from 1.9% to 10.9%(95% CI 0.6%–18.1%). In ventricular ejection force calculations, the mean variabilitywas 7.3% (95% CI 3.5%–11.0%).

54
In study V, reproducibility and intra-observer variability of PIV calculations for theDV and IVC were analyzed in control group fetuses (n=31). The mean intra-observervariability of DV calculations was 9.3% (95% CI 6.2–12.4%), and in IVC calculations, itwas 8.6% (95% CI 5.3–11.8%).
5.1.2 Assays of NT-proANP and cTnT
Umbilical artery and vein NT-proANP concentrations (n=62) demonstrated a significantcorrelation (R=0.71, p<0.001), while no correlation between umbilical artery NT-proANPconcentrations and maternal cubital vein NT-proANP values (n=62) was found (R=–0.07,p=0.75) (II). There was no significant difference in NT-proANP concentrations of theumbilical artery and vein. The median (range) umbilical artery [580 pmol/l (226–3000pmol/l)] and vein [679 pmol/l (210–2730 pmol/l)] NT-proANP concentrations were bothhigher (p<0.02) than in the maternal cubital vein [479 pmol/l (229–1359 pmol/l)] (II). Nosignificant difference between umbilical vein and artery cTnT values was detected.Maternal vein cTnT concentrations were less than 0.10 ng/ml in every case (I). Mode ofthe delivery and gestational age did not affect NTproANP and cTnT concentrations, atleast after 27 gestational weeks (I–III). In neonates born after uncomplicated pregnancyand labor, the +2SD level of umbilical artery NT-proANP was 1145 pmol/l (II), whichwas chosen to represent the cut-off level in study III. The cut-off level for umbilicalartery cTnT was set at 0.10 ng/ml in studies I and III, which has been used as a level ofclinically significant myocardial cell damage in adult patients.
5.2 Increased NT-proANP production with normal cTnT concentration
5.2.1 Clinical outcome
In 6 out of 12 fetuses with placental insufficiency and normal NT-proANP (<1145pmol/l) concentrations, cesarean delivery was performed due to signs of fetal distress,while the corresponding number was 8 out of 11 cesarean deliveries among 25 fetuseswith increased NT-proANP levels (>1145 pmol/l) (III). No differences were found inantenatal and perinatal data in pregnancies complicated by placental insufficiency witheither normal or elevated NT-proANP levels (Table 2) (III). Newborns withuncomplicated pregnancy and fetal acidemia during labor had lower 5-minute Apgarscores and umbilical artery pH values than newborns with uncomplicated pregnancy andlabor. In addition, newborns with acidemia during labor had lower pH values compared tonewborns of pregnancies complicated by maternal hypertensive disorders.

55
Table
2. P
erin
atal
dat
a in
the
diffe
rent
stu
dies
of t
his
rese
arch
pro
toco
l. In
stu
dies
III
–V, a
ll ex
cept
con
trol f
etus
es s
uffe
red
from
feta
lgr
owth
rest
rict
ion
and/
or p
lace
ntal
insu
ffici
ency
. All
valu
es a
re g
iven
as m
ean
(SD
) or m
edia
n (r
ange
).
GA
at s
tudy
ent
ry
(wee
ks)
GA
at d
eliv
ery
(wee
ks)
Birt
h w
eigh
t (g
ram
s)U
A p
H5
min
Apg
arN
T–pr
oAN
P(p
mol
/l)cT
nT
(ng/
ml)
Stud
y I
C
ontro
ls40
.0 (1
.2)
3713
(408
)
9
(7–1
0)
0 (0
–0.1
4)N
o U
V p
ulsa
tion
37.6
(3.4
)†27
94 (9
88)†
9
(3–1
0)
0 (0
–0.1
6)U
V p
ulsa
tions
29.8
(3.0
)‡66
0 (2
69)‡
6 (2
–7)‡
0.18
(0.1
1–0.
35)‡
Stud
y II
Con
trols
39.9
(1.1
)
36
60 (3
84)
7.23
(0.0
8)
9 (7
–10)
562
(226
–161
4)N
o U
V p
ulsa
tion
37.8
(3.0
)*
28
19 (9
44)†
7.20
(0.0
6)
9 (5
–10)
89
8 (3
20–3
000)
†U
V p
ulsa
tions
29.8
(2.9
)†66
0 (2
69)†
7.
26 (0
.09)
6
(2–7
) †
46
25 (3
510–
1603
0)†
Feta
l aci
dem
ia (p
H<7
.10)
du
ring
labo
r 40
.2 (1
.5)
3391
(510
)
7.05
(0.0
6) †
8 (5
–10)
* 92
5 (2
55–2
430)
†
Stud
y II
IG
roup
134
.7 (3
.7)
35.2
(3.1
)20
86 (6
77)
7.26
(0.0
6)9
(8–1
0)73
7 (4
02–1
101)
0.03
(0.0
3–0.
09)
Gro
up 2
32.9
(4.8
)33
.7 (3
.5)
1698
(578
)7.
25 (0
.05)
9 (3
–10)
1652
(116
0–74
50)
0.03
(0.0
0–0.
09)
Gro
up 3
27.9
(4.6
)**
28.1
(4.7
)**
846
(471
)†7.
21 (0
.10)
3 (1
–9) †
5179
(175
5–16
030)
†0.
35 (0
.11–
1.77
)†St
udy
IVC
ontro
ls30
.5 (4
.0)
39.5
(1.4
)32
67 (4
44)
9 (8
–10)
Ant
egra
de A
OI
29.9
(5.9
)30
.8 (4
.0)‡
1308
(724
)‡7.
24 (0
.05)
9 (2
–9)*
R
etro
grad
e A
OI
32.1
(3.8
)32
.5 (3
.9)‡
1252
(540
)‡7.
26 (0
.06)
8 (1
–9)*
*St
udy
VC
ontro
ls29
.9 (4
.9)
39.8
(1.4
)34
87 (3
51)
9 (8
–10)
Ant
egra
de A
OI
29.9
(5.9
)30
.8 (4
.0)‡
1282
(674
)‡7.
24 (0
.05)
9 (3
–9)*
Ret
rogr
ade
AO
I32
.1 (3
.8)
32.5
(3.9
)‡12
52 (5
40)‡
7.26
(0.0
6)8
(1–9
)**
* p<
0.05
vs c
ontro
ls (I
I, IV
, V),
** p
<0.0
1 vs
gro
ups 1
and
2 (I
II),
vs c
ontro
ls (I
V, V
), †
p<0.
001
vs g
roup
s 1 a
nd 2
(III
), vs
con
trols
(I, I
I), ‡
p<0
.000
1 vs
con
trols
(I, I
V, V
).

56
5.2.2 Fetal heart
5.2.2.1 Volumetric blood flows
Weight-indexed RVCO, LVCO and CCO, and RVCO% and LVCO% of CCO in fetuseswith placental insufficiency and increased NT-proANP levels did not differ from thosefetuses who suffered from placental insufficiency but had normal NT-proANP levels(Table 3).
5.2.2.2 Systolic and diastolic function of fetal heart
Weight-indexed RVeFo and LVeFo did not differ between fetuses with placentalinsufficiency and with either normal or elevated NT-proANP levels (Table 3). Rightventricular contractility as assessed by TR dP/dT was similar among these fetuses, with amean value of 717 mmHg/s and 820 mmHg/s in fetuses with normal and elevatedNT-proANP levels, respectively.
The left ventricular IRT%, the total TVIs of TV and MV, and their E/A TVI- and ATVI/total TVI- ratios did not differ between the groups. The index of myocardialperformance was similar between the groups (Table 3).
5.2.2.3 Afterload
The CC/TC ratio, RVFS and LVFS did not differ between fetuses with normal andelevated NT-proANP levels. The detection rate of TR was similar in both groups (Table3)

57
Table 3. Results of ultrasonographic measurements of cardiac function in fetuses withplacental insufficiency, and a) normal NT-proANP and cTnT, b) increased NT-proANPand normal cTnT, and c) increased cTnT levels.
5.2.3 Fetal arterial circulation
In pregnancies complicated by preeclampsia, fetuses with abnormal umbilical arteryblood velocity waveforms and normal umbilical vein blood flow profile had significantlyhigher umbilical artery NT-proANP concentrations than fetuses with normal umbilicalartery blood velocity waveforms (Table 2) (II). When fetuses suffering from placental
Normal NT-proANP(≤1145pmol/l) (n=12)
IncreasedNT-proANP (>1145 pmol/l) (n=25)
Increased cTnT (>0.10ng/ml) (n=11)
Volumetric blood flow
CCO (ml/min/kg) 523 (72) 548 (121) 499 (189)
RVCO (ml/min/kg) 299 (40) 327 (90) 272 (129)
LVCO (ml/min/kg) 223 (51) 221 (42) 226 (77)
RVCO% 57.2 (5.2) 59.1 (4.5) 53.3 (13.1)*
LVCO% 42.8 (5.2) 40.9 (4.5) 46.7 (13.0)*
Systolic function
RVeFo (mN/kg) 6.56 (1.57) 7.35 (2.91) 5.40 (3.50)
LVeFo (mN/kg) 6.18 (1.94) 6.12 (1.88) 5.96 (2.75)
Diastolic function
IRT% 13.2 (2.1) 12.6 (1.9) 13.4 (2.8)
Tricuspid valve
total TVI (cm) 5.8 (0.9) 5.5 (1.3) 5.0 (1.0)
E/A TVI 0.61 (0.29) 0.62 (0.27) 0.62 (0.35)
A TVI/total TVI 0.63 (0.13) 0.63 (0.14) 0.67 (0.18)
Mitral valve
Total TVI (cm) 5.6 (1.1) 5.0 (1.1) 4.7 (1.5)
E/A TVI 0.79 (0.22) 0.80 (0.27) 0.88 (0.35)
A TVI/total TVI 0.56 (0.08) 0.57 (0.08) 0.55 (0.09)
IMP 0.59 (0.11) 0.54 (0.09) 0.60 (0.13)
Afterload
CC/TC (%) 57.0 (4.4) 57.5 (3.3) 61.3 (6.1)*
RVFS (%) 22.6 (7.6) 26.2 (5.0) 13.2 (11.4)*
LVFS (%) 29.0 (7.3) 32.8 (6.7) 26.2 (12.0)
TR 3/12 (25%) 3/25 (12%) 6/11 (55%)*
*p<0.05 vs Increased NT-proANP

58
insufficiency were divided into the subgroups using umbilical artery NT-proANP cut-offvalue of 1145 pmol/l, no difference in PI values of umbilical artery, MCA and DAo wasfound between fetuses with normal and elevated NT-proANP levels (Table 4) (III).Neither did the DAo/ MCA PI- ratio differ between these fetuses (III). The detection rateof retrograde net blood flow in the aortic isthmus was similar among these fetuses (III).In addition, no difference between the groups was found in the visualization rate ofcoronary artery circulation (Table 4) (III).
5.2.4 Fetal venous circulation
In study III, PIV values in DV, LHV and IVC did not differ significantly between fetuseswith normal NT-proANP concentrations and fetuses with elevated NT-proANPconcentrations without myocardial cell damage (Table 4). No difference in the incidenceof atrial pulsations in the umbilical vein was detected between these two groups (Table 4)(III).
Table 4. Ultrasonographic parameters describing arterial and venous circulations infetuses with placental insufficiency, and a) normal NT-proANP and cTnT, b) increasedNT-proANP and normal cTnT, and c) increased cTnT levels.
Normal NT-proANP(≤1145pmol/l) (n=12)
Increased NT-proANP (>1145 pmol/l) (n=25)
Increased cTnT (>0.10ng/ml), (n=11)
Arterial circulation
UA PI 1.34 (0.42) 1.70 (0.88) 3.13 (1.45)**
MCA PI 1.43 (0.43) 1.42 (0.36) 1.52 (0.45)
DAo PI 2.37 (0.50) 2.58 (0.51) 2.77 (0.86)
DAo/MCA PI 1.76 (0.56) 1.95 (0.65) 2.19 (1.32)
Retrograde AOI 4/11 (36%) 7/20 (35%) 5/10 (50%)
Coronary artery visualization 4/12(33%) 4/25 (16%) 3/11 (27%)
Venous circulation
IVC PIV 2.32 (1.60-4.36) 2.33 (1.02-5.66) 4.95 (2.46-6.45)*
LHV PIV 2.50 (1.06-7.28) 3.86 (1.76-9.37) 5.84 (3.29-46.59)*
DV PIV 0.59 (0.34-1.14) 0.55 (0.26-1.24) 1.17 (0.57-3.81)**
IA UV pulsations 2/12 (17%) 4/25 (16%) 9/11 (82%)*
*p<0.01 vs groups 1 and 2, ** p<0.001 vs groups 1 and 2

59
5.2.5 NT-proANP and acidemia during the labor
Newborns with uneventful pregnancy and acidemia during labor had significantly(p<0.001) higher NT-proANP concentration than newborns with uncomplicatedpregnancy and labor (Table 2) (II). The umbilical artery NT-proANP concentrations innewborns with acidemia during labor did not differ from those detected in newborns ofhypertensive pregnancies with normal nonpulsatile umbilical vein blood velocitywaveform profile (Table 2) (II).
5.3 Increased fetal cTnT concentration
5.3.1 Clinical outcome
Gestational age at delivery, birth weights and 5-minute Apgar scores were significantlylower in fetuses with elevated cTnT (>0.10 ng/ml) levels than in fetuses with normalcTnT values (<0.10 ng/ml) (Table 2). The umbilical artery pH values of the newborns didnot differ between the groups (Table 2). Cesarean delivery was performed due to signs offetal distress in 8 out of 11 cases (III).
5.3.2 Fetal heart
Fetuses with myocardial cell damage had significantly higher neonatal umbilical arteryNT-proANP concentrations than fetuses with normal umbilical artery cTnTconcentrations (Table 2) (III).
5.3.2.1 Volumetric blood flows
No difference in weight-indexed RVCO, LVCO and CCO was found between fetuseswith placental insufficiency and either elevated or normal cTnT levels (Table 3) (III).However, in fetuses with elevated cTnT levels, the LVCO% was greater (p<0.05) and theRVCO% was less (p<0.05) than in fetuses with only increased NT-proANP levels (Table3) (III).

