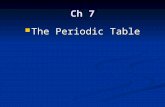
Periodic Table of the Elements Chemistry Reference Sheet...
Transcript of Periodic Table of the Elements Chemistry Reference Sheet...
Periodic Table of the Elements Chemistry Reference Sheet California Standards Test
Sodium 22.99
Na 11
Element symbol
* Element name
Hydrogen 1.01
H 1
Lithium 6.94
Li 3
Sodium 22.99
Na 11
39.10
19
K 58.69
Ni 28
Rubidium 85.47
Rb 37
140.91
Pr 59
(261)
Rf 104
95.94
Mo 42
(258)
Md 101
72.61
Ge 32
1 1A
2 2A
1
2
3
4
7 7B
11 1B
12 2B
13 3A
16 6A
8
5
6
7
9 8B
10
14 4A
15 5A
17 7A
18 8A
3 3B
4 4B
5 5B
6 6B
Copper 63.55
Cu 29
Cobalt 58.93
Co 27
Helium 4.00�
He 2
Boron 10.81
B 5
Carbon 12.01�
C 6
Nitrogen 14.01
N 7
Oxygen 16.00
O 8
19.00�
F 9
Neon 20.18�
Ne 10
26.98
Al 13
Silicon 28.09�
Si 14
30.97
P 15
Sulfur 32.07
S 16
35.45
Cl 17
Argon 39.95
Ar 18
Calcium 40.08
Ca 20
Scandium 44.96
Sc 21
Titanium 47.87
Ti 22
Chromium 52.00
Cr 24
Iron 55.85
Fe 26
Zinc 65.39
Zn 30
Gallium 69.72
Ga 31
Arsenic 74.92
As 33
Selenium 78.96
Se 34
Bromine 79.90
Br 35
83.80
Kr 36
Strontium 87.62
Sr 38
88.91
Y 39
Zirconium 91.22
Zr 40
Niobium 92.91
Nb 41
(98)
Tc 43
Ruthenium 101.07
Ru 44
Rhodium 102.91
Rh 45
106.42
46
107.87
Ag 47
Cadmium 112.41
Cd 48
Indium 114.82
In 49
Tin 118.71
Sn 50
121.76
Sb 51
127.60
52
Iodine 126.90
I 53
Xenon 131.29
Xe 54
Cesium 132.91
Cs 55
137.33
Ba 56
138.91
La 57
Hafnium 178.49
Hf 72
180.95
73
183.84
W 74
Rhenium 186.21
Re 75
Osmium 190.23
Os 76
192.22
Ir 77
195.08
Pt 78
Gold 19 7
79
200.59
Hg 80
Thallium 204.38
Tl 81
Lead 207.2
Pb 82
208.98
Bi 83
(209)
84
Astatine (210)
At 85
Pd
Radon (222)
Rn 86
(223)
Fr 87
Radium (226)
Ra 88
Actinium (227)
Ac 89
Dubnium (262)
Db 105
Seaborgium (266)
Sg 106
(264)
Bh 107
Hassium (269)
Hs 108
(268)
Mt 109
140.12
Ce 58
Neodymium 144.24
Nd 60
Promethium (145)
Pm 61
150.36
Sm 62
Europium 151.96
Eu 63
Gadolinium 157.25
Gd 64
158.93
Tb 65
Dysprosium 162.50
Dy 66
Holmium 164.93
Ho 67
Erbium 167.26
Er 68
Thulium 168.93
Tm 69
Ytterbium 173.04
Yb 70
Lutetium 174.97
Lu 71
232.04
Th 90
Protactinium 231.04
91
238.03
U 92
Neptunium (237)
Np 93
Plutonium (244)
Pu 94
(243)
Am 95
(247)
Cm 96
(247)
Bk 97
(251)
Cf 98
Einsteinium (252)
Es 99
(257)
Fm 100
Nobelium (259)
No 102
(262)
Lr 103
Magnesium 24.31
Mg 12
9.01
Be 4
50.94
V 23
Manganese 54.94
Mn 25
*
Atomic number
Average atomic mass
Potassium Nickel
Praseodymium
Rutherfordium
Molybdenum
Mendelevium
Germanium
Key
Fluorine
Aluminum Phosphorus Chlorine
Krypton
Yttrium Technetium Palladium Silver Antimony Tellurium Te
Barium Lanthanum Tantalum Ta
Tungsten Iridium Platinum 6.9
Au Mercury Bismuth Polonium
Po
Francium Bohrium Meitnerium
Cerium Samarium Terbium
Thorium Pa
Uranium Americium Curium Berkelium Californium Fermium Lawrencium
Beryllium
Vanadium
If this number is in parentheses, then it refers to the atomic mass of the most stable isotope.
