Ozone Solubility Chart
description
Transcript of Ozone Solubility Chart

High Efficiency In-Line Pressurized Ozone ContactingWith
The GDT™ Process
Presented at:
International Ozone AssociationEuropean African Group1997 Annual Conference
Berlin, GermanyApril 22, 1997
COURTESY OF:
GDT Corporation •• 20805 North 19th Avenue •• Suite 1 •• Phoenix, AZ 85027 •• Phone (623) 587-8858http://www.gdt-h2o.com

1
High Efficiency In-Line Pressurized Ozone ContactingWith
The GDT™ Process
Paul OverbeckGDT Corporation
Phoenix, Arizona USA
Angelo MazzeiMazzei Injector CorporationBakersfield, California USA
ABSTRACT
The development of high concentration (4-14%) ozone generators has created a need for highlyefficient contacting systems to transfer ozone to solution. The patented GDT™ Process utilizes venturiinjectors, minimal detention and entrained gas separation and removal under pressurized conditions tomaximize ozone mass transfer. The dynamic, high energy mixing at the injector benefits highconcentration ozone processes with typical low gas to liquid ratios. The GDT™ ( Gas DegasTechnology) process can be used in full flow and side stream applications to achieve transfer rates inexcess of 95%. The process has demonstrated high dissolved ozone levels (above 15 mg/l) in as littleas 12 seconds detention time.
This paper will focus on the effects of various operating parameters on ozone mass transfer at variousoperating conditions. This data can be used for selection and optimization of operating parameters forwater and wastewater applications.
Introduction
Ozone has gained global acceptance as an oxidant, disinfectant and microflocculant in many of the mostdemanding water and wastewater treatment applications1,2. Ozone generator manufacturers haveexpended considerable development resources to design and manufacture ozone generators capable ofenergy efficient production of high concentration ozone gas3. Today, generators are available tocontinuously produce ozone at 10-14% by weight from oxygen feed gas to compete on a totalevaluated cost basis with chlorine and chlorine dioxide.
In order to fully utilize the advantages of high concentration ozone generators, it is important that aneffective process with high mass transfer efficiency be employed for the transfer of ozone from the gasphase to solution. Options available include bubble column reactors, mechanically stirred reactors andpacked towers which can be further categorized as fine bubble diffusion, deep U-tube, turbine mixers,injectors, etc., contacting4.

2
This paper will concentrate on the newly developed GDT™ process for high efficiency mass transfer ofgas to liquid5. Ozone gas is aspirated via high efficiency Mazzei® injectors in the patented GDT™process. The dynamic mixing and mass transfer that occurs at the Mazzei® injector is enhanced in asmall reaction vessel providing added pressurized detention time. A patented "degas separator" anddegas relief valve remove entrained unreacted ozone and feed gases for reuse or ozone destructprocessing. The total process takes place under pressure and is controlled by various methods ofpressure regulation to maximize mass transfer and minimize electrical operating cost.
The GDT™ process produces a stream of water containing a high concentration ofdissolved ozone free of entrained gases. Dissolved ozone levels can exceed 15 mg/l5. The process isreceiving strong acceptance for side stream ozonation in conjunction with high concentration oxygen fedozone generators followed by in-line pipe contacting for C • t disinfection credit1,2. Operationalimprovements are also attained in full stream treatment applications with air fed ozone generators. TheGDT™ process detention times are short at 10-120 seconds to achieve high transfer rates at variousoperating parameters.
With the GDT™ process, ozone can be used in a very cost effective system for all ozone to liquidapplications. High reaction rate applications such as sulfide ion oxidation, natural organic matterassociated color and textile wastewater decolorization are driven by the high energy mixing and rapiddissolution of ozone7,8. Municipal utility systems can reduce capital, space and operating costs byemploying in-line pipe side stream ozone contactors in place of fine bubble diffuser contactors6.
This report, restates the study and paper by Mazzei, Meyer and Bollyky as carried out to determine themass transfer efficiency of the GDT™ process using high concentration ozone under various operatingconditions5,9. The data generated by this study demonstrated the utility and operational flexibility of theGDT™ process for ozone mass transfer. The effects of the most important operating parameters forozone mass transfer efficiency were identified and evaluated.
Background Information5,9:
The driving force for mass transfer is the difference between the solubility and the already dissolvedozone concentration. The solubility is increased by increasing the pressure10. The solubility of a gas inwater can be described by Henry's law. Henry's law states that the solubility of a gas is very nearlydirectly proportional to its partial pressure in the gas phase11,12.
Strictly speaking this law applies only to gases that do not undergo chemical reaction with water duringthe mass transfer. Dalton's law states that the partial pressure of a gas is equivalent to its volumetricconcentration in the gas phase multiplied by the absolute pressure of the system11,12. One form ofHenry's law that expresses dissolved gas concentration in units of mg/l is as follows12:
Cs = B x M x Pg
Cs = Dissolved Gas Concentration, mg/l M = Gas Phase Density, mg/lB = Bunsen Absorption Coefficient Pg = Partial Pressure In Atmospheres