60
5.3.2.2 Systolic and diastolic function of fetal heart
Weight-indexed RVeFo and LVeFo of fetuses with elevated cTnT levels did not differfrom those of fetuses with normal cTnT concentrations (Table 3) (III). In addition, rightventricular contractility assessed by TR dP/dT was similar in fetuses with myocardial celldamage (605 mmHg/s) to fetuses with normal cTnT levels (III).
No difference in the left ventricular IRT%, the total TVIs of TV and MV, and their E/ATVI- and A TVI/total TVI- ratios was found between the groups (Table 3) (III). Inaddition, IMP was similar among these fetuses (Table 3) (III).
5.3.2.3 Afterload
Fetuses with myocardial cell damage had a greater (p<0.05) CC/TC ratio than fetuseswith normal cTnT levels (Table 3) (III). The presence of TR was more common (p<0.03)in fetuses with a rise in cTnT concentrations (55%) than in fetuses with only increasedNT-proANP levels (12%) (Table 3) (III). Three out of 11 fetuses with myocardial celldamage had MR (III). The RVFS of fetuses with myocardial cell damage was less thanthat in fetuses with only increased NT-proANP level (p<0.05), while LVFS did not differbetween the groups (Table 3) (III).
5.3.3 Fetal arterial circulation
Fetuses with myocardial cell damage had higher (p<0.001) umbilical artery PI valuesthan fetuses with normal cTnT levels (Table 4) (III). No differences in PI values of MCAand DAo, and the DAo/ MCA PI-ratio were found between the groups (Table 4) (III). Thedetection rate of retrograde net blood flow in the aortic isthmus was similar among thegroups (Table 4) (III). In addition, no difference between the groups was found in thevisualization rate of coronary artery circulation (Table 4) (III).
5.3.4 Fetal venous circulation
In venous circulation, PIV values in DV, LHV and IVC were higher (p<0.01) in fetuseswith myocardial cell damage than in fetuses with normal cTnT values (Table 4) (III).When all the fetuses in study III were combined (n= 48), DV, LHV and IVC PIV valuesdemonstrated a significant positive correlation with umbilical artery NT-proANPconcentrations (Fig. 8). Fetuses with myocardial cell damage demonstrated atrialpulsations in umbilical vein more often than fetuses with normal cTnT values (p<0.01)(Table 4) (I, III).

61
Fig.
8. C
orre
latio
n of
DV
, LH
V a
nd IV
C P
IV v
alue
s with
um
bilic
al a
rter
y N
T-p
roA
NP
conc
entr
atio
ns in
fetu
ses w
ith p
lace
ntal
insu
ffic
ienc
y.N
orm
al N
T-p
roA
NP
and
cTnT
=gro
up 1
, in
crea
sed
NT
-pro
AN
P an
d no
rmal
cT
nT=g
roup
2,
and
incr
ease
d c
TnT
=gro
up 3
. (D
VPI
V=0
.000
15xN
T-p
roA
NP+
0.42
, R
=0.7
6,
p=0.
0001
; L
HV
PI
V=0
.002
0xN
T-p
roA
NP+
0.76
, R
=0.7
6,
p=0.
0001
; IV
CPI
V=0
.000
23xN
T-p
roA
NP+
2.34
, R=0
.57,
p=0
.000
1).

62
5.4 Retrograde net blood flow in the aortic isthmus
5.4.1 Clinical outcome
Fetuses with placental insufficiency and either antegrade or retrograde aortic isthmus netblood flow were delivered earlier (p<0.0001) and with lower birth weight (p<0.0001)than control fetuses with no difference as regards to the direction of aortic isthmus netblood flow (Table 2) (IV, V). Apgar scores at 5-minute age did not differ between thestudy groups but were significantly lower than in the control group (IV, V). Fetuses withretrograde aortic isthmus net blood flow were delivered via cesarean route more often(p<0.01) due to signs of fetal distess than fetuses with antegrade net blood flow (IV, V).No difference in umbilical artery pH and pO2 was detected between the groups (Table 2)(IV, V).
5.4.2 Fetal heart
5.4.2.1 Volumetric blood flows
Fetuses with placental insufficiency and either antegrade or retrograde aortic isthmus netblood flow had lower weight-indexed CCO, LVCO, RVCO (p<0.05) compared to thecontrol group (Table 5) (IV). Weight-indexed QP was lower (p<0.01) in fetuses withantegrade aortic isthmus net blood flow than in the control group fetuses, whileweight-indexed QFO was higher (p<0.05) than in fetuses with retrograde aortic isthmusnet blood flow (Table 5, Fig. 9) (IV). The LVCO% and RVCO% did not differsignificantly between the study groups (Table 5, Fig. 9) (IV). Fetuses with antegradeaortic isthmus net blood flow had greater QDA% (p<0.05), and QP% was less (p<0.05)than in the control group (IV). Furthermore, in fetuses with antegrade aortic isthmus netblood flow, QFO% of CCO was higher (p<0.01) than in fetuses with retrograde aorticisthmus net blood flow and in the control group (Table 5, Fig. 9) (IV). The proportion ofQFO of LVCO was greater (p<0.05) in fetuses with antegrade aortic isthmus net bloodflow (71%) than in the control group (49%) or in the fetuses with retrograde aorticisthmus net blood flow (48%) (IV).

63
Fig.
9. D
istr
ibut
ion
of fe
tal c
ardi
ac o
utpu
t in
the c
ontr
ol g
roup
fetu
ses (
Nor
mal
), in
fetu
ses w
ith a
nteg
rade
aor
tic is
thm
us n
et b
lood
flow
(Gro
up1)
, and
in fe
tuse
s with
ret
rogr
ade
aort
ic is
thm
us n
et b
lood
flow
(Gro
up 2
).

64
Table 5. Cardiac volumetric blood flow and their proportions (%) of combined cardiacoutput in control fetuses and in fetuses with placental insufficiency and either antegradeor retrograde aortic isthmus (AOI) net blood flow.
Controls Antegrade AOI Retrograde AOI
Volumetric blood flow
CCO (ml/min/kg) 647 (113) 529 (141)* 549 (153)*
RVCO (ml/min/kg) 391 (74) 287 (57)* 317 (106)*
LVCO (ml/min/kg) 298 (67) 242 (94)* 231 (59)*
QP (ml/min/kg) 150 (63) 99 (48)† 125 (74)
QDA (ml/min/kg) 244 (50) 208 (49) 208 (77)
QFO (ml/min/kg) 153 (74) 163 (79)‡ 105 (56)
Proportions (%) of CCO
RVCO% 56.8 (4.7) 55.1 (5.2) 57.2 (6.3)
LVCO% 43.2 (4.7) 44.9 (5.2) 42.8 (6.3)
QP % 23.4 (7.7) 17.8 (6.8) * 21.4 (10.5)
QDA % 35.1 (6.8) 42.2 (18.3)* 37.4 (11.5)
QFO % 23.5 (11.1) 32.0 (16.9)** 19.7 (10.8)
Systolic function
RVeFo (mN/kg) 7.1 (2.1) 5.5 (1.6) 6.4 (3.0)
LVeFo (mN/kg) 7.0 (2.4) 6.4 (3.7) 5.9 (2.3)
Diastolic function
IRT% 8.8 (1.2) 13.4 (1.8)† 13.2 (1.9)†
Tricuspid valve
total TVI (cm) 6.5(1.1) 4.9 (0.8)† 4.9 (0.8)†
E/A TVI 0.61 (0.17) 0.69 (0.24) 0.84 (0.87)
Mitral valve
Total TVI (cm) 6.5 (1.1) 4.4 (0.8)† 4.9 (0.1)†
E/A TVI 0.71 (0.15) 0.76 (0.17) 1.08 (0.75)* §
IMP 0.35 (0.06) 0.56 (0.09)† 0.57 (0.07)†
Afterload
CC/TC (%) 50.0 (1.9) 57.6 (3.7)† 60.8 (4.5)* §
RVFS % 30.1 (7.0) 17.7 (8.0)§
LVFS % 30.4 (5.1) 28.2 (5.2)
TR 2/18 (11%) 7/11 (64%)§
* p< 0.05 vs control group, † p< 0.01 vs control group, ‡ p< 0.05 vs retrograde AOI** p< 0.01 vs control group and retrograde AOI, § p< 0.05 vs antegrade AOI

65
5.4.2.2 Systolic and diastolic function of fetal heart
Weight-indexed RVeFo and LVeFo did not differ between the control group and fetuseswith placental insufficiency and either antegrade or retrograde aortic isthmus net bloodflow (Table 5) (IV). In addition, the ejection forces did not differ between the right andleft ventricles in any of the groups. Fetuses with either antegrade or retrograde aorticisthmus net blood flow demonstrated greater left ventricular IRT% (p<0.01) than didcontrol group fetuses (Table 5) (IV).
In fetuses with placental insufficiency and either antegrade or retrograde aortic isthmusnet blood flow, the total TVIs of TV and MV were less (p<0.001) than in the controlgroup (Table 5) (IV). Fetuses with retrograde aortic isthmus net blood flow demonstratedgreater (p<0.05) E/A TVI ratio of MV compared to control group fetuses and fetuses withantegrade aortic isthmus net blood flow (IV). No difference in E/A TVI ratio of TV wasfound between these groups (IV). Fetuses with placental insufficiency and eitherantegrade or retrograde aortic isthmus net blood flow demonstrated increased IMP values(p<0.0001) compared to the control group (Table 5) (IV).
5.4.2.3 Afterload
The mean (SD) CC/TC ratio of fetuses with placental insufficiency and retrograde aorticisthmus net blood flow (60.8% (4.5%)) was greater than that of normal fetuses (50.0%(1.9%), p< 0.001) and fetuses with placental insufficiency and antegrade aortic isthmusnet blood flow (57.6% (3.7%), p<0.05) (V). Fetuses with retrograde aortic isthmus netblood flow had lower RVFS values than fetuses with antegrade aortic isthmus net bloodflow (p<0.01), while no difference in LVFS values was noticed (Table 5) (V). Tricuspidregurgitation was present in seven of 11 (64%) fetuses with retrograde aortic isthmus netblood flow, and in two of 18 (11%) fetuses with antegrade aortic isthmus net blood flow(p<0.003) (Table 5) (V).
5.4.3 Fetal arterial circulation
Umbilical artery, DAo and DA PI values were significantly higher, and the PIs of theMCA were significantly lower in fetuses with placental insufficiency with no differenceas regards to the direction of aortic isthmus net blood flow than in the control fetuses(Table 6) (V). Fetuses with retrograde aortic isthmus net blood flow had greater PI of thePPA (p<0.001) than the control fetuses and fetuses with antegrade aortic isthmus netblood flow (Table 6) (V). The DAo/MCA PI ratios were higher (p<0.0001) in fetuseswith placental insufficiency (Table 6) (V). However, no difference between fetuses withantegrade and retrograde aortic isthmus net blood flow was observed (V). Coronary

66
artery blood velocity waveforms were visualized in seven of 11 (64%) fetuses withretrograde aortic isthmus net blood flow, and in four of 18 (22%) fetuses with antegradeaortic isthmus net blood flow (p<0.003) (Table 6) (V).
5.4.4 Fetal venous circulation
The PIV of the DV was significantly higher in fetuses with retrograde aortic isthmus netblood flow than in the control fetuses and in fetuses with antegrade aortic isthmus netblood flow (Table 6) (V). In IVC blood velocity waveforms, PIV values did not differbetween the groups (Table 6) (V). Atrial pulsations in the intra-abdominal umbilical veinwere noted in seven of 11 (64%) fetuses with retrograde aortic isthmus net blood flow,and in seven of 18 (39%) fetuses with antegrade aortic isthmus net blood flow (p=0.20)(Table 6) (V).
Table 6. Arterial and venous circulations in the control fetuses and fetuses with placentalinsufficiency and either antegrade or retrograde aortic isthmus net blood flow.
Controls Antegrade AOI Retrograde AOI
Arterial circulation
UA PI 1.01 (0.18) 2.57 (1.44)† 2.80 (1.91)†
MCA PI 1.96 (0.29) 1.44 (0.43)‡ 1.33 (0.25)‡
PPA PI 3.33 (0.26) 4.51 (1.44) * 7.55 (3.87)†,§
DA PI 2.67 (0.29) 3.05 (0.42)† 3.15 (0.72)†
DAo PI 2.03 (0.22) 2.67 (0.60)* 3.13 (1.08)†
DAo/MCA PI 1.05 (0.17) 2.06 (0.90)‡ 2.36 (0.63)‡
Coronary artery visualization 0/31 (0%) 4/18 (22%) 7/11 (64%)§
Venous circulation
IVC PIV 2.15 (1.13-2.89) 2.46 (1.02-6.19) 3.30 (1.76-11.94)
DV PIV 0.56 (0.30-0.71) 0.60 (0.26-1.24) 1.10 (0.33-3.81)†,§
IA UV pulsations 0/31 (0%) 7/18 (39%) 7/11 (64%)
* p< 0.05 vs control group, † p< 0.01vs control group, ‡ p< 0.0001vs control group, § p< 0.001vs group 1

6 Discussion
6.1 Validation of methodology
6.1.1 Ultrasonographic parameters
In a sheep model, volumetric blood flow measurements through the abdominal aorta andumbilical vein obtained by Doppler ultrasonography have been shown to be valid indifferent flow rates by using radionuclide-labeled microspheres and electromagnetic flowtransducers (Schmidt et al. 1991). Meijboom et al. demonstrated that the intra- andinterobserver variabilities in Doppler ultrasonographic measurements of volume bloodflow across TV were less than 5% in invasively monitored dogs and in childrenundergoing cardiac catheterization (Meijboom et al. 1985). Previously, it has been shownthat Doppler-determined maximal velocity tracings across cardiac valves vary by lessthan 10% (Reed et al. 1986). In a randomized blinded study on human fetuses, intra-observer variability in volumetric blood flow measurements of RVCO, LVCO, QDA andQP has been shown to be less than 9% (Rasanen et al. 1998). In addition, an excellentcorrelation between two independent calculations of RVCO from PV and QP+QDA hasbeen shown in human fetuses (Rasanen et al. 1998). In the present study, the anglebetween the Doppler beam and the vessel was kept at <15 degrees to minimizemethodological errors in volume blood flow calculations. Vessel diameters weremeasured at least three times by using the well-established leading edge-to-leading edgemethod. The mean intra-observer variabilities of volumetric blood flow calculations ofRVCO, LVCO, QDA and QP were comparable with previous reports. In this study,indirect estimation of QP was also found to correlate significantly with direct QPcalculation.
In human fetal studies, intra-observer variability in RVeFo and LVeFo calculationsbetween 18 and 38 weeks of gestation has been less than 10% (Rizzo et al. 1995a,Rasanen et al. 1997), and the corresponding interobserver variability less than 13%(Rizzo et al. 1995a). In this study, intra-observer variability of fetal ventricular ejectionforce calculations was 7 – 8%, and thus comparable with previous reports.