Copyright © 2003 California Department of Education
Fe3+
Fe2+iron (III)
iron (II)
26atomicnumber
ionchargeionname
symbol (IUPAC)
KEY
58 59 60 61 62 63 64 65 66 67 68 69 70 71
90 91 92 93 94 95 96 97 98 99 100 101 102 103
Ce3+
ceriumPr3+
praseodymiumNd3+
neodymiumPm3+
promethium
Sm3+samarium(III)
samarium(II)
Eu3+
Eu2+europium (III)
europium (II)
Th4+
thorium
Pa5+
Pa4+protactinium(V)
protactinium(IV)
U6+
U4+uranium (VI)
uranium (IV)
Gd3+
gadoliniumTb3+
terbiumDy3+
dysprosiumHo3+
holmiumEr3+
erbiumTm3+
thulium
Yb3+
Yb3+ytterbium(III)
ytterbium(II)Sm2+
Lu3+
lutetium
Np5+ Pu4+
Pu6+plutonium(IV)
Am3+
Am4+americium(IV)
americium(III)neptunium
Bk3+
Bk4+berkelium(IV)
berkelium(III)Cm3+
curiumCf3+
californiumEs3+
einsteiniumFm3+
fermium
Md2+
Md3+mendelevium (II)
mendelevium (III)
Lr3+
lawrencium
No2+
No3+nobelium(II)
nobelium(III)plutonium(VI)
PERIODIC TABLE OF IONSacetatearsenatearsenitebenzoateboratebromatecarbonatechloratechloridechloritechromatecyanatecyanidedichromate
CH3COO–
AsO43–
AsO33–
C6H5COO–
BO33–
BrO3 –
CO32–
ClO3 –
Cl–
ClO2 –
CrO42–
CNO–
CN–
Cr2O72–
oxalateperchlorateperiodatepermanganateperoxidephosphatepyrophosphatesulfatesulfitethiocyanatethiosulfate
ammoniumhydronium
C2O42–
ClO4 –
IO4 –
MnO4 –
O22–
PO43–
P2O74–
SO42–
SO32–
SCN–
S2O32–
NH4+
H3O+
POSITIVE POLYATOMIC IONS
TABLE OF POLYATOMIC IONS
H2PO4 –
HCO3 –
HC2O4 –
HSO4 –
HS–
HSO3 –
OH–
ClO–
IO3 –
HPO42–
NO3 –
NO2 –
SiO44–
hydrogen carbonatehydrogen oxalatehydrogen sulfatehydrogen sulfidehydrogen sulfitehydroxidehypochloriteiodate
nitratenitriteorthosilicate
monohydrogen phosphate
dihydrogen phosphate
1 2
3 4 5 6 7 8 9 10
13 14 15 16 17 1811 12
19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36Ti4+titanium (IV)
Ti3+titanium (III)
V3+vanadium(III)
V5+vanadium (V)
Cr3+
Cr2+chromium (III)
chromium (II)
Mn2+
Mn4+manganese(II)
manganese(IV)
Fe3+
Fe2+iron (III)
iron (II)
Co2+
Co3+cobalt (II)
cobalt (III)
Ni2+
Ni3+nickel (II)
nickel (III)
Cu2+
Cu+copper (II)
copper (I)
Ga3+
galliumSc3+
scandium
Y3+
yttrium
La3+
lanthanum
Ac3+
actinium
Zr4+
zirconium
Hf4+
hafnium
Nb5+
Nb3+niobium (V)
niobium(III)
37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54
Mo6+molybdenum
Rh3+
rhodium
Ru3+
Ru4+ruthenium(III)
ruthenium(IV)
Pd2+
Pd4+paladium(II)
paladium(IV)
Ag+
silverCd2+
cadmium
Pt4+
Pt2+platinum(IV)
platinum(II)
55 56 57 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86Au3+
Au+gold (III)
gold (I)
Hg2+
Hg+mercury (II)
mercury (I)
Tl+
Tl3+thallium (I)
thallium(III)
Pb2+
Pb4+lead (II)
lead (IV)
Bi3+
Bi5+bismuth(III)
bismuth(V)
Sn4+
Sn2+tin (IV)