3
A number of parameters influence the solubility of ozone in water. They are: temperature, pH, ionicstrength and the decomposition of ozone in the gas and liquid phases. Much research has been done todetermine the Bunsen absorption coefficient for ozone under various conditions12. The curve on Figure1 is an average of published data of the Bunsen absorption coefficient for ozone versus temperature12.Figures 2 and 3 illustrate the effect of partial pressure and total pressure on the calculated ozonesolubility13. It can be seen that increasing both partial and total pressure, increases the calculated ozonesolubility dramatically.
Mass transfer efficiency (TE) can be calculated as follows:
TE = ((Ozone In - Ozone Out)/Ozone In) x 100
When the amount of gas entering the system is about the same as the amount leaving, the TE can becalculated as follows:
TE = ((Ozone Conc. In - Ozone Conc. Out)/Ozone Conc. In) x 100
The ozone dose is calculated as :
Ozone Dosage = Ozone In - Ozone Out
Experimental Procedure:
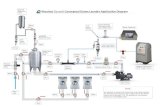
The experimental set up for this study is shown in Figure 4. The experiments were carried out in thelaboratory and pilot plant facilities of Bollyky Associates Inc. of Stamford ,CT. USA.
The injector was a Mazzei Injector Corp. Model 584. The GDT™ degassing separator was a Model DS-100-SS-AP and the off-gas discharge valve was a GDT™ Model DRV-48-SS. The ozone generator wasan Emery-Trailigaz Model SORBIOS unit fed by bottled dry oxygen.
The dissolved ozone monitors were Anseros Model OZOMAT MP, IN USA Model AFX Model W1and Rosemont Model 1054AOZ. They were calibrated by using the Indigo-Trisulfonate method ofBader and Hoigne14. The gas phase monitors were PCI Ozone Corp. UV type monitors Model HC, LCunits. The water was Stamford, CT city water directly from the tap. Water quality was monitoredbefore and after ozone treatment. Typical water quality data are shown in Table 1.
The experiments were carried under continuous flow conditions in the system shown in Figure 4.During each test run the system was operated and monitored continuously until equilibrium was reached.Typically, it took about 25 minutes to reach equilibrium.
The effect of detention time was evaluated by varying the residence time in the system. This objectivewas accomplished by operating with or without various combinations of 1.5 or 8 gallon up-flow holdingtanks.

4
Mass transfer efficiency (TE) was calculated from the amount of ozone in the feed gas and in the off-gas. Gas flow measurements indicated a good material balance between the feed gas and captured off-gas. Therefore TE was calculated as follows:
TEoff-gas = 100 X {1 - (Ozone Out / Ozone In)}
The operating parameters were evaluated in the ranges indicated below:
Gas/Liquid Volumetric Ratio (Vg/Vl) : 0.023-0.364
Injector Discharge Pressure : 10-38 PSIG
Ozone Feed : 2.1-20.4 mg/l
Ozone Gas Phase Concentration : 4.2-14.4% wt.
Detention Time : 10 - 60 seconds
The Effect Of Ozone Concentration And Gas/Liquid Ratio
On Mass Transfer Efficiency:
The ozone concentration has a powerful effect on the mass transfer efficiency. In addition theVolumetric Gas/Liquid ratio (Vg/Vl) affects ozone mass transfer efficiency also. The high ozoneconcentration facilitates low Vg/Vl ratios. The ozone concentration and Vg/Vl ratio are notindependent parameters. In fact, the effect of ozone concentration cannot be separated from theeffect of Vg/Vl in our experiments.
This fact is demonstrated by Figures 5, 6 and 7. The significance of low Vg/Vl ratios is underlined bythe observation that a finer dispersion of bubbles is produced under those conditions in our experiments.The smaller bubbles produce a greater interfacial surface area for mass transfer. The agitation created bythe injector aggressively promotes continuous renewal of the gas and liquid phases at the gas/liquidinterface.
The Figures 5, 6 and 7 demonstrate the improvement in mass transfer efficiency that can be realizedwith the high concentration ozone. The higher TE is the function of higher gas phase ozoneconcentration (higher partial pressure) and lower Vg/Vl ratio.
The above figures also demonstrate the lower mass transfer efficiency obtained at high ozoneconcentration when the applied ozone dosage is high. At a 5 mg/l applied ozone dosage (dissolvedconcentration 3.6 mg/l) a mass transfer efficiency greater than 95% was achieved. In comparison with a14-16 mg/l applied dosage (dissolved ozone concentration 11.2 mg/l), the mass transfer efficiency wasapproximately 90%. The reason for this reduced transfer efficiency at the higher applied ozone dose isthat the dissolved concentration is approaching the solubility of ozone.