68
The intra-observer variability as regards PI calculations in the human fetal arterialcirculation has been shown to be less than 4% (Rasanen et al. 1998). In the present study,PI and PIV calculations showed intra-observer variability of less than 6%, which iscomparable with previous reports.
Other potential limitation of this study could be that some of the ultrasonographicallyobtained cardiovascular parameters change with advancing gestation in normaluncomplicated pregnancies (Rizzo et al. 1988, Tulzer et al. 1994, Rizzo et al. 1995a,Tsutsumi et al. 1999). However, in study III, in which all the pregnancies werecomplicated by placental insufficiency, cardiovascular parameters obtained by Dopplerultrasonography did not correlate with gestational age, which is in agreement with earlierstudies (Rizzo et al. 1988, Rizzo et al. 1995a, Tsutsumi et al. 1999). In studies IV and V,the gestational age at study entry was similar among the studied groups.
6.1.2 Cardiac biochemical markers
The sensitivity of the NT-proANP assay is 30 pmol/l. The intra-assay and interassaycoefficients of variation are < 10% and < 15%, respectively (Vuolteenaho et al. 1992). Inexperimental studies on rats, it has been shown that ANP does not cross the placenta(Mulay & Varma 1989). Shilo et al. found no correlation between maternal and neonatalANP concentrations, which supports the view that it does not cross the human placenta(Shilo et al. 1989). The molecular size of NT-proANP (98 amino acids) is several timesgreater than that of ANP (28 amino acids), making it very likely that it does not cross theplacenta either. The mode of delivery does not influence umbilical artery or vein plasmaANP concentrations (Kingdom et al. 1992). All these findings are in agreement with theresults in study II. When umbilical artery and vein blood samples were compared, NT-proANP concentrations did not differ significantly. However, maternal cubital vein NT-proANP concentrations were significantly lower than corresponding umbilical artery andvein concentrations. In addition, maternal cubital vein NT-proANP values showed nocorrelation to newborn umbilical artery NT-proANP, while a significant correlation wasfound between newborn umbilical artery and vein concentrations. These resultsdemonstrate that umbilical artery NT-proANP was of fetal origin. In addition, it has beenshown previously that fetal ANP concentrations do not change after 16 weeks ofgestation (Ville et al. 1994).
The cTnT assay has shown excellent reproducibility and a statistically significantability to distinguish between values differing in the low range by 0.01 ng/ml (Lipshultzet al. 1997). The cTnT was measurable in children of all ages with myocyte damage andthe smallest detectable troponin T elevations were 0.03 ng/ml (Lipshultz et al. 1997). Thisminimum detectable level was based on validation of the performance of the cTnT assayin the submyocardial infarction range of 0 to 0.10 ng/ml. A cTnT concentration equal toor more than 0.10 ng/ml was selected as the level of a clinically significant increase inthis study, as used earlier in adults (Ohman et al. 1996). The measuring range of the usedcTnT kit varies from 0 to 15 ng/ml, and the lower detection limit is 0.02 ng/ml. Themonoclonal antibodies used are highly specific for cTnT. No measurable cross-reactionhas been observed with skeletal muscle TnT or other myocardial filamentary proteins

69
(Katus et al. 1991). Cardiac troponin T concentrations start to rise within 4 to 12 hoursafter myocardial cell destruction and they remain high for over a week after the injury(Katus et al. 1991). Maternal cTnT concentrations were normal in our series, even incases with increased newborn cTnT levels, demonstrating that umbilical artery cTnTmeasured in newborns was not of maternal origin. In addition, our results show thatgestational age at delivery does not appear to affect newborn cTnT levels, at least after 27weeks of gestation. It has been shown that during and after delivery, maternal troponin Ilevels remain undetectable (Shivvers et al. 1999). Troponin I levels were not affected byobstetric anesthesia, prolonged labor, or operative delivery. As regards cTnT, it wasshown in study I that mode of delivery did not affect newborn cTnT levels.
6.2 Increased fetal NT-proANP levels without myocardial cell damage (II, III)
Newborns with acidemia during labor had higher umbilical artery NT-proANP levels thanneonates after uncomplicated pregnancy and labor, although the course of pregnancy wasuneventful, the birth weights were within the normal reference range and fetal heart ratetracings were normal when the subjects were admitted to the labor unit. These findingsshow that relatively acute changes in fetal acid/base status are reflected in thecardiovascular system as an increase in the release of NT-proANP. Previously, inverselyrelated newborn umbilical vein ANP concentrations to umbilical artery pH values havebeen thought to indicate fetal cardiac response to the stress of acidosis (Kingdom et al.1992). Studies on animals have shown that ANP release and production in the fetus areincreased by acute hypoxemia and a rise in atrial pressure. Immunohistochemical studieshave shown that ANP is present in the atria, the ventricular impulse-conducting system,and also in working ventricular cardiocytes (Jougasaki et al. 1989). Similarly, pro-ANP isexpressed in human fetal ventricles, in which the number of myofibers containing ANPgranules is greater in the subendocardial than in the subepicardial region (Tsuchimochi etal. 1988). In human fetuses, the connection between acute hypoxemia and increased ANPproduction is not so evident. Newborn pO2 did not show any independent relationshipwith ANP concentrations (Kingdom et al. 1992). According to our study, acute fetaldistress with acidemia during labor is capable of triggering fetal cardiac dysfunctionindicated by a rise in a release of NT-proANP.
Newborns with abnormal umbilical artery blood velocity waveform pattern duringfetal life had higher NT-proANP levels than those with a normal umbilical artery bloodflow velocity pattern, indicating an activated ANP system in fetuses suffering fromplacental insufficiency. In placental insufficiency, the afterload faced by the fetal rightventricle is increased, and these fetuses tend to be hypertensive (Stale et al. 1991). Thus,ANP secretion seems to be activated in response to an increase in afterload. It has beenshown during intravascular red cell transfusion in utero that human fetal ANPconcentrations increase promptly in response to an acute vascular volume expansion andan acute rise in the afterload (Panos et al. 1989). The NT-proANP levels in studies II andIII show that in hypertensive pregnancies, atrial wall stretch of the fetal heart is increased,

70
and it rises with further deterioration of placental function. Capponi et al. found nodifference in ANP levels between appropriately grown fetuses and growth-restrictedfetuses with abnormal umbilical artery and normal inferior vena cava Dopplerultrasonographic findings (Capponi et al. 1997). This is not contradictory to our findings.The half-life of NT-proANP, which is released in equimolar amounts with ANP, is longerthan that of ANP, and thus it is reflected in a larger increase in the NT-proANP levelcompared with that of ANP.
Fetuses with increased umbilical artery NT-proANP concentrations had similarweight-indexed LVCO, RVCO and CCO compared to fetuses with normal NT-proANPlevels. In addition, the distribution of cardiac output did not differ between these twogroups. No differences were observed in parameters describing systolic and diastolicfunctions of the fetal heart. Significantly increased neonatal and umbilical venous ANPlevels have been reported in anemic, acidemic, hydropic and growth-restricted fetuses(Hatjis et al. 1989, Panos et al. 1989, Kingdom et al. 1992, Ville et al. 1994). However,no significant correlation was found between fetal ANP levels and reductions in theumbilical artery S/D-ratio (Kingdom et al. 1991). Our Doppler findings of fetuses withincreased NT-proANP levels demonstrate the large functional capacity of the fetal heart inpregnancies complicated by placental insufficiency.
6.3 Fetal myocardial cell damage (I, III)
In normal pregnancies with uncomplicated deliveries, newborn cTnT was not clinicallysignificantly (> 0.10 ng/ml) increased. Maternal hypertensive disorders with or withoutsigns of placental insufficiency and with normal umbilical venous return are notassociated with significant fetal myocardial cell damage.
Based on increased umbilical artery PI values, decreased RVFS, more frequentvisualization of TR and increased cardiac size, it seems that the right ventricular afterloadis increased in fetuses with elevated cTnT levels. The right ventricle mainly reflects thecirculation and resistance in the fetal lower body, placenta and pulmonary bed, while theleft ventricle is responsible for the circulation in the coronary and cerebral arteries and thefetal upper body. In acute experiments on fetal lambs, increase in right ventricularafterload by means of ductal occlusion significantly decreased RVFS, and TR wasimmediately present. All these changes resolved after ductal occlusion was released(Tulzer et al. 1991a). In human fetuses with increased vascular impedance in thedescending aorta, RVFS was found to be decreased with no change in the left ventricle(Rasanen et al. 1989). In addition, in our study III, the RVCO% was less and LVCO%was greater in fetuses with placental insufficiency and increased cTnT levels than infetuses with only a rise in NT-proANP production. This suggests that fetuses withincreased right ventricular afterload shift their cardiac output from the right to the leftventricle. However, no difference in weight-indexed CCO was found. This finding is inagreement with the results of al-Ghazali et al., who reported that the RVCO% was about58% in growth restricted fetuses with relatively mild placental insufficiency, but it wassignificantly lower in fetuses with more severe placental insufficiency and FGR (al-Ghazali et al. 1989). In study III, the increased right ventricular afterload seemed to

71
originate from the placenta. Fetuses with elevated cTnT levels demonstrated significantlyhigher incidence of TR (54.5%) than reported previously during the second half ofgestation (6.8%) (Respondek et al. 1994). However, similar TR dP/dT between fetuseswith increased cTnT levels and fetuses with only increased NT-proANP concentrationssuggest that the right ventricle is able to maintain its contractility despite the increase inright ventricular afterload.
In animal studies, it has been previously shown that ejection forces are not affected byafterload (Noble et al. 1966). During the second half of gestation, human fetal ejectionforces of both ventricles increase with no difference between the ventricles (Rasanen etal. 1997). However, fetuses with severe ductal constriction or occlusion demonstratedlower RVeFo values than fetuses with patent DA (Rasanen et al. 1997). Based on similarweight-indexed ventricular ejection forces, fetuses with elevated cTnT levels in our seriesseem to be able to maintain adequate systolic function of the heart. The diastolic functionof the fetal heart described by the IRT% was also similar between fetuses with onlyelevated NTpro-ANP levels and fetuses with increased cTnT concentrations. Thus, itseems that global myocardial function and ventricular compliance are not changed evenin conditions with significant fetal myocardial cell damage.
Redistribution of fetal arterial circulation, as determined by the DAo/MCA PI-ratio,the incidences of retrograde net blood flow in the aortic isthmus and visualization ofcoronary artery blood flow, did not differ between fetuses with elevated cTnT levels andfetuses who had only increased NT-proANP levels. Retrograde net blood flow in theaortic isthmus has been associated with diminished oxygen delivery to the cerebralcirculation even though the volume blood flow is maintained (Fouron et al. 1999).Growth-restricted fetuses with easily visualized coronary artery blood flow have beensuggested to be at a high risk of intrauterine death and postpartum circulatory failure(Baschat et al. 1998). Fetuses with elevated cTnT levels in study III, demonstratedincreased right ventricular afterload. A rise in pressure causes a greater demand foroxygen in the myocardium than an increase in volume load. A rise in right ventricularafterload increases the wall stress, thus increasing oxygen consumption by themyocardium. This is also supported by the fact that the right ventricular systolic diameterremained proportionally greater in fetuses with elevated cTnT levels. However, it seemsthat fetal compensatory mechanisms, including coronary artery vasodilatation andincreased blood flow, are able to meet the increased oxygen demand because theincidences of demonstrable coronary artery circulation were similar in fetuses withelevated cTnT levels and fetuses with only increased NT-proANP concentrations. Studieson fetal lambs have also shown large coronary vascular reserves in response todiminished oxygen content of the blood (Reller et al. 1995). Increased right ventricularafterload and similarity in demonstrable coronary artery circulation in fetuses withelevated cTnT levels suggest that fetal myocardial cell damage is more related toincreased afterload and pressure than diminished oxygen delivery to the myocardium.The increase in wall tension is greater in the subendocardial than in the subepicardialregion. In the coronary circulation, the reserve of the subendocardial region is less thanthat of more superficial layers. Thus, the subendocardial area could be more vulnerable tohypoxemia in the human fetus.