tin (II)
Sb3+
Sb5+antimony(III)
antimony(V)
Tc7+
Ta5+
tantalumW6+
tungstenRe7+
rheniumOs4+
osmiumIr4+
iridium
87 88 89
H+hydrogen
Li+lithium
Be2+beryllium
Na+sodium
Mg2+magnesium
K+
potassiumCa2+
Rb+
rubidiumSr2+
strontium
Cs+cesium
Ba2+
calcium
barium
Fr+francium
Ra2+radium
Bboron
Ccarbon nitride
N3-oxideO2-
fluorideF-
neonNe
Al3+aluminum
Sisilicon phosphide
P3-sulfiide
S2-chloride
Cl-argonAr
heliumHe
Zn2+
zinc
In3+
indium
Ge4+
germaniumAs3-
arsenide selenideSe2-
bromideBr-
kryptonKr
tellurideTe2-
Po2+polonium(II)
polonium(IV)Po4+
iodideI-
xenonXe
astatideAt-
radonRn
hydrideH-
1
1
2
3 4 5 6 7 8 9 10 11 12
13 14 15 16
17 18
technetium
List of Common Multivalent Ions
The following elements form multivalent ions, and therefore require a
Roman numeral charge when writing the name of the compound. Rare and
synthetic elements are not included in this list.
Element Sym. Possible Charges
Element Sym. Possible Charges
Titanium Ti +2, +3, +4 Tin Sn +2, +4 Vanadium V +2, +3, +4, +5 Rhenium Re +4, +6, +7 Chromium Cr +2, +3, +6 Osmium Os +3, +4 Manganese Mn +2, +3, +4, +7 Iridium Ir +3, +4
Iron Fe +2, +3 Platinum Pt +2, +4 Cobalt Co +2, +3 Gold Au +1, +3 Nickel Ni +2, +3 Mercury Hg +1, +2 Copper Cu +1, +2 Thallium Tl +1, +3
Niobium Nb +2, +5 Lead Pb +2, +4 Molybdenum Mo +3, +6 Bismuth Bi +3, +5
Palladium Pd +2, +4 Polonium Po +2, +4
Steps to Determine Charge from the Chemical Formula
1. Find total negative charge on all anions.
2. Divide value by number of cations to give charge on one multivalent
cation.
Lab Safety
Safety is essential in a science lab, due to the serious risks that can occur from mishandled chemicals,
glassware and other material. It is vital that all students are aware of the lab’s safety equipment, in
particular where it is stored and how to use it. Additionally, students need to act in a conscientious and
cautious manner when conducting an experiment or working in the lab.
Safety Equipment
Eyewash Station
An eyewash is used to flush out one or both eyes if they have been contacted with a chemical or
abrasive substance. In serious cases, flushing should occur immediately for at least five minutes (or
more, depending on the nature of the substance) using a water faucet.
First Aid Kit
1. The first aid kit contains a number of items for dealing with minor cuts, burns and scrapes. Inform
Ms. Hayduk of any injuries incurred during a lab, regardless of how severe they are.
Fire Extinguisher
Fire extinguishers should be used for putting out small to medium sized fires that are uncontrolled.