5
The Effect Of Injector Discharge Pressure On Mass Transfer Efficiency
The effect of injector discharge pressure on mass transfer efficiency was measured while operating at aset ozone gas concentration of 10% wt , applied high ozone dosage of 15 mg/l and Vg/Vl ratio of 0.1.The data in Figure 8 indicate mass transfer efficiencies in the range of 88% to 95% with injector outletpressures in the range of 25-34 PSIG.
Transfer efficiencies as high as 99% were measured for many test runs with injector outlet pressuresbetween 25-35 PSIG with ozone dosages of 3-5 mg/l.
The Effect Of Gas/Liquid Ratio (Vg/Vl) On Mass Transfer Efficiency:
The data in Figure 9 indicates the effect of Vg/Vl ratios as well as injector outlet pressure on transferefficiency. The effect of increased injector outlet pressure from 10-25 PSIG resulted in increased transferefficiency from 89% to 91% at a Vg/Vl ratio of 0.1 and 91% to 94% at a Vg/Vl ratio of 0.07.
The Effect of Detention Time On Mass Transfer Efficiency:
Figure 10 illustrates the effect of detention time on mass transfer efficiency at an applied dose rate of15-18 mg/l, a Vg/Vl ratio of 0.1 and injector outlet pressure of 25 PSIG. The data in Figure 10 indicatethat the transfer efficiency of 88% can be achieved with a detention time of just 12 seconds. However ,transfer efficiency increased to 95% with 55 seconds of detention time and 97% at 114 secondsdetention time.
This ability to achieve very high transfer efficiencies with very short detention times even at a very highapplied dosage makes this process eminently suitable for side stream ozonation.
The Effect Of Ozone Feed On Mass Transfer Efficiency:
Ozone feed is the amount of ozone introduced into the system. The ozone dose or dosage is defined asthe ozone feed less the amount of ozone lost as off-gas from the system. The ozone feed and Vg/Vl ratiocan not be varied independently in our experiments if a constant ozone concentration is maintained in theozone feed.
Figure 11 demonstrates the effect of ozone feed on transfer efficiency under the conditions of 12%ozone concentration. The data in Figure 11 indicate that the mass transfer efficiency ranged from nearly100% with the 2 mg/l feed to 94-95% with the 20 mg/l feed.

6
Dissolved Ozone Concentrations:
Dissolved ozone concentration in the effluent from the ozone contactor is a very important processparameter, especially for side stream ozonation. Dissolved ozone concentrations measured during theseexperiments varied through a broad range depending on the operating conditions of the test.
Measured dissolved ozone concentrations varied in the range of 2.3-15.6 mg/l depending on operatingconditions. The effect of the individual operating parameters on dissolved ozone concentration closelyparalleled their effect on mass transfer efficiency.
Figure 10 demonstrates the positive effect of longer detention time. When it is increased from 12 to 55seconds, the transfer efficiency and the dissolved ozone concentration increased substantially from 88%to 97% and from 10.2 to 12.1 mg/l respectively .