72
Fetuses with myocardial cell damage had increased pulsatility in the blood velocitywaveforms of the DV, LHV and IVC. Their NT-proANP concentrations were higher thanin fetuses with no signs of myocardial cell damage. The PIV values in DV, LHV and IVChad a significant positive correlation with umbilical artery NT-proANP concentrationsdemonstrating that DV, LHV and IVC PIV values reflect fetal systemic venous pressure.Studies on fetal lambs have shown that a rise in systemic venous pressure is associatedwith increased pulsatility in the systemic venous blood velocity waveforms(Gudmundsson et al. 1999). It has also been shown that changes in umbilical venousvelocities originate in the fetal venous system and are transmitted towards the placenta(Reed & Anderson 2000). Fouron et al. showed in a lamb model that an increase in fetalright ventricular pressure is associated with a marked accumulation of products ofreactive O2 species generation in the right ventricular myocardium (Fouron et al. 2001a).The findings in studies I and III demonstrate that the presence of increased pulsatility insystemic veins and atrial pulsations in the fetal intra-abdominal umbilical vein areassociated with biochemically detectable myocardial cell damage. In study I, serum cTnTconcentrations were increased above the clinically significant level in every newborn whoexperienced atrial pulsations in the intra-abdominal umbilical vein during fetal life.
6.4 Retrograde aortic isthmus net blood flow (IV, V)
In placental insufficiency, the parallel circulatory system of the fetal heart ensures ahighly oxygenated blood supply to the coronary and cerebral circulations: well-oxygenated blood entering from the placenta flows through the ductus venosus and lefthepatic vein via the foramen ovale to the left atrium and ventricle (Kiserud et al. 1991)and blood from the fetal lower body enters the right atrium and ventricle through theinferior vena cava and other hepatic veins (Fig. 1). The aortic isthmus has a dynamic rolein connecting these two parallel circulatory systems in the fetus. Based on the findings inan acute fetal lamb model, Fouron et al. showed that oxygen delivery to the brain doesnot decrease until the net blood flow through the aortic isthmus becomes retrograde(Fouron et al. 1999). The volume blood flow in the cerebral circulation is maintainedeven in the presence of retrograde aortic isthmus net blood flow.
In our study population, placental function, as assessed by placental vascularimpedance, seemed to be equally impaired, and umbilical artery pH and PO2 values atbirth were similar in fetuses with either antegrade or retrograde net blood flow in theaortic isthmus. It is important to note that retrograde aortic isthmus net blood flow couldbe detected in the presence of normal looking umbilical artery blood velocity waveformprofiles. This is in agreement with the results of studies on fetal lambs, which have shownthat the aortic isthmus blood flow profile can change prior to deterioration of theumbilical artery blood velocity waveform pattern (Bonnin et al. 1993).
In pregnancies complicated by placental insufficiency, fetal weight-indexed RVCO andLVCO were significantly reduced with no difference as regards to direction of aorticisthmus net blood flow. The RVCO% and LVCO% were similar in fetuses with eitherretrograde or antegrade aortic isthmus net blood flow. However, in fetuses with antegradeaortic isthmus net blood flow, the QDA% was increased, and the QP% was decreased. This

73
demonstrates a shift in the distribution of RVCO from the pulmonary to the systemiccirculation. Human fetal studies have shown that the QP% increases and the QFO%decreases significantly with no change in the QDA% during the second half of gestation(Rasanen et al. 1996a). Furthermore, the proportion of umbilical volume blood flowshunted through the DV has been shown to decrease significantly from 40% to 15%between 20 and 38 weeks of gestation (Bellotti et al. 2000). In addition, animal andhuman studies have demonstrated that during the last trimester of pregnancy, fetalpulmonary circulation is under acquired vasoconstriction which is affected by fetaloxygen tension (Lewis et al. 1976, Morin et al. 1988, Rasanen et al. 1998). In placentalinsufficiency, oxygen delivery from the placenta can be impaired, leading to loweroxygen content of the fetal blood. As a result, vasoconstriction of the pulmonary arterialbed and a drop in pulmonary volume blood flow could occur. In addition, the QFO% wasgreater in fetuses with antegrade aortic isthmus net blood flow compared with fetuseswith retrograde aortic isthmus net blood flow, thus increasing its role in making up leftventricular cardiac output. In this way, in placental insufficiency, fetuses with antegradenet blood flow in the aortic isthmus are able to ensure highly oxygenated blood to thecoronary and cerebral circulations. Fetuses with retrograde net blood flow in the aorticisthmus failed to reorganize the distribution of right and left ventricular cardiac outputs,and thus increase volume blood flow through the foramen ovale to the same magnitude asfetuses with antegrade net blood flow. In fetuses with retrograde aortic isthmus net bloodflow, oxygen delivery to the coronary and cerebral circulations is diminished compared tofetuses with antegrade aortic isthmus net blood flow, even in the presence of similar pO2in the umbilical vein. This may predispose these fetuses to coronary and cerebralhypoxemia. This is supported by the fact that fetuses with retrograde aortic isthmus netblood flow were delivered more frequently by cesarean section due to signs of fetaldistress.
The weight-indexed RVeFo and LVeFo in fetuses with placental insufficiency andeither antegrade or retrograde net blood flow in the aortic isthmus did not differ fromthose measured in the control fetuses. Rizzo et al. demonstrated that the ejection forces ofboth ventricles were significantly and symmetrically decreased in growth-restrictedfetuses. Adverse obstetric outcome was observed in growth-restricted fetuses in which theejection forces of both ventricles were below the 5th centile of the normal limits forgestation. In addition, a relationship between the severity of acidosis and diminishedRVeFo and LVeFo was demonstrated. The differences between these studies can beexplained by a different study design. In this study, weight-indexed ejection force valueswere used because fetuses with placental insufficiency tend to be of a lower weight. Inaddition, umbilical artery pH values at delivery were similar among the groups,suggesting that fetuses in our study were delivered prior to development of severe fetalacidosis. Consequently, these findings show that the fetal heart is able to preserve itssystolic function even in the presence of retrograde net blood flow in the aortic isthmus.
The proportion of IRT of the total cardiac cycle was greater in fetuses with placentalinsufficiency than in control fetuses with no difference as regards to the direction of aorticisthmus net blood flow. This suggests impaired diastolic function of the fetal heart inplacental insufficiency. Increased pressure gradient between systemic and atrial levelsmight also explain the longer IRT proportion of the cardiac cycle, because fetuses withplacental insufficiency tend to be hypertensive (Stale et al. 1991). However, significantly

74
higher IMP values suggest impaired global cardiac function in these fetuses compared tothe control group, as shown earlier by Tsutsumi et al. (Tsutsumi et al. 1999). Lower totalTVIs of the TV and MV in fetuses with placental insufficiency might be a consequence oflower cardiac output in these fetuses. The tricuspid valve E/A TVI- ratio did not differbetween fetuses with either retrograde or antegrade aortic isthmus net blood flow.However, the MV E/A TVI- ratio demonstrated a shift towards the early passive fillingperiod of the ventricle in fetuses with retrograde aortic istmus net blood flow. The E/ATVI- ratios of TV and MV increase with advancing gestation, which is thought to reflectimprovement in the diastolic function of the fetal heart (Rizzo et al. 1988). However,loading conditions of the heart may affect E/A TVI- ratios. Elevation in atrial pressureincreases the E- component of the inflow velocity waveform. Thus, the greater E/A TVI-ratio of MV in fetuses with retrograde aortic isthmus net blood flow may indicateincreased left atrial pressure. Increased left atrial pressure and impaired left ventriculardiastolic function could also reduce the blood stream through the foramen ovale.
Fetuses with retrograde net blood flow in the aortic isthmus had higher rightventricular afterload than fetuses with antegrade net blood flow. The greatest CC/TC-ratioin fetuses with placental insufficiency and retrograde net blood flow in the aortic isthmusdemonstrates the increase in relative heart size. Fetal right ventricular afterload is mainlya result of vascular resistance in the fetal lower body, placenta and pulmonary arterialbed. No difference in vascular impedances in the umbilical artery and DAo was found,and DA blood velocity waveform profiles showed no signs of ductal constriction infetuses with either retrograde or antegrade aortic isthmus net blood flow. In fetuses withretrograde net blood flow in the aortic isthmus, RVFS was decreased and the incidence ofTR was the highest. This indicates altered loading conditions of the right ventricle andsuggests that the pulmonary arterial circulation has an important role in the regulation ofright ventricular afterload. Consequently, increased cardiac afterload, and, thus, increasedventricular pressure and wall tension, may lead to an increased oxygen demand of themyocardium.
Redistribution of fetal arterial circulation, as determined by the DAo/MCA PI ratio,was similar regardless of the direction of aortic isthmus net blood flow. This findingdemonstrates that there is already a maximal redistribution of cerebral circulation in thepresence of antegrade aortic isthmus net blood flow, which does not further increase infetuses with retrograde aortic isthmus net blood flow. Thus, in fetuses with placentalinsufficiency, vascular impedance in the middle cerebral artery does not reflect thedirection of aortic isthmus net blood flow and the oxygen content of the blood enteringthe cerebral circulation. However, fetuses with retrograde aortic isthmus net blood flowdemonstrated higher proximal pulmonary artery PI values than fetuses with antegrade netblood flow. Circulation in the fetal pulmonary arterial bed is regulated by fetal oxygentension (Rasanen et al. 1998). Hypoxemia causes vasoconstriction and increasespulmonary arterial vascular resistance, and the effects of hyperoxemia are opposite(Morin et al. 1988). In addition, an increase in pulmonary arterial pressure is capable ofinducing vasoconstriction of the pulmonary arterial bed after 26 weeks of gestation(Rasanen et al. 1999). In placental insufficiency, impaired oxygen delivery from theplacenta to the fetus could lower the oxygen content of the fetal blood, and lead to

75
vasoconstriction of the pulmonary arterial circulation. In addition, these fetuses tend to behypertensive and their pulmonary arterial pressure may be increased, which could lead tovasoconstriction of the pulmonary arterial bed.
Fetuses with retrograde net blood flow in the aortic isthmus demonstrated increasedpulsatility in DV compared with fetuses with antegrade net blood flow. Pulsatility in IVCdid not differ between the groups. The finding that fetuses with retrograde net blood flowin the aortic isthmus are unable to increase QFO could explain the increased pulsatility inthe DV blood velocity waveforms. In addition, fetuses with retrograde net blood flow hadsigns of elevated left atrial pressure. Furthermore, diminished placental volume bloodflow could make the DV blood flow profile more sensitive to changes in atrial pressure.
These results suggest that the oxygen content of the blood entering the left ventricle isdiminished in fetuses with retrograde aortic isthmus net blood flow compared with fetuseswith antegrade net blood flow, even in the presence of similar umbilical vein pO2 values.This is also supported by the fact that visualization of coronary artery blood flow,described earlier as a heart-sparing effect (Baschat et al. 1997), was significantly morecommon in fetuses with retrograde net blood flow in the aortic isthmus, suggestingdiminished oxygen delivery to the myocardium and, thus vasodilatation of the coronaryarteries. This may cause subtle changes in myocardial performance and an increase in leftatrial pressure. In addition, oxygen consumption of the fetal heart could be increased inpregnancies complicated by placental insufficiency.
6.5 Clinical implications
In pregnancies complicated by placental insufficiency, the optimal timing of delivery isstill an issue to be resolved. In a longitudinal study with growth-restricted fetuses after 24weeks of gestation, the DV pulsatility index and short-term variation of fetal heart ratewere important indicators for the optimal timing of delivery prior to 32 weeks ofgestation. Delivery should be considered if one of these parameters becomes persistentlyabnormal (Hecher et al. 2001). The results of the present study demonstrate that in thepresence of abnormal systemic venous blood velocity waveform patterns, the fetusalready has biochemically detectable myocardial cell damage. The clinical significance ofthe fetal myocardial cell damage must be addressed in future studies. Based on ourfindings, the most optimal timing of the delivery in pregnancies complicated by placentalinsufficiency might be before the systemic venous blood flow patterns become abnormal.
The first report of the clinical significance of the direction of aortic isthmus net bloodflow suggests that children who had retrograde aortic isthmus net blood flow during thefetal period have increased risk for neurodevelopmental deficit at least at the ages of 2and 4 years (Fouron et al. 2001b). Our results show that fetuses with retrograde aorticisthmus net blood flow are delivered more often by cesarean section because of signs offetal distress. In addition, the oxygen content of the blood entering the coronary andcerebral circulations is less than in fetuses with antegrade net blood flow in the aorticisthmus. Doppler examination of cerebral circulation does not reveal the direction of

76
aortic isthmus net blood flow. The investigation of aortic isthmus net blood flow shouldbe incorporated in the evaluation of fetal well-being in pregnancies complicated byplacental insufficiency.

7 Conclusions
1. Fetuses with placental insufficiency and atrial pulsations in their umbilical vein haveincreased umbilical artery NT-proANP and cTnT levels, demonstrating that atrialpulsations in the umbilical vein reflect increased systemic venous pressure andmyocardial cell damage (I-II).
2. In placental insufficiency, umbilical artery NT-proANP levels correlate significantlywith pulsatility index values for veins in the ductus venosus, left hepatic vein andinferior vena cava (III). Fetal myocardial cell damage, as regards to elevatedumbilical artery cTnT levels, is associated with a shift in cardiac output in favor of theleft ventricle, an increase in right ventricular afterload and increased pulsatility in thesystemic venous blood velocity waveforms, the latter indicating a rise in systemicvenous pressure, (III).
3. Fetuses with placental insufficiency and antegrade aortic isthmus net blood flowdemonstrate a shift in their right ventricular cardiac output from the pulmonary to thesystemic circulation, and foramen ovale volume blood flow makes up the majority oftheir left ventricular output. Fetuses with retrograde aortic isthmus net blood flow failto demonstrate these changes, and they have signs of increased left atrial pressure(IV).
4. Fetuses with retrograde aortic isthmus net blood flow demonstrate a rise in rightventricular afterload and increased pulsatility in ductus venosus blood velocitywaveforms with no difference in the redistribution of the arterial circulationcompared with fetuses with antegrade aortic isthmus net blood flow (V).