Before using a fire extinguisher, it is important to ensure it is appropriate for the type of fire.
Class Use Symbol
A Ordinary combustible materials (e.g. paper,
wood, cardboard and most plastics) Green triangle
B Flammable or combustible liquids (e.g.
gasoline, kerosene, grease and oil) Red square
C Electrical equipment Blue circle
D Combustible metals (e.g. magnesium,
potassium and sodium) Yellow decagon
K Kitchen fires (e.g. cooking oil, trans-fats, fats) Black hexagon
An ABC fire extinguisher, or an all-purpose fire extinguisher, is the most common, because it will put
out the most common types of fire.
Spill Kit
2. A spill kit contains a number of absorbent substances that can be used to neutralize and safety clean
up chemical spills in the lab. A serious chemical spill includes substances that cannot be handled
safely (highly corrosive) or an unknown mixture of chemicals. If a serious chemical spill occurs, find
Mrs. Hayduk immediately; do not attempt to clean up the spill. Ensure that other students remain
clear of the area.
Personal Protective Equipment
3. Personal protective equipment refers to all clothing, helmets and eyewear designed to protect the
wearer from injury. Prior to any lab, Ms. Hayduk will inform you which equipment is required to be
worn during the activity. Students must wear all of this equipment for the duration of the lab, even
if they have completed the activity.
Specific Emergency Procedures
Fire
If the fire is small, use the appropriate fire extinguisher on the fire or extinguish it with a lid or
blanket.
To use a fire extinguisher, follow the acronym PASS: pull out the pin, aim the nozzle at the base of
the fire, squeeze the trigger and sweep the spray along the base of the fire until it is extinguished.
If the fire is large and cannot be controlled, leave the room immediately and pull the fire alarm. All
students should file out of the building in a calm manner to the designated meeting spot outside.
The last student out of the room should shut the door to impede spread of the fire.
If a fire alarm goes off during an experiment, students should shut off all gas and heat sources
before exiting the lab.
Spill
If the spill is a harmless substance (e.g. water, vinegar), clean it up immediately with rags or paper
towel.
If the spill is a more hazardous substance, inform Ms. Hayduk immediately. She will clean the spill
with the spill kit. If possible, block the spill from spreading and remove any books, bags or personal
items from the area.
If more than one chemical spills in the same area, tell Ms. Hayduk and evacuate the room
immediately. She will assess the danger and take steps to decontaminate the area
Injury
For minor injuries, use the first aid kit. Make sure to clean any cuts or scrapes before applying a
bandage.
For serious injuries, inform Ms. Hayduk immediately and use a cell phone or the office phone to call
911. Make sure to tell EMS if the injury was caused by contact with a chemical (or broken glass
contaminated with chemical).
Laboratory Safety Procedures
This list of safety procedures is general, and does not cover all aspects of safety in the lab. It is important
that students use common sense and caution when working in the science lab, and ask for help when
instructions or procedures are unclear.
1. Behave in a calm, professional, responsible manner at all times.
2. No food in the lab at any time.
Beverages are allowed provided they are in re-sealable containers.
Never eat any materials being used for experiments.
3. Use the appropriate personal protective equipment for the activity you (or others) are performing.
Do not remove your PPE until you are instructed to do so by the teacher.
4. Keep yourself, your equipment and your workstation clean before, during and after the lab.
Handle equipment with care.
Wash glassware thoroughly with soap and water.
After handling chemicals, wash hands thoroughly with soap and water.
Keep aisles and table tops clear of bags and books.
5. Dispose of materials properly.
Do not dump any chemicals down the drain unless instructed to by the teacher.
Do not put any solid material in the drains.
Sharp materials (e.g. dissection pins, broken glass) should be disposed of in the proper
waste container – never in the garbage can.
6. Do not touch any chemicals or equipment you have not been instructed to handle.
Do not smell or taste chemicals.
Do not try any unauthorized experiments.
Do not enter the science storage room.
7. Never leave your lab station unattended.
8. Dress appropriately.
Tie back long hair.