8 References
Adamcova M, Kokstein Z, Palicka V, Vavrova J, Kostal M, Podholova M & Kalous P (1999) Cardiactroponin T in pregnant women having intravenous tocolytic therapy. Arch Gynecol Obstet 262(3–4): 121–126.
Adams J, 3rd (1999) Impact of troponins on the evaluation and treatment of patients with acutecoronary syndromes. Current Opin Cardiol 14(4): 310–313.
Alfirevic Z & Neilson JP (1995) Doppler ultrasonography in high-risk pregnancies: systematicreview with meta-analysis. Am J Obstet Gynecol 172(5): 1379–1387.
al-Ghazali W, Chita SK, Chapman MG & Allan LD (1989) Evidence of redistribution of cardiacoutput in asymmetrical growth retardation. Br J Obstet Gynaecol 96(6): 697–704.
Almstrom H & Sonesson SE (1996) Doppler echocardiographic assessment of fetal blood flowredistribution during maternal hyperoxygenation. Ultrasound Obstet Gynecol 8(4): 256–261.
Arduini D, Rizzo G, Soliani A & Romanini C (1991) Doppler velocimetry versus nonstress test in theantepartum monitoring of low-risk pregnancies. J Ultrasound Med 10(6): 331–335.
Arduini D, Rizzo G & Romanini C (1993) The development of abnormal heart rate patterns afterabsent end-diastolic velocity in umbilical artery: analysis of risk factors. Am J Obstet Gynecol168(1 Pt 1): 43–50.
Arjamaa O & Vuolteenaho O (1985) Sodium ion stimulates the release of atrial natriureticpolypeptides (ANP) from rat atria. Biochem Biophys Res Commun 132(1): 375–381.
Bäcklund T, Palojoki E, Gronholm T, Eriksson A, Vuolteenaho O, Laine M, Tikkanen I &2001;44:411–418 DioaceaneboirivPR (2001) Dual inhibition of angiotensin converting enzymeand neutral endopeptidase by omapatrilat in rat in vivo. Pharmacol Res 44:411–418.
Barker DJ, Gluckman PD, Godfrey KM, Harding JE, Owens JA & Robinson JS (1993) Fetal nutritionand cardiovascular disease in adult life. Lancet 341(8850): 938–941.
Barnett SB, Rott HD, ter Haar GR, Ziskin MC & Maeda K (1997) The sensitivity of biological tissueto ultrasound. Ultrasound Med Biol 23(6): 805–812.
Baschat AA, Gembruch U, Reiss I, Gortner L & Diedrich K (1997) Demonstration of fetal coronaryblood flow by Doppler ultrasound in relation to arterial and venous flow velocity waveforms andperinatal outcome – the 'heart-sparing effect'. Ultrasound Obstet Gynecol 9(3): 162–172.
Baschat AA, Gembruch U & Harman CR (1998) Coronary blood flow in fetuses with intrauterinegrowth restriction. J Perinatal Med 26(3): 143–156.
Behrman RE, Lees MH, Peterson EN, De Lannoy CW & Seeds AE (1970) Distribution of thecirculation in the normal and asphyxiated fetal primate. Am J Obstet Gynecol 108(6): 956–969.
Bellamy RF (1978) Diastolic coronary artery pressure-flow relations in the dog. Circ Res 43(1): 92–101.

79
Bellotti M, Pennati G, Pardi G & Fumero R (1998) Dilatation of the ductus venosus in human fetuses:ultrasonographic evidence and mathematical modeling. Am J Physiol 275(5 Pt 2): H1759–1767.
Bellotti M, Pennati G, De Gasperi C, Battaglia FC & Ferrazzi E (2000) Role of ductus venosus indistribution of umbilical blood flow in human fetuses during second half of pregnancy. Am JPhysiol Heart Circ Physiol 279(3): H1256–1263.
Berman HJ & Gamble WJ (1975) Myocardial oxygen usage: its measurement regulation andmeaning. Microvasc Res 9(1): 127–135.
Bewley S, Campbell S & Cooper D (1989) Uteroplacental Doppler flow velocity waveforms in thesecond trimester. A complex circulation. Br J Obstet Gynaecol 96(9): 1040–1046.
Bilardo CM, Nicolaides KH & Campbell S (1990) Doppler measurements of fetal and uteroplacentalcirculations: relationship with umbilical venous blood gases measured at cordocentesis. Am JObstet Gynecol 162(1): 115–120.
Boatwright RB, Downey HF, Bashour FA & Crystal GJ (1980) Transmural variation inautoregulation of coronary blood flow in hyperperfused canine myocardium. Circ Res 47(4): 599–609.
Bocking AD, White SE, Homan J & Richardson BS (1992) Oxygen consumption is maintained infetal sheep during prolonged hypoxaemia. J Develop Physiol 17(4): 169–174.
Bonds DR, Crosby LO, Cheek TG, Hagerdal M, Gutsche BB & Gabbe SG (1986) Estimation ofhuman fetal-placental unit metabolic rate by application of the Bohr principle. J Develop Physiol8(1): 49–54.
Bonnin P, Fouron JC, Teyssier G, Sonesson SE & Skoll A (1993) Quantitative assessment ofcirculatory changes in the fetal aortic isthmus during progressive increase of resistance toumbilical blood flow. Circulation 88(1): 216–222.
Boomsma F, Bhaggoe UM, Man in 't Veld AJ & Schalekamp MA (1996) Comparison of N-terminalpro-atrial natriuretic peptide and atrial natriuretic peptide in human plasma as measured withcommercially available radioimmunoassay kits. Clin Chim Acta 252(1): 41–49.
Brar HS & Platt LD (1988) Reverse end-diastolic flow velocity on umbilical artery velocimetry inhigh-risk pregnancies: an ominous finding with adverse pregnancy outcome. Am J ObstetGynecol 159(3): 559–561.
Brenner BM, Ballermann BJ, Gunning ME & Zeidel ML (1990) Diverse biological actions of atrialnatriuretic peptide. Physiol Rev 70(3): 665–699.
Burns PN (1993) The Physics of Doppler. In: (eds Chervenak FA, Isaacson GC, Campbell S)Ultrasound in Obstetrics and Gynecology. Library of Congress Cataloging-in-Publication Data,Boston, Toronto, London, 33-54.
Capponi A, Rizzo G, De Angelis C, Arduini D & Romanini C (1997) Atrial natriuretic peptide levelsin fetal blood in relation to inferior vena cava velocity waveforms. Obstet Gynecol 89(2): 242–247.
Clark SJ, Newland P, Yoxall CW & Subhedar NV (2001) Cardiac troponin T in cord blood. Arch DisChild Fetal Neonatal Ed 84(1): F34–37.
Cohn HE, Sacks EJ, Heymann MA & Rudolph AM (1974) Cardiovascular responses to hypoxemiaand acidemia in fetal lambs. Am J Obstet Gynecol 120(6): 817–824.
Cohn HE, Piasecki GJ & Jackson BT (1980) The effect of fetal heart rate on cardiovascular functionduring hypoxemia. Am J Obstet Gynecol 138(8): 1190–1199.
Committee on Technical Bulletins of the American College of Obstetricians and Gynecologists(1996) Hypertension in pregnancy 1996 Jan. ACOG technical bulletin No:219 (replaces No: 91,1986 Jan). Intern J Gynaecol Obstet 53(2): 175–183.
Court DJ, Parer JT, Block BS & Llanos AJ (1984) Effects of beta-adrenergic blockade on blood flowdistribution during hypoxaemia in fetal sheep. J Develop Physiol 6(4): 349–358.
Davies JA, Lee A & Spencer JA (1990) Variability of continuous-wave Doppler flow velocitywaveform indices from the umbilical artery. Obstet Gynecol 76(3 Pt 1): 366–369.

80
de Bold AJ, Borenstein HB, Veress AT & Sonnenberg H (1981) A rapid and potent natriureticresponse to intravenous injection of atrial myocardial extract in rats. Life Sciences 28(1): 89–94.
Del Carlo CH & O'Connor CM (1999) Cardiac troponins in congestive heart failure. Am Heart J138(4 Pt 1): 646–653.
Delivoria-Papadopoulos M & McGowan JE (1998) Oxygen transport and delivery. In: (eds Polin RA,Fox WW) Fetal and Neonatal Physiology. W. B. Saunders Company, Philadelphia, 1105-1117.
den Ouden M, Cohen-Overbeek TE & Wladimiroff JW (1990) Uterine and fetal umbilical artery flowvelocity waveforms in normal first trimester pregnancies. Br J Obstet Gynaecol 97(8): 716–719.
DeVore GR, Siassi B & Platt LD (1984) Fetal echocardiography. IV. M-mode assessment ofventricular size and contractility during the second and third trimesters of pregnancy in the normalfetus. Am J Obstet Gynecol 150(8): 981–988.
Dickey RP & Hower JF (1995) Ultrasonographic features of uterine blood flow during the first 16weeks of pregnancy. Hum Reprod 10(9): 2448–2452.
Dickey RP (1997) Doppler ultrasound investigation of uterine and ovarian blood flow in infertilityand early pregnancy. Hum Reprod Update 3(5): 467–503.
Divon MY (1996) Umbilical artery Doppler velocimetry: clinical utility in high-risk pregnancies. AmJ Obstet Gynecol 174(1 Pt 1): 10–14.
Dole WP & Bishop VS (1982) Influence of autoregulation and capacitance on diastolic coronaryartery pressure-flow relationships in the dog. Circ Res 51(3): 261–270.
Donnelly R & Millar-Craig MW (1998) Cardiac troponins: IT upgrade for the heart. Lancet351(9102): 537–539.
Donner C, Vermeylen D, Kirkpatrick C, de Maertelaer V & Rodesch F (1995) Management of thegrowth-restricted fetus: the role of noninvasive tests and fetal blood sampling. Obstet Gynecol85(6): 965–970.
Edelstone DI & Rudolph AM (1979) Preferential streaming of ductus venosus blood to the brain andheart in fetal lambs. Am J Physiol 237(6): H724–729.
Edelstone DI, Darby MJ, Bass K & Miller K (1989) Effects of reductions in hemoglobin-oxygenaffinity and hematocrit level on oxygen consumption and acid-base state in fetal lambs. Am JObstet Gynecol 160(4): 820–826.
Eriksson JG, Forsen T, Tuomilehto J, Osmond C & Barker DJP (2001) Early growth and coronaryheart disease in later life: longitudinal study. BMJ 322:949–953.
European Committee for Medical Ultrasound Safety (ECMUS). (1999a) Thermal teratology. Eur JUltrasound 9(3): 281–283.
European Committee for Medical Ultrasound Safety (ECMUS) (1999b) Acoustic cavitation andcapillary bleeding. Eur J Ultrasound 9(3): 277–280.
Farine D, Kelly EN, Ryan G, Morrow RJ & Ritchie JWK (1994) Reverse and end-diastolic flowvelocity on umbilical artery velocimetry in high-risk pregnancies: An omnious finding withadverse pregnancy outcome. In: (Copel JA, Reed KL) Doppler ultrasound in obstetrics andgynecology. Raven Press, New York, 187-197.
Ferrazzi E, Rigano S, Bozzo M, Giovannini N, Galan H & Battaglia FC (2000) Umbilical vein bloodflow in growth-restricted fetuses. Ultrasound Obstet Gynecol 16(5): 432–438.
Fleischer A, Schulman H, Farmakides G, Bracero L, Grunfeld L, Rochelson B & Koenigsberg M(1986) Uterine artery Doppler velocimetry in pregnant women with hypertension. Am J ObstetGynecol 154(4): 806–813.
Flynn TG, de Bold ML & de Bold AJ (1983) The amino acid sequence of an atrial peptide with potentdiuretic and natriuretic properties. Biochem Biophys Res Commun 117(3): 859–865.
Forouzan I, Cohen AW & Arger P (1991) Measurement of systolic-diastolic ratio in the umbilicalartery by continuous-wave and pulsed-wave Doppler ultrasound: comparison at different sites.Obstet Gynecol 77(2): 209–212.

81
Fouron JC, Zarelli M, Drblik P & Lessard M (1994) Flow velocity profile of the fetal aortic isthmusthrough normal gestation. Am J Cardiol 74(5): 483–486.
Fouron JC, Skoll A, Sonesson SE, Pfizenmaier M, Jaeggi E & Lessard M (1999) Relationshipbetween flow through the fetal aortic isthmus and cerebral oxygenation during acute placentalcirculatory insufficiency in ovine fetuses. Am J Obstet Gynecol 181(5 Pt 1): 1102–1107.
Fouron JC, Chemtob S, Chartrand C, Russo P, Haswani P, Sonesson SE, Skoll A, Teyssier G &Castor S (2001a) Generation of reactive O2 species in the myocardium of newborn lambsfollowing intrauterine increase in right ventricular pressure. Pediatr Cardiol 22(2): 143–146.
Fouron JC, Gosselin J, Amiel-Tison C, Infante-Rivard C, Fouron C, Skoll A & Veilleux A (2001b)Correlation between prenatal velocity waveforms in the aortic isthmus and neurodevelopmentaloutcome between the ages of 2 and 4 years. Am J Obstet Gynecol 184(4): 630–636.
Gembruch U & Baschat AA (1996) Demonstration of fetal coronary blood flow by color-coded andpulsed wave Doppler sonography: a possible indicator of severe compromise and impendingdemise in intrauterine growth retardation. Ultrasound Obstet Gynecol 7(1): 10–16.
Gennser G (1992) Fetal ductus venosus and its sphincter mechanism. Lancet 339(8785): 132.Gerhardt W, Katus H, Ravkilde J, Hamm C, Jorgensen PJ, Peheim E, Ljungdahl L & Lofdahl P
(1991) S-troponin T in suspected ischemic myocardial injury compared with mass and catalyticconcentrations of S-creatine kinase isoenzyme MB. Clin Chem 37(8): 1405–1411.
Gilbert RD (1982) Effects of afterload and baroreceptors on cardiac function in fetal sheep. J DevelopPhysiol 4(5): 299–309.
Giles WB, Trudinger BJ & Baird PJ (1985a) Fetal umbilical artery flow velocity waveforms andplacental resistance: pathological correlation. Br J Obstet Gynaecol 92(1): 31–38.
Giles WB, Trudinger BJ & Cook CM (1985b) Fetal umbilical artery flow velocity-time waveformsin twin pregnancies. Br J Obstet Gynaecol 92(5): 490–497.
Gill RW, Trudinger BJ, Garrett WJ, Kossoff G & Warren PS (1981) Fetal umbilical venous flowmeasured in utero by pulsed Doppler and B-mode ultrasound. I. Normal pregnancies. Am J ObstetGynecol 139(6): 720–725.
Gill RW (1987) Doppler ultrasound – physical aspects. Semin Perinatol 11(4): 292–299.Gudmundsson S, Huhta JC, Wood DC, Tulzer G, Cohen AW & Weiner S (1991) Venous Doppler
ultrasonography in the fetus with nonimmune hydrops. Am J Obstet Gynecol 164(1 Pt 1): 33–37.Gudmundsson S, Fairlie F, Lingman G & Marsal K (1990) Recording of blood flow velocity
waveforms in the uteroplacental and umbilical circulation: reproducibility study and comparisonof pulsed and continuous wave Doppler ultrasonography. J Clin Ultrasound 18(2): 97–101.
Gudmundsson S, Tulzer G, Huhta JC & Marsal K (1996) Venous Doppler in the fetus with absentend-diastolic flow in the umbilical artery. Ultrasound Obstet Gynecol 7(4): 262–267.
Gudmundsson S, Gunnarsson GO, Hokegard KH, Ingemarsson J & Kjellmer I (1999) VenousDoppler velocimetry in relationship to central venous pressure and heart rate during hypoxia inthe ovine fetus. J Perinatal Med 27(2): 81–90.
Hadlock FP, Harrist RB, Carpenter RJ, Deter RL & Park SK (1984) Sonographic estimation of fetalweight. The value of femur length in addition to head and abdomen measurements. Radiology150(2): 535–540.
Harrington K, Carpenter RG, Nguyen M & Campbell S (1995) Changes observed in Doppler studiesof the fetal circulation in pregnancies complicated by pre-eclampsia or the delivery of a small-for-gestational-age baby. I. Cross-sectional analysis. Ultrasound Obstet Gynecol 6(1): 19–28.
Harrington K, Cooper D, Lees C, Hecher K & Campbell S (1996) Doppler ultrasound of the uterinearteries: the importance of bilateral notching in the prediction of pre-eclampsia, placentalabruption or delivery of a small-for-gestational-age baby. Ultrasound Obstet Gynecol 7(3): 182–188.
Harvey W (1628) Movement of the Heart and Blood in Animals, T. Translated from the original Latinby K. J. Franklin. Oxford, Blackwell Scientific Publications, 1957 44–60.

82
Hatjis CG, Greelish JP, Kofinas AD, Stroud A, Hashimoto K & Rose JC (1989) Atrial natriureticfactor maternal and fetal concentrations in severe preeclampsia. Am J Obstet Gynecol 161(4):1015–1019.
Hawkins J, Van Hare GF, Schmidt KG & Rudolph AM (1989) Effects of increasing afterload on leftventricular output in fetal lambs. Circ Res 65(1): 127–134.
Hecher K, Campbell S, Doyle P, Harrington K & Nicolaides K (1995a) Assessment of fetalcompromise by Doppler ultrasound investigation of the fetal circulation. Arterial, intracardiac,and venous blood flow velocity studies. Circulation 91(1): 129–138.
Hecher K, Snijders R, Campbell S & Nicolaides K (1995b) Fetal venous, intracardiac, and arterialblood flow measurements in intrauterine growth retardation: relationship with fetal blood gases.Am J Obstet Gynecol 173(1): 10–15.
Hecher K & Hackeloer BJ (1997) Cardiotocogram compared to Doppler investigation of the fetalcirculation in the premature growth-retarded fetus: longitudinal observations. Ultrasound ObstetGynecol 9(3): 152–161.
Hecher K, Bilardo CM, Stigter RH, Ville Y, Hackeloer BJ, Kok HJ, Senat MV & Visser GHA (2001)Monitoring of fetuses with intrauterine growth restriction: a longitudinal study. Ultrasound ObstetGynecol 18:564–570.
Hess DS & Bache RJ (1976) Transmural distribution of myocardial blood flow during systole in theawake dog. Circ Res 38(1): 5–15.
Hofstaetter C, Dubiel M & Gudmundsson S (2001) Two types of umbilical venous pulsations andoutcome of high-risk pregnancy. Early Hum Dev 61(2): 111–117.
Holtz J, Grunewald WA, Manz R, von Restorff W & Bassenge E (1977) Intracapillary hemoglobinoxygen saturation and oxygen consumption in different layers of the left ventricular myocardium.Pflugers Arch 370(3): 253–258.
Huhta JC (1997) Deciphering the hieroglyphics of venous Doppler velocities. Ultrasound ObstetGynecol 9(5): 300–301.
Huhta JC, Weil-Chalker S & Pagotto LT (2000) Fetal congestive heart failure. In: (Allan L,Hornberger L, Sharland G) Textbook of fetal cardiology. Greenwich Medical Media Limited,London, 565-575.
Indik JH, Chen V & Reed KL (1991) Association of umbilical venous with inferior vena cava bloodflow velocities. Obstet Gynecol 77(4): 551–557.
Inglis GC, Kingdom JC, Nelson DM, Lindop GB, Whittle MJ & Connell JM (1993) Atrial natriuretichormone: a paracrine or endocrine role within the human placenta? J Clin Endocrinol Metab76(4): 1014–1018.
Isaaz K, Ethevenot G, Admant P, Brembilla B & Pernot C (1989) A new Doppler method of assessingleft ventricular ejection force in chronic congestive heart failure. Am J Cardiol 64(1): 81–87.
Itoh H, Nakao K, Sugawara A, Saito Y, Mukoyama M, Morii N, Yamada T, Shiono S, Arai H,Hosoda K & Imura H (1988) Gamma-atrial natriuretic polypeptide (gamma ANP)-derivedpeptides in human plasma: cosecretion of N-terminal gamma ANP fragment and alpha ANP. JClin Endocrinol Metab 67(3): 429–437.
Itskovitz J & Rudolph AM (1982) Denervation of arterial chemoreceptors and baroreceptors in fetallambs in utero. Am J Physiol 242(5): H916–920.
Itskovitz J, LaGamma EF & Rudolph AM (1983) The effect of reducing umbilical blood flow on fetaloxygenation. Am J Obstet Gynecol 145(7): 813–818.
Itskovitz J, LaGamma EF & Rudolph AM (1987) Effects of cord compression on fetal blood flowdistribution and O2 delivery. Am J Physiol 252(1 Pt 2): H100–109.
Johnson P, Maxwell DJ, Tynan MJ & Allan LD (2000) Intracardiac pressures in the human fetus.Heart 84(1): 59–63.

83
Jougasaki M, Yasue H, Okumura K, Mukoyama M, Saito Y, Nakao K & Takahashi K (1989) Atrialnatriuretic peptide in the ventricles of patients with dilated cardiomyopathy and human foetuses.Histochem J 21(12): 715–720.
Jouppila P & Kirkinen P (1984) Increased vascular resistance in the descending aorta of the humanfetus in hypoxia. Br J Obstet Gynaecol 91(9): 853–856.
Karim MA, Shinn MS, Oskarsson H, Windle J & Deligonul U (1995) Significance of cardiac troponinT release after percutaneous transluminal coronary angioplasty. Am J Cardiol 76(7): 521–523.
Katus HA, Remppis A, Neumann FJ, Scheffold T, Diederich KW, Vinar G, Noe A, Matern G &Kuebler W (1991) Diagnostic efficiency of troponin T measurements in acute myocardialinfarction. Circulation 83(3): 902–912.
Kenny JF, Plappert T, Doubilet P, Saltzman DH, Cartier M, Zollars L, Leatherman GF & St. JohnSutton MG (1986) Changes in intracardiac blood flow velocities and right and left ventricularstroke volumes with gestational age in the normal human fetus: a prospective Dopplerechocardiographic study. Circulation 74(6): 1208–1216.
Kettunen RV, Leppaluoto J, Jounela A & Vuolteenaho O (1994) Plasma N-terminal atrial natriureticpeptide in acute myocardial infarction. Am Heart J 127(6): 1449–1455.
Kieler H, Haglund B, Waldenstrom U & Axelsson O (1997) Routine ultrasound screening inpregnancy and the children's subsequent growth, vision and hearing. Br J Obstet Gynaecol104(11): 1267–1272.
Kieler H, Axelsson O, Haglund B, Nilsson S & Salvesen KA (1998) Routine ultrasound screening inpregnancy and the children's subsequent handedness. Early Hum Dev 50(2): 233–245.
Kingdom JC, Ryan G, Whittle MJ, McNay MB, Bowman AW, Doyle J & Connell JM (1991) Atrialnatriuretic peptide: a vasodilator of the fetoplacental circulation? Am J Obstet Gynecol 165(4 Pt1): 791–800.
Kingdom JC, McQueen J, Connell JM & Whittle MJ (1992) Maternal and fetal atrial natriureticpeptide levels at delivery from normal and growth retarded pregnancies. Br J Obstet Gynaecol99(10): 845–849.
Kirkpatrick SE, Pitlick PT, Naliboff J & Friedman WF (1976) Frank-Starling relationship as animportant determinant of fetal cardiac output. Am J Physiol 231(2): 495–500.
Kiserud T, Eik-Nes SH, Blaas HG & Hellevik LR (1991) Ultrasonographic velocimetry of the fetalductus venosus. Lancet 338(8780): 1412–1414.
Kiserud T, Saito T, Ozaki T, Rasmussen S & Hanson MA (1999) Validation of diametermeasurements by ultrasound: intraobserver and interobserver variations assessed in vitro and infetal sheep. Ultrasound Obstet Gynecol 13(1): 52–57.
Kiserud T, Rasmussen S & Skulstad S (2000) Blood flow and the degree of shunting through theductus venosus in the human fetus. Am J Obstet Gynecol 182(1 Pt 1): 147–153.
Kiserud T (2000) Fetal venous circulation- an update on hemodynamics. J Perinatal Med 28(2): 90–96.
Kiserud T, Jauniaux E, West D, Ozturk O & Hanson MA (2001) Circulatory responses to maternalhyperoxaemia and hypoxaemia assessed non-invasively in fetal sheep at 0.3–0.5 gestation in acuteexperiments. Br J Obstet Gynaecol 108:359–364.
Klocke FJ (1976) Coronary blood flow in man. Progress in Cardiovascular Diseases 19(2): 117–166.Kofinas AD, Espeland M, Swain M, Penry M & Nelson LH (1989) Correcting umbilical artery flow
velocity waveforms for fetal heart rate is unnecessary. Am J Obstet Gynecol 160(3): 704–707.Kossoff G (1997) Contentious issues in safety of diagnostic ultrasound. Ultrasound Obstet Gynecol
10(3): 151–155.Kremkau W (1990) Doppler Ultrasound: Principles and Instruments. Philadelphia, London. W.B.
Saunders.Kremkau FW (1992) Doppler color imaging. Principles and instrumentation. Clinics in Diagnostic
Ultrasound 27:7–60.

84
Kukkonen P, Vuolteenaho O & Ruskoaho H (1992) Basal and volume expansion-stimulated plasmaatrial natriuretic peptide concentrations and hemodynamics in conscious rats: effects of SCH39.370, an endopeptidase inhibitor, and C-ANF-(4-23), a clearance receptor ligand.Endocrinology 130(2): 755–765.
Kurkinen-Raty M, Kivela A & Jouppila P (1997) The clinical significance of an absent end-diastolicvelocity in the umbilical artery detected before the 34th week of pregnancy. Acta Obstet GynecolScand 76(5): 398–404.
Lang RE, Tholken H, Ganten D, Luft FC, Ruskoaho H & Unger T (1985) Atrial natriuretic factor--acirculating hormone stimulated by volume loading. Nature 314(6008): 264–266.
Leiva MC, Tolosa JE, Binotto S, Weiner S, Huppert L, Denis AL & Huhta JC (1999) Fetal cardiacdevelopment and hemodynamics in the first trimester. Ultrasound Obstet Gynecol 14): 169–174.
Lerman A, Gibbons RJ, Rodeheffer RJ, Bailey KR, McKinley LJ, Heublein DM & Burnett JC, Jr.(1993) Circulating N-terminal atrial natriuretic peptide as a marker for symptomless left-ventricular dysfunction . Lancet 341(8853): 1105–1109.
Leskinen H, Vuolteenaho O, Leppaluoto J & Ruskoaho H (1995) Role of nitric oxide on cardiachormone secretion: effect of NG-nitro-L-arginine methyl ester on atrial natriuretic peptide andbrain natriuretic peptide release. Endocrinology 136(3): 1241–1249.
Leskinen H, Vuolteenaho O & Ruskoaho H (1997) Combined inhibition of endothelin andangiotensin II receptors blocks volume load-induced cardiac hormone release. Circ Res 80(1):114–123.
Lew RA & Baertschi AJ (1989) Mechanisms of hypoxia-induced atrial natriuretic factor release fromrat hearts. Am J Physiol 257(1 Pt 2): H147–156.
Lewis AB, Heymann MA & Rudolph AM (1976) Gestational changes in pulmonary vascularresponses in fetal lambs in utero. Circ Res 39(4): 536–541.
Lingman G & Marsal K (1987) Fetal cardiac arrhythmias: Doppler assessment. Semin Perinatol11(4): 357–361.
Lipshultz SE, Rifai N, Sallan SE, Lipsitz SR, Dalton V, Sacks DB & Ottlinger ME (1997) Predictivevalue of cardiac troponin T in pediatric patients at risk for myocardial injury. Circulation 96(8):2641–2648.
Mäntymaa P, Leppäluoto J & Ruskoaho H (1990) Endothelin stimulates basal and stretch-inducedatrial natriuretic peptide secretion from the perfused rat heart. Endocrinology 126(1): 587–595.
Mari G & Deter RL (1992) Middle cerebral artery flow velocity waveforms in normal and small-for-gestational-age fetuses. Am J Obstet Gynecol 166(4): 1262–1270.
Mari G, Abuhamad AZ, Uerpairojkit B, Martinez E & Copel JA (1995) Blood flow velocitywaveforms of the abdominal arteries in appropriate- and small-for-gestational-age fetuses.Ultrasound Obstet Gynecol 6(1): 15–18.
Marsal K, Laurin J, Lindblad A & Lingman G (1987) Blood flow in the fetal descending aorta. SeminPerinatol 11(4): 322–334.
McQueen J, Kingdom JC, Whittle MJ & Connell JM (1993) Characterization of atrial natriureticpeptide receptors in human fetoplacental vasculature. Am J Physiol 264(3 Pt 2): H798–804.
Meijboom EJ, Horowitz S, Valdes-Cruz LM, Sahn DJ, Larson DF & Oliveira Lima C (1985) ADoppler echocardiographic method for calculating volume flow across the tricuspid valve:correlative laboratory and clinical studies. Circulation 71(3): 551–556.
Michener ML, Gierse JK, Seetharam R, Fok KF, Olins PO, Mai MS & Needleman P (1986)Proteolytic processing of atriopeptin prohormone. Mol Pharmacol 30(6): 552–557.
Misono KS, Fukumi H, Grammer RT & Inagami T (1984) Rat atrial natriuretic factor: completeamino acid sequence and disulfide linkage essential for biological activity. Biochem Biophys ResCommun 119(2): 524–529.