Avoid wearing loose or dangling clothes or jewelry around chemicals or open flames.
Wear closed-toed shoes.
9. Report any accident or incident immediately.
Students who do not follow these safety procedures will be asked to leave the lab, and will be given an
alternate written assignment to complete.
WHMIS
WHMIS stands for Workplace Hazardous Material Information System. It is a program designed to protect
workers (e.g. students and teachers) who are handling chemicals on a regular basis. There are three key
elements to WHMIS:
1. Labels,
2. Material safety data sheets; and,
3. Education and training.
As a student, your primary concern will be with labels and material safety data sheets (MSDS).
Labels
The purpose of a WHMIS label is to identify the product as controlled and alert the user to the hazards and
safe handling procedures of the product. The label provides a summary of the important information about
the substance, including:
1. Name of chemical and common name
2. Hazard symbols
3. Risks associate with the substance and precautions to take when using it
4. First aid measures
5. Supplier information
6. Reference to MSDS, stating that more information is available
Chemicals that are going to be stored in beakers, flasks or test tubes should be labelled with a name, date
and some identifier of what is contained in it. If you are doing work in the lab and are planning to store
chemicals, you must ensure this is done.
Hazard Symbols
Class Controlled Product Dangers
A
Compressed Gas
It is a gas kept under pressure
Heat may cause the container to explode
Drop or impact may cause the container to explode
Compressed gases can be dangerous because the contents are
under pressure, but also because the gas may be poisonous
B
Flammable and Combustible Material
Material may burn at a low temperature, and may catch on fire
from sparks, flame or friction
May burst into flame in air or release a flammable gas if it comes
into contact with water
C
Oxidizing Material
Substance is a fire or explosion risk near flammable or
combustible material
May not burn itself, but could release oxygen or other gases that
might make other materials burn more easily
D
Poisonous and Infectious Material
Material is a potentially fatal poisonous substance that could kill
or cause permanent damage if inhaled, swallowed or absorbed
through skin
Division 1: Material Causing Immediate and Serious Toxic Effects
Immediately dangerous to life and health
Division 2: Materials Causing Other Toxic Effects
Material is poisonous but not immediately dangerous
May cause death or permanent damage as a result of repeated
exposure over time
May cause irritation
Division 3: Biohazardous Infectious Material
An organism and the toxins they produce that may cause disease
E
Corrosive Material Acidic or caustic materials that can eat through skin or corrode
metals
F
Dangerously Reactive Material
Substance could undergo dangerous reactions when subject to
heat, pressure, shock or contact with other materials
International Hazard Symbols
Not all products and substances are controlled by WHMIS, so they may not have WHMIS labels or
symbols. These are other symbols you may see on other household products. The border of the
symbol represents the level of danger, and the symbol inside represents the specific hazard.
“Danger”, shown with an octagon, is the biggest threat. “Caution”, given by an upside down triangle,
is a smaller threat but should still be considered dangerous!
Danger Warning Caution
Poison
Flammable
Explosive
Corrosive
Material Safety Data Sheets
Material safety data sheets (MSDS) are used to give more detailed information about the product
than the information on the WHMIS label. The information includes:
A description of the chemical
Hazardous ingredients
Physical and chemical data
Fire and explosive hazard (how easily it will catch on fire or explode)
Reactivity data
Toxicological properties (how toxic it is to humans and other animals)
Preventative measures to take when handling and storing it
First aid measures to be taken if exposed
When it was made and who to contact for more information
MSDS are available online, as well as in a binder in the school. They are all current within three
years for all of the chemicals available in the school. It is expected that all students will review the
MSDS for important chemicals prior to every lab so they know the safety hazards and the personal
protective equipment they should wear.
Experimental Error and Measurements
Accuracy and Precision
Experimental error is the difference between a measurement and the true value, or between two
measured values. This is measured by accuracy and precision.
Accuracy tells how close a measured value is to the theoretical, accepted or “true” value. If this
value is unknown, then the accuracy of a measurement cannot be determined.