85
Moise KJ, Jr., Huhta JC, Sharif DS, Ou CN, Kirshon B, Wasserstrum N & Cano L (1988)Indomethacin in the treatment of premature labor. Effects on the fetal ductus arteriosus. N Engl JMed 319(6): 327–331.
Morin FC, 3rd & Egan EA (1992) Pulmonary hemodynamics in fetal lambs during development atnormal and increased oxygen tension. J Appl Physiol 73(1): 213–218.
Morin FCD, Egan EA, Ferguson W & Lundgren CE (1988) Development of pulmonary vascularresponse to oxygen. Am J Physiol 254(3 Pt 2): H542–546.
Morrow RJ, Adamson SL, Bull SB & Ritchie JW (1989) Effect of placental embolization on theumbilical arterial velocity waveform in fetal sheep. Am J Obstet Gynecol 161(4): 1055–1060.
Morrow RJ, Adamson SL, Bull SB & Ritchie JW (1990) Hypoxic acidemia, hyperviscosity, andmaternal hypertension do not affect the umbilical arterial velocity waveform in fetal sheep. Am JObstet Gynecol 163(4 Pt 1): 1313–1320.
Muijsers GJ, Hasaart TH, van Huisseling H & de Haan J (1990) The response of the umbilical arterypulsatility index in fetal sheep to acute and prolonged hypoxaemia and acidaemia induced byembolization of the uterine microcirculation. J Develop Physiol 13(4): 231–236.
Mulay S & Varma DR (1989) Placental barrier to atrial natriuretic peptide in rats. Can J PhysiolPharmacol 67(1): 1–4.
Mulay S, Conliffe PR & Varma DR (1995) Increased natriuretic peptides in fetal hearts of diabeticrats. J Endocrinol 146(2): 255–259.
Nadal–Ginard B & Mahdavi V (1993) Basic mechanisms of cardiac gene expression. Eur Heart J14(Suppl J): 2–11.
Naeye RL (1989) Pregnancy hypertension, placental evidences of low uteroplacental blood flow, andspontaneous premature delivery. Hum Pathol 20(5): 441–444.
Nakai Y, Imanaka M, Nishio J & Ogita S (1997) Umbilical venous pulsation associated withhypercoiled cord in growth-retarded fetuses. Gynecol Obstet Invest 43(1): 64–67.
Narin N, Cetin N, Kilic H, Basbug M, Narin F, Kafali M, Uzum K, Genc E & Ustunbas HB (1999)Diagnostic value of troponin T in neonates of mild pre-eclamptic mothers. Biol Neonate 75(2):137–142.
Newnham JP, Evans SF, Michael CA, Stanley FJ & Landau LI (1993) Effects of frequent ultrasoundduring pregnancy: a randomised controlled trial. Lancet 342(8876): 887–891.
Nicolini U, Nicolaidis P, Fisk NM, Vaughan JI, Fusi L, Gleeson R & Rodeck CH (1990) Limited roleof fetal blood sampling in prediction of outcome in intrauterine growth retardation. Lancet336(8718): 768–772.
Noble MIM, Trenchard D & Guz A (1966) Left ventricular ejection in conscious dogs: II.Determinants of stroke volume. Circ Res 19:148–152.
Noble MIM (1968) The contribution of blood momentum to left ventricular ejection in the dog. CircRes 23:663–670.
Oberst L, Zhao G, Park JT, Brugada R, Michael LH, Entman ML, Roberts R & Marian AJ (1998)Dominant-negative effect of a mutant cardiac troponin T on cardiac structure and function intransgenic mice. J Clin Invest 102(8): 1498–1505.
Ohman EM, Armstrong PW, Christenson RH, Granger CB, Katus HA, Hamm CW, O’Hanesian MA,Wagner GS, Kleiman NS, Harrell FE, Jr., Califf RM & Topol EJ (1996) Cardiac troponin T levelsfor risk stratification in acute myocardial ischemia. GUSTO IIA Investigators. N Engl J Med335(18): 1333–1341.
O'Rourke MF (1982) Vascular impedance in studies of arterial and cardiac function. Physiol Rev62(2): 570–623.
Panos MZ, Nicolaides KH, Anderson JV, Economides DL, Rees L & Williams R (1989) Plasma atrialnatriuretic peptide in human fetus: response to intravascular blood transfusion. Am J ObstetGynecol 161(2): 357–361.

86
Pantely GA, Ladley HD & Bristow JD (1984) Low zero-flow pressure and minimal capacitance effecton diastolic coronary arterial pressure-flow relationships during maximum vasodilation in swine.Circulation 70(3): 485–494.
Peeters LL, Sheldon RE, Jones MD, Makowski EL & Meschia G (1979) Blood flow to fetal organsas a function of arterial oxygen content. Am J Obstet Gynecol 135(5): 637–646.
Pickoff AS (1998) Developmental electrophysiology in the fetus and neonate. In: (eds Polin RA, FoxWW) Fetal and Neonatal Physiology. W. B. Saunders Company, Philadelphia, 891-913.
Pool PE, Chandler BM, Seagren SC & Sonnenblick EH (1968) Mechanochemistry of cardiac muscle.II. The isotonic contraction. Circ Res 22(4): 465–472.
Rasanen J, Kirkinen P & Jouppila P (1988) Fetal aortic blood flow and echocardiographic findings inhuman pregnancy. Eur J Obstet Gynecol Reprod Biol 27(2): 115–124.
Rasanen J, Kirkinen P & Jouppila P (1989) Right ventricular dysfunction in human fetal compromise.Am J Obstet Gynecol 161(1): 136–140.
Rasanen J, Wood DC, Weiner S, Ludomirski A & Huhta JC (1996a) Role of the pulmonarycirculation in the distribution of human fetal cardiac output during the second half of pregnancy.Circulation 94(5): 1068–1073.
Rasanen J, Huhta JC, Weiner S, Wood DC & Ludomirski A (1996b) Fetal branch pulmonary arterialvascular impedance during the second half of pregnancy. Am J Obstet Gynecol 174(5): 1441–1449.
Rasanen J, Debbs RH, Wood DC, Weiner S, Weil SR & Huhta JC (1997) Human fetal rightventricular ejection force under abnormal loading conditions during the second half of pregnancy.Ultrasound Obstet Gynecol 10(5): 325–332.
Rasanen J, Wood DC, Debbs RH, Cohen J, Weiner S & Huhta JC (1998) Reactivity of the humanfetal pulmonary circulation to maternal hyperoxygenation increases during the second half ofpregnancy: a randomized study. Circulation 97(3): 257–262.
Rasanen J, Debbs RH, Wood DC, Weiner S & Huhta JC (1999) The effects of maternal indomethacintherapy on human fetal branch pulmonary arterial vascular impedance. Ultrasound ObstetGynecol 13(2): 112–116.
Reed KL, Meijboom EJ, Sahn DJ, Scagnelli SA, Valdes-Cruz LM & Shenker L (1986) CardiacDoppler flow velocities in human fetuses. Circulation 73(1): 41–46.
Reed KL (1997) Doppler--the fetal circulation. Clinical Obstet Gynecol 40(4): 750–754.Reed KL & Anderson CF (2000) Changes in umbilical venous velocities with physiologic
perturbations. Am J Obstet Gynecol 182(4): 835–838. Reller MD, Morton MJ, Reid DL & Thornburg KL (1987) Fetal lamb ventricles respond differently
to filling and arterial pressures and to in utero ventilation. Pediatr Res 22(6): 621–626.Reller MD, Morton MJ, Giraud GD, Wu DE & Thornburg KL (1992a) Maximal myocardial blood
flow is enhanced by chronic hypoxemia in late gestation fetal sheep. Am J Physiol 263(4 Pt 2):H1327–1329.
Reller MD, Morton MJ, Giraud GD, Wu DE & Thornburg KL (1992b) Severe right ventricularpressure loading in fetal sheep augments global myocardial blood flow to submaximal levels.Circulation 86(2): 581–588.
Reller MD, Burson MA, Lohr JL, Morton MJ & Thornburg KL (1995) Nitric oxide is an importantdeterminant of coronary flow at rest and during hypoxemic stress in fetal lambs. Am J Physiol269(6 Pt 2): H2074–2081.
Respondek M, Respondek A, Huhta JC & Wilczynski J (1992) 2D echocardiographic assessment ofthe fetal heart size in the 2nd and 3rd trimester of uncomplicated pregnancy. Eur J Obstet GynecolReprod Biol 44(3): 185–188.
Respondek ML, Kammermeier M, Ludomirsky A, Weil SR & Huhta JC (1994) The prevalence andclinical significance of fetal tricuspid valve regurgitation with normal heart anatomy. Am J ObstetGynecol 171(5): 1265–1270.

87
Reuss ML, Parer JT, Harris JL & Krueger TR (1982) Hemodynamic effects of alpha-adrenergicblockade during hypoxia in fetal sheep. Am J Obstet Gynecol 142(4): 410–415.
Reuss ML, Rudolph AM & Dae MW (1983) Phasic blood flow patterns in the superior and inferiorvenae cavae and umbilical vein of fetal sheep. Am J Obstet Gynecol 145(1): 70–78.
Richardson B, Nodwell A, Webster K, Alshimmiri M, Gagnon R & Natale R (1998) Fetal oxygensaturation and fractional extraction at birth and the relationship to measures of acidosis. Am JObstet Gynecol 178(3): 572–579.
Rizzo G, Arduini D, Romanini C & Mancuso S (1988) Doppler echocardiographic assessment ofatrioventricular velocity waveforms in normal and small-for-gestational-age fetuses. Br J ObstetGynaecol 95(1): 65–69.
Rizzo G & Arduini D (1991) Fetal cardiac function in intrauterine growth retardation. Am J ObstetGynecol 165(4 Pt 1): 876–882.
Rizzo G, Arduini D & Romanini C (1992) Umbilical vein pulsations: a physiologic finding in earlygestation. Am J Obstet Gynecol 167(3): 675–677.
Rizzo G, Capponi A, Rinaldo D, Arduini D & Romanini C (1995a) Ventricular ejection force ingrowth-retarded fetuses. Ultrasound Obstet Gynecol 5(4): 247–255.
Rizzo G, Capponi A, Arduini D & Romanini C (1995b) The value of fetal arterial, cardiac and venousflows in predicting pH and blood gases measured in umbilical blood at cordocentesis in growthretarded fetuses. Br J Obstet Gynaecol 102(12): 963–969.
Rizzo G, Capponi A, Soregaroli M, Arduini D & Romanini C (1995c) Umbilical vein pulsations andacid-base status at cordocentesis in growth-retarded fetuses with absent end-diastolic velocity inumbilical artery. Biol Neonate 68(3): 163–168.
Rochelson B, Schulman H, Farmakides G, Bracero L, Ducey J, Fleischer A, Penny B & Winter D(1987a) The significance of absent end-diastolic velocity in umbilical artery velocity waveforms.Am J Obstet Gynecol 156(5): 1213–1218.
Rochelson BL, Schulman H, Fleischer A, Farmakides G, Bracero L, Ducey J, Winter D & Penny B(1987b) The clinical significance of Doppler umbilical artery velocimetry in the small forgestational age fetus. Am J Obstet Gynecol 156(5): 1223–1226.
Romero T, Covell J & Friedman WF (1972) A comparison of pressure-volume relations of the fetal,newborn, and adult heart. Am J Physiol 222(5): 1285–1290.
Rooke GA & Feigl EO (1982) Work as a correlate of canine left ventricular oxygen consumption, andthe problem of catecholamine oxygen wasting. Circ Res 50(2): 273–286.
Rosenzweig A & Seidman CE (1991) Atrial natriuretic factor and related peptide hormones. AnnuRev Biochem 60:229–255.
Rudolph AM & Heymann MA (1970) Circulatory changes during growth in the fetal lamb. Circ Res26(3): 289–299.
Rudolph AM (1985) Distribution and regulation of blood flow in the fetal and neonatal lamb. CircRes 57(6): 811–821.
Ruskoaho H, Toth M & Lang RE (1985) Atrial natriuretic peptide secretion: synergistic effect ofphorbol ester and A23187. Biochem Biophys Res Commun 133(2): 581–588.
Ruskoaho H (1992) Atrial natriuretic peptide: synthesis, release, and metabolism. Pharmacol Rev44(4): 479–602.
Saba Z, Nassar R, Ungerleider RM, Oakeley AE & Anderson PA (1996) Cardiac troponin T isoformexpression correlates with pathophysiological descriptors in patients who underwent correctivesurgery for congenital heart disease. Circulation 94(3): 472–476.
Sagnella GA (1998) Measurement and significance of circulating natriuretic peptides incardiovascular disease. Clin Sci 95(5): 519–529.
Salafia CM, Minior VK, Lopez-Zeno JA, Whittington SS, Pezzullo JC & Vintzileos AM (1995)Relationship between placental histologic features and umbilical cord blood gases in pretermgestations. Am J Obstet Gynecol 173(4): 1058–1064.