Precision measures how close two or more measurements are to each other. Precision can also be
called “repeatability” or “reproducibility”. A measurement that is highly precise, or highly
reproducible, will give values that are very close.
Experimental Error
Human errors are avoidable mistakes that come from mistakes, blunders or miscalculations. These
are not considered sources of experimental error, since they can be eliminated by careful technique
and by repeating the procedure correctly. Examples include spilling chemicals, using the wrong
calculation or missing a step in the procedure.
Systematic errors, or bias, are a type of experimental error that affect accuracy of a measurement.
They are generally caused by flaws in equipment or in reading measurements, and are generally
difficult to detect. These errors are “one-sided”, meaning they will usually give results that are close
to each other, but are not close to the true value. Systematic errors can be caused by poor
calibration of measuring instruments, poorly maintained or poorly constructed instruments and
faulty reading of measurements by the user (e.g. reading volume at an angle).
Random errors affect the precision of a measurement. These errors are “two-sided” because they
can fluctuate above or below the true value in repeated trials. Random errors can be reduced by
repeating the procedure and taking average values or by using better quality instruments. Common
sources of random errors include estimating a measurement that is between graduations on an
instrument or recording a value that fluctuates during the reading.
Sample Sources of Error
Limited accuracy of measuring tools (how many decimal places the measurement can reasonably have)
Accidental spills or residue from pouring from one container to another
Contaminants on equipment or in chemicals Lots of measurements that increase uncertainty in calculations
Measuring tools not calibrated properly Not reading volumes and temperatures directly at eye-level
Impurities in chemicals Reaction does not go to completion
Not enough trials or data Volumes are too small to read easily
Calculating Experimental Error
Accuracy of Equipment
All measuring equipment has precision depending on the smallest unit of measurement on the
instrument. Unless otherwise stated, the precision of a measurement can stated be up to 1/2 of the
smallest unit, and the precision of that measurement is ±0.5 of the unit. For example, a ruler that
has graduations of 1 mm can be used to measure a line that is 12.5 mm long, and that length is
precise to ±0.5 mm. This is stated as 12.5 ±0.5 mm, which means the true value of the length is
between 12 and 13 mm.
Digital equipment and chemistry glassware generally has a specified accuracy that is written on the
instrument. With glassware, some equipment is deliberately very precise (e.g. volumetric flasks),
while others are not really intended to be used for measurements (e.g. beakers).
Percent Error
Percent error is the difference in accuracy between a measured, or experimental, value and the
true, or accepted, value. It is calculated as follows:
% 𝐸𝑟𝑟𝑜𝑟 = |𝐸𝑥𝑝𝑒𝑟𝑖𝑚𝑒𝑛𝑡𝑎𝑙 − 𝐴𝑐𝑐𝑒𝑝𝑡𝑒𝑑|
𝐴𝑐𝑐𝑒𝑝𝑡𝑒𝑑× 100
The vertical lines on the top of the equation indicates absolute value, which means that negative
signs are ignored. If you get a negative answer, record the value without the negative sign.
Percent error can only be used if the true value is known, or can be calculated.
Percent Difference
Percent difference is used in place of percent error when the true value is unknown. It is used to
find the precision of repeated measurements (experimental values, E). The equation used is:
% 𝐷𝑖𝑓𝑓𝑒𝑟𝑒𝑛𝑐𝑒 = |𝐸1 − 𝐸2|
(𝐸1 + 𝐸2
2 )× 100
A Short Guide to Significant Figures
What is a “significant figure”?
The number of significant figures in a result is simply the number of figures that are known with somedegree of reliability. The number 13.2 is said to have 3 significant figures. The number 13.20 is said tohave 4 significant figures.
Rules for deciding the number of significant figures in a measured quantity:
(1) All nonzero digits are significant:
1.234 g has 4 significant figures,1.2 g has 2 significant figures.
(2) Zeroes between nonzero digits are significant:
1002 kg has 4 significant figures,3.07 mL has 3 significant figures.
(3) Zeroes to the left of the first nonzero digits are not significant; such zeroes merely indicate theposition of the decimal point:
0.001o C has only 1 significant figure,0.012 g has 2 significant figures.
(4) Zeroes to the right of a decimal point in a number are significant:
0.023 mL has 2 significant figures,0.200 g has 3 significant figures.
(5) When a number ends in zeroes that are not to the right of a decimal point, the zeroes are notnecessarily significant:
190 miles may be 2 or 3 significant figures, 50,600 calories may be 3, 4, or 5 significant figures. Thepotential ambiguity in the last rule can be avoided by the use of standard exponential, or ”scientific,”notation. For example, depending on whether 3, 4, or 5 significant figures is correct, we could write50,6000 calories as:
5.06× 104 calories (3 significant figures)5.060× 104 calories (4 significant figures), or5.0600× 104 calories (5 significant figures).
What is a ”exact number”?
Some numbers are exact because they are known with complete certainty.
Most exact numbers are integers: exactly 12 inches are in a foot, there might be exactly 23 studentsin a class. Exact numbers are often found as conversion factors or as counts of objects.
Exact numbers can be considered to have an infinite number of significant figures. Thus, number ofapparent significant figures in any exact number can be ignored as a limiting factor in determining thenumber of significant figures in the result of a calculation.
Rules for mathematical operations
In carrying out calculations, the general rule is that the accuracy of a calculated result is limited bythe least accurate measurement involved in the calculation.
1
(1) In addition and subtraction, the result is rounded off to the last common digit occurring furthest tothe right in all components. For example, 100 (assume 3 significant figures) + 23.643 (5 significantfigures) = 123.643, which should be rounded to 124 (3 significant figures).
(2) In multiplication and division, the result should be rounded off so as to have the same number ofsignificant figures as in the component with the least number of significant figures. For example, 3.0(2 significant figures ) 12.60 (4 significant figures) = 37.8000 which should be rounded off to 38 (2significant figures).
Rules for rounding off numbers
(1) If the digit to be dropped is greater than 5, the last retained digit is increased by one. For example,
12.6 is rounded to 13.
(2) If the digit to be dropped is less than 5, the last remaining digit is left as it is. For example,
12.4 is rounded to 12.
(3) If the digit to be dropped is 5, and if any digit following it is not zero, the last remaining digit isincreased by one. For example,
12.51 is rounded to 13.
(4) If the digit to be dropped is 5 and is followed only by zeroes, the last remaining digit is increased byone if it is odd, but left as it is if even. For example,
11.5 is rounded to 12,12.5 is rounded to 12.
This rule means that if the digit to be dropped is 5 followed only by zeroes, the result is alwaysrounded to the even digit. The rationale is to avoid bias in rounding: half of the time we round up,half the time we round down.
General guidelines for using calculators
When using a calculator, if you work the entirety of a long calculation without writing down anyintermediate results, you may not be able to tell if a error is made and, even if you realize that one hasoccurred, you may not be able to tell where the error is.
In a long calculation involving mixed operations, carry as many digits as possible through the entireset of calculations and then round the final result appropriately. For example,
(5.00 / 1.235) + 3.000 + (6.35 / 4.0)=4.04858... + 3.000 + 1.5875=8.630829...
The first division should result in 3 significant figures; the last division should result in 2 significantfigures; the three numbers added together should result in a number that is rounded off to the lastcommon significant digit occurring furthest to the right (which in this case means the final result shouldbe rounded with 1 digit after the decimal). The correct rounded final result should be 8.6. This finalresult has been limited by the accuracy in the last division.
Warning: carrying all digits through to the final result before rounding is critical for many math-ematical operations in statistics. Rounding intermediate results when calculating sums of squares canseriously compromise the accuracy of the result.
2
Unit Conversions and Scientific Notation
Metric Unit Conversions
To convert between metric units in the same “category”, use the staircase method:
Tera Giga Mega Kilo Hecto Deca BASE Deci Centi Milli Micro Nano Pico
T G M k h da d c m µ n p
1012 109 106 1000 100 10 1 0.1 0.01 0.001 10-6 10-9 10-12
To move to a smaller unit, multiply by the number of steps it takes to get to the right prefix.