88
Salafia CM (1997) Placental pathology of fetal growth restriction. In: (Pitkin, R. M. Scott, J. R)Clinical Obstetrics and Gynecology. Lippincott - Raven Publishers, Philadelphia, 740-749.
Salvesen KA & Eik-Nes SH (1999) Ultrasound during pregnancy and birthweight, childhoodmalignancies and neurological development. Ultrasound Med Biol 25(7): 1025–1031.
Sarnoff SJ (1958) Hemodynamic determinants of oxygen consumption of the heart with specialreference to the tension-time index. Am J Physiol 192:148-156.
Schmidt KG, Di Tommaso M, Silverman NH & Rudolph AM (1991) Doppler echocardiographicassessment of fetal descending aortic and umbilical blood flows. Validation studies in fetal lambs.Circulation 83(5): 1731–1737.
Schulman H, Fleischer A, Farmakides G, Bracero L, Rochelson B & Grunfeld L (1986) Developmentof uterine artery compliance in pregnancy as detected by Doppler ultrasound. Am J ObstetGynecol 155(5): 1031–1036.
Sestier FJ, Mildenberger RR & Klassen GA (1978) Role of autoregulation in spatial and temporalperfusion heterogeneity of canine myocardium. Am J Physiol 235(1): H64–71.
Severi FM, Rizzo G, Bocchi C, D’Antona D, Verzuri MS & Arduini D (2000) Intrauterine growthretardation and fetal cardiac function. Fetal Diagn Ther 15(1): 8–19.
Shelton SD, Fouse BL, Holleman CM, Sedor FA & Herbert WN (1999) Cardiac troponin T levels inumbilical cord blood. Am J Obstet Gynecol 181(5 Pt 1): 1259–1262.
Shilo L, Dolev S & Shenkman L (1989) Atrial natriuretic peptide and digoxin-like factor in theperipartum period. J Clin Pharmacol 29(12): 1106–1107.
Shivvers SA, Wians FH, Jr., Keffer JH & Ramin SM (1999) Maternal cardiac troponin I levels duringnormal labor and delivery. Am J Obstet Gynecol 180(1 Pt 1): 122.
Sonnenblick EH, Ross J, Covell JW, Kaiser GA & Braunwald E (1965) Velocity of contraction as adeterminant of myocardial oxygen consumption. Am J Physiol 209(5): 919–927.
Sonnenblick EH & Skelton CL (1971) Myocardial energetics: basic principles and clinicalimplications. N Engl J Med 285(12): 668–675.
Soothill PW, Nicolaides KH, Rodeck CH & Campbell S (1986) Effect of gestational age on fetal andintervillous blood gas and acid-base values in human pregnancy. Fetal Therapy 1(4): 168–175.
Soregaroli M, Rizzo G, Danti L, Arduini D & Romanini C (1993) Effects of maternalhyperoxygenation on ductus venosus flow velocity waveforms in normal third-trimester fetuses.Ultrasound Obstet Gynecol 3:115–119.
Spaan JA (1985) Coronary diastolic pressure-flow relation and zero flow pressure explained on thebasis of intramyocardial compliance. Circ Res 56(3): 293–309.
Stale H, Marsal K, Gennser G, Benthin M, Dahl P & Lindstrom K (1991) Aortic diameter pulse wavesand blood flow velocity in the small, for gestational age, fetus. Ultrasound Med Biol 17(5): 471–478.
Sudoh T, Kangawa K, Minamino N & Matsuo H (1988) A new natriuretic peptide in porcine brain.Nature 332(6159): 78–81.
Sudoh T, Minamino N, Kangawa K & Matsuo H (1990) C-type natriuretic peptide (CNP): a newmember of natriuretic peptide family identified in porcine brain. Biochem Biophys Res Commun168(2): 863–870.
Suga S, Nakao K, Itoh H, Komatsu Y, Ogawa Y, Hama N & Imura H (1992) Endothelial productionof C-type natriuretic peptide and its marked augmentation by transforming growth factor-beta.Possible existence of "vascular natriuretic peptide system". J Clin Invest 90(3): 1145–1149.
Sundsfjord JA, Thibault G, Larochelle P & Cantin M (1988) Identification and plasma concentrationsof the N-terminal fragment of proatrial natriuretic factor in man. J Clin Endocrinol Metab 66(3):605–610.
Sutton MS, Theard MA, Bhatia SJ, Plappert T, Saltzman DH & Doubilet P (1990) Changes inplacental blood flow in the normal human fetus with gestational age. Pediatr Res 28(4): 383–387.

89
Sutton MG, Plappert T & Doubilet P (1991a) Relationship between placental blood flow andcombined ventricular output with gestational age in normal human fetus. Cardiovasc Res 25(7):603–608.
Sutton MS, Gill T, Plappert T, Saltzman DH & Doubilet P (1991b) Assessment of right and leftventricular function in terms of force development with gestational age in the normal human fetus.Br Heart J 66(4): 285–289.
Sutton MS, Groves A, MacNeill A, Sharland G & Allan L (1994) Assessment of changes in bloodflow through the lungs and foramen ovale in the normal human fetus with gestational age: aprospective Doppler echocardiographic study. Br Heart J 71(3): 232–237.
Tarantal AF & Hendrickx AG (1989a) Evaluation of the bioeffects of prenatal ultrasound exposurein the cynomolgus macaque (Macaca fascicularis): I. Neonatal/infant observations. Teratology39(2): 137–147.
Tarantal AF & Hendrickx AG (1989b) Evaluation of the bioeffects of prenatal ultrasound exposurein the cynomolgus macaque (Macaca fascicularis): II. Growth and behavior during the first year.Teratology 39(2): 149–162.
Tarantal AF, O’Brien WD & Hendrickx AG (1993) Evaluation of the bioeffects of prenatalultrasound exposure in the cynomolgus macaque (Macaca fascicularis): III. Developmental andhematologic studies. Teratology 47(2): 159–170.
Tarantal AF, Gargosky SE, Ellis DS, O’Brien WD, Jr. & Hendrickx AG (1995) Hematologic andgrowth-related effects of frequent prenatal ultrasound exposure in the long-tailed macaque(Macaca fascicularis). Ultrasound Med Biol 21(8): 1073–1081.
Tei C, Ling LH, Hodge DO, Bailey KR, Oh JK, Rodeheffer RJ, Tajik AJ & Seward JB (1995) Newindex of combined systolic and diastolic myocardial performance: a simple and reproduciblemeasure of cardiac function – a study in normals and dilated cardiomyopathy. J Cardiol 26(6):357–366.
Teitel DF (1998) Physiologic development of the cardiovascular system in the fetus. In: (eds PolinRA, Fox WW) Fetal and Neonatal Physiology. W. B. Saunders Company, Philadelphia, 827-836.
Tekay A & Jouppila P (2000) Fetal adrenal artery velocimetry measurements in appropriate-for-gestational age and intrauterine growth-restricted fetuses. Ultrasound Obstet Gynecol 16(5): 419–424.
Tessler FN, Kimme-Smith C, Sutherland ML, Schiller VL, Perrella RR & Grant EG (1990) Inter- andintra-observer variability of Doppler peak velocity measurements: an in-vitro study. UltrasoundMed Biol 16(7): 653–657.
Thaler I, Weiner Z & Itskovitz J (1992) Systolic or diastolic notch in uterine artery blood flowvelocity waveforms in hypertensive pregnant patients: relationship to outcome. Obstet Gynecol80(2): 277–282.
Thibault G, Murthy KK, Gutkowska J, Seidah NG, Lazure C, Chretien M & Cantin M (1988) NH2-terminal fragment of rat pro-atrial natriuretic factor in the circulation: identification,radioimmunoassay and half-life. Peptides 9(1): 47–53.
Thompson RS (1987) Blood flow velocity waveforms. Semin Perinatol 11(4): 300–310.Thompson RS & Trudinger BJ (1990) Doppler waveform pulsatility index and resistance, pressure
and flow in the umbilical placental circulation: an investigation using a mathematical model.Ultrasound Med Biol 16(5): 449–458.
Thornburg KL & Morton MJ (1983) Filling and arterial pressures as determinants of RV strokevolume in the sheep fetus. Am J Physiol 244(5): H656–663.
Townsend PJ, Farza H, MacGeoch C, Spurr NK, Wade R, Gahlmann R, Yacoub MH & Barton PJ(1994) Human cardiac troponin T: identification of fetal isoforms and assignment of the TNNT2locus to chromosome 1q. Genomics 21(2): 311–316.
Trudinger BJ, Giles WB & Cook CM (1985) Uteroplacental blood flow velocity-time waveforms innormal and complicated pregnancy. Br J Obstet Gynaecol 92(1): 39–45.

90
Trudinger BJ, Stevens D, Connelly A, Hales JR, Alexander G, Bradley L, Fawcett A & ThompsonRS (1987) Umbilical artery flow velocity waveforms and placental resistance: the effects ofembolization of the umbilical circulation. Am J Obstet Gynecol 157(6): 1443–1448.
Tsuchimochi H, Kurimoto F, Ieki K, Koyama H, Takaku F, Kawana M, Kimata S & Yazaki Y (1988)Atrial natriuretic peptide distribution in fetal and failed adult human hearts. Circulation 78(4):920–927.
Tsutsumi T, Ishii M, Eto G, Hota M & Kato H (1999) Serial evaluation for myocardial performancein fetuses and neonates using a new Doppler index. Pediatr Int 41(6): 722–727.
Tsyvian P, Malkin K & Wladimiroff JW (1995) Assessment of fetal left cardiac isovolumic relaxationtime in appropriate and small-for-gestational-age fetuses. Ultrasound Med Biol 21(6): 739–743.
Tulzer G, Gudmundsson S, Rotondo KM, Wood DC, Yoon GY & Huhta JC (1991a) Acute fetalductal occlusion in lambs. Am J Obstet Gynecol 165(3): 775–778.
Tulzer G, Gudmunsson S, Rotondo KM, Wood DC, Cohen AW & Huhta JC (1991b) Doppler in theevaluation and prognosis of fetuses with tricuspid regurgitation. J Matern Fetal Invest 1:15–18.
Tulzer G, Khowsathit P, Gudmundsson S, Wood DC, Tian ZY, Schmitt K & Huhta JC (1994)Diastolic function of the fetal heart during second and third trimester: a prospective longitudinalDoppler-echocardiographic study. Eur J Pediatr 153(3): 151–154.
Ursem NT, Struijk PC, Hop WC, Clark EB, Keller BB & Wladimiroff JW (1998) Heart rate and flowvelocity variability as determined from umbilical Doppler velocimetry at 10–20 weeks ofgestation. Clin Sci 95(5): 539–545.
van den Wijngaard JA, Groenenberg IA, Wladimiroff JW & Hop WC (1989) Cerebral Dopplerultrasound of the human fetus. Br J Obstet Gynaecol 96(7): 845–849.
van Splunder IP, Stijnen T & Wladimiroff JW (1995) Fetal pressure gradient estimations across theductus venosus in early pregnancy using Doppler ultrasonography. Ultrasound Obstet Gynecol6(5): 334–339.
van Splunder P, Stijnen T & Wladimiroff JW (1996) Fetal atrioventricular flow-velocity waveformsand their relation to arterial and venous flow-velocity waveforms at 8 to 20 weeks of gestation.Circulation 94(6): 1372–1378.
Vatner SF (1984) Alpha-adrenergic tone in the coronary circulation of the conscious dog. FederationProceedings 43(14): 2867–2872.
Veille JC, Smith N & Zaccaro D (1999) Ventricular filling patterns of the right and left ventricles innormally grown fetuses: a longitudinal follow-up study from early intrauterine life to age 1 year.Am J Obstet Gynecol 180(4): 849–858.
Ville Y, Proudler A, Abbas A & Nicolaides K (1994) Atrial natriuretic factor concentration in normal,growth-retarded, anemic, and hydropic fetuses. Am J Obstet Gynecol 171(3): 777–783.
Vuolteenaho O, Koistinen P, Martikkala V, Takala T & Leppaluoto J (1992) Effect of physicalexercise in hypobaric conditions on atrial natriuretic peptide secretion. Am J Physiol 263(3 Pt 2):R647–652.
Vyas S, Nicolaides KH, Bower S & Campbell S (1990) Middle cerebral artery flow velocitywaveforms in fetal hypoxaemia. Br J Obstet Gynaecol 97(9): 797–803.
Walther T, Stepan H & Faber R (2001) Dual natriuretic peptide response to volume load in the fetalcirculation. Cardiovasc Res 49(4): 817–819.
Wladimiroff JW, Tonge HM & Stewart PA (1986) Doppler ultrasound assessment of cerebral bloodflow in the human fetus. Br J Obstet Gynaecol 93(5): 471–475.
Wladimiroff JW, van den Wijngaard JA, Degani S, Noordam MJ, van Eyck J & Tonge HM (1987)Cerebral and umbilical arterial blood flow velocity waveforms in normal and growth-retardedpregnancies. Obstet Gynecol 69(5): 705–709.
Wladimiroff JW, Huisman TW & Stewart PA (1991) Fetal and umbilical flow velocity waveformsbetween 10–16 weeks' gestation: a preliminary study. Obstet Gynecol 78(5 Pt 1): 812–814

91
Woo JS, Liang ST, Lo RL & Chan FY (1987) Middle cerebral artery Doppler flow velocitywaveforms. Obstet Gynecol 70(4): 613–616.
Yan W, Wu F, Morser J & Wu Q (2000) Corin, a transmembrane cardiac serine protease, acts as apro-atrial natriuretic peptide-converting enzyme. Proc Natl Acad Sci U S A 97(15): 8525–8529.
Yandle TG (1994) Biochemistry of natriuretic peptides. J Intern Med 235(6): 561–576.