For example, 5 km = (5×10000) cm = 50000 cm, since it takes five steps to get from kilo to centi.
To move to a larger unit, divide by the number of steps it takes to get to the right prefix. For example, 30 mg = (30÷1000) g = 0.03 g, since it takes three steps to get from milli to “base”.
Scientific Notation
Scientific notation is used for very large or very small numbers, which are common in science! It is written
as a product (multiplication) between a number between 1 and 10 and a power of 10.
3.45 × 105 is a big number. It has a positive exponent.
2.31 × 10-4 is a small number. It has a negative exponent.
Many scientific calculators have a button that allows the user to enter numbers in scientific notation. It may
be “EXP”, “×10x”, “EE” or “y1/x”. It is important that students learn to use this function on their own
calculator, to make calculations with scientific notation easier.
To convert to scientific notation:
1. Create a number between 1 and 10 by moving the decimal (for whole numbers, it is after the last
digit) to the left. There should be only one non-zero number before the decimal.
2. Count the number of spaces the decimal moves to determine the exponent on the 10.
a. If the decimal moves left, the exponent is positive.
b. If the decimal moves right, the exponent is negative.
Example: 3 346 000 000 = 3.346 × 109
The decimal moved from the right of the last zero nine places to the left, which gives the exponent
of 9. This number has four significant digits, but more could be added by putting zeros or
removed by rounding (e.g. 3.346 to 3.35).
To convert to standard form:
1. Multiply the two terms (the decimal between 1 and 10 and the power of 10) together; to ensure you
are multiplying or dividing properly. Ask, “Is it reasonable?” after doing a calculation. If you get an
answer of 1000 km in 1 m, and you know kilometres are much bigger than metres, you should
recognize that the answer does not make sense and there was an error in the calculation.
Comparing Values of Scientific Notation
Numbers with higher exponents on the 10 are greater:
10 > 3
4 > -1
-2 > -5
For numbers with the same exponent, numbers with a larger decimal value are greater:
6.43 × 105 > 2.17 × 105
3 × 10-2 > 1 × 10-2
Factor-Label Method
The factor-label method, also called dimensional analysis, is a problem solving method used for unit
conversions and stoichiometric calculations. It is based on the idea of “cancelling” units to get the desired
result. To get from one unit to another, conversion factors are used to change the units to the correct ones.
Example: How many inches are in 32 km?
The unit conversions here are:
1.6 km = 1 mile 1 mile = 5280 ft 1 ft = 12 inches
A grid is used to show the work – horizontal lines indicate “divide” (like in a fraction) and vertical lines
indicate “multiply” (multiplication signs can also be used).
32 km 1 mile 5280 ft 12 inches 1.6 km 1 mile 1 ft
Units that are on both the top and the bottom can be crossed out, like this:
32 km 1 mile 5280 ft 12 inches 1.6 km 1 mile 1 ft
This leaves the answer in inches. To get the value, multiply the numbers on the top together, and divide by
the numbers on the bottom:
= (32 × 1 × 5280 × 12) ÷ (1.6 × 1 × 1)
= 2027520 ÷ 1.6
= 1 267 200 inches
Remember to ALWAYS include units in the final answer!!
Common Unit Conversions
Length 1 in = 2.54 cm 3.28 ft = 1 m
1 mile = 1.61 km
1 mile = 5280 ft 1 ft = 12 in
Mass 1 oz = 28.3 g 1 kg = 2.20 lb
1 lb = 16 oz
Volume 1 fl oz = 29.6 mL 1 cup = 237 mL
1 tbsp = 3 tsp 1 cup = 16 tbsp = 8 fl oz
1 US pint = 2 cups 1 US quart = 4 cups
1 US gallon = 16 cups
Temperature °𝐶 = (°𝐹 − 32) ×5
9 °𝐹 =
9
5× °𝐶 + 32