7
Conclusions:
High mass transfer efficiencies (85 - 99%) can be achieved readily even at high ozonedosages, (3 - 15 mg/l) when high concentration ozone (10 - 14% wt) is used for the mass transfer in theGDT™ process. The operating parameters shown to be most important for the high transfer efficiencyinclude: ozone concentration, applied ozone dosage, injector discharge pressure and detention time.
The combination of the GDT™ process and high concentration ozone generators provide a very viableand effective means for producing high dissolved ozone concentration in water streams. The very lowentrained gas content resulting from off gas removal facilitates the use of ozone for a wide variety oftreatment processes. This combination is particularly suitable for side stream ozonation.
The results of this study identify the parameters for optimum operation of the GDT™ process incombination with high concentration ozone generators. The design of any system requires the carefulconsideration of many factors in order to produce an efficient operating system. The most importantfactors to be considered for high mass transfer efficiency are.
Ø Ozone concentration and applied dosage. High ozone concentration facilitates high ozonesolubility, and this high solubility is the driving force for ozone mass transfer. When a high applied ozonedose is used the dissolved ozone concentration becomes high and begins to limit the mass transfer. Eventhough it is easier to achieve higher mass transfer at lower doses, mass transfer efficiencies of about 90%can be achieved at applied ozone doses as high as 10-14 mg/l utilizing high concentration (14%) ozonegenerators. Ø Injector Discharge Pressure: Similarly, the higher injector discharge pressure increases thesolubility of ozone. An injector outlet pressure of 25-30 PSIG will facilitate mass transfer efficienciesgreater than 95% depending on the other factors involved. However, injector outlet pressures as low as10 PSIG can provide very adequate transfer efficiencies under some conditions while at the same timeutilizing less energy for pumping. Injector outlet pressures greater that 30 psi will often result in veryhigh, 95% or greater, transfer efficiencies, but, at the expense of high energy use for pumping.
Ø Detention Time: Detention times as low as 12 seconds can provide excellent mass transferefficiency (> 95%) depending on the other factors involved. If possible, a detention time of one minuteor greater can provide incrementally better transfer efficiency.
Ø Agitation: The injector introduces the ozone gas into the water stream as a fine dispersion ofbubbles with a great interfacial surface area. The agitation created by the injector aggressively promotescontinuous renewal of the gas and liquid interface.

8
References
1: Pontius, F.W.An Update Of The Federal Drinking Water RegsJournal AWWA February 1995pages 48-58
2: Finch, G. R., Black, E. K., Gyurek, L., Belosevic, M.Ozone Disinfection OF Girardia And CryptosporidiumAWWA Research Foundation and American Water Works Association
3: Loeb, B.L., Uhlig, P.Latest Improvements in Commercial Ozone Generation TechnologyAWWA 1995 Annual Conference Proceedings
4: Ozone In Water Treatment, Application And EngineeringAWWA Research Foundation, 1991, Lewis Publishers
5: Mazzei, A.L., Meyer, M., Bollyky, L.J.Mass Transfer of High Concentration Ozone with High Efficiency Injectors and Degassing SeparatorsInternational Ozone Association, Pan American GroupCambridge, Massachusetts November 1995 Proceedings
6: Price, M., CH2M HillIn Line Injection Improves Oxidation EfficiencyConference Proceedings IOA, Advances In the Application Of OzoneIn Water And Waste Water Treatment, September 1994
7: Overbeck, P.K.Ground Water Color and Sulfide Reduction with OzoneAWWA 1995 Annual Conference Proceedings
8: Overbeck, P.K., Mazzei, A.L., Meyer, M., Mullin, J.Advanced Water Processing with the Cost Effective GDT™ Process AWWA 1996 Annual Conference Proceedings
9: Bollyky Associates Inc."Study Of Ozone Mass Transfer Efficiency With The Mazzei Injector And Degassing
Separator Using High Concentration Ozone" July 10, 1995.

9
10: Bollyky, L.J.The Mass Transfer of Ozone Into Water: Energy Requirements State-OfThe-Art Ozone Science And Engineering Vol. 3 181-210, 1981
11: CRC Handbook Of Chemistry And Physics, 58th Edition 1977-78
12: Ozone In Water Treatment, Application And EngineeringAWWA Research Foundation, 1991, Lewis Publisherspages 112-116
13: Mazzei Injector CorporationCalculated From Data In Reference 12 Above
14: Bader, H., Hoigne, J.Determination Of Ozone In Water By The Indigo MethodOzone Science And Engineering Vol. 4 169-

10
Figure 1 – Bunsen Absortion Coefficient
Figure 2 – Ozone Solubility Relative to Pressure
BUNSEN ABSORPTION COEFFICIENTFOR OZONE vs TEMPERATURE
40 50 60 70 80 90 1000
0.1
0.2
0.3
0.4
0.5
(4.4) (10) (15.6) (21.1) (26.7) (32.2) (37.8)

11
Figure 3 – Ozone Solubility
Typical Water Quality Data
ParameterBeforeOzone
AfterOzone
pH 7.290 7.310
Alkalinity 32.000 32.000Mg/las CaCO3
Total Hardness 51.000 51.000mg/las CaCO3
Iron 0.013 0.014 mg/l
Manganese 0.000 0.000 mg/l
Dissolved Oxygen 8.900 11.500 mg/l
Chlorine 0.970 0.020 mg/l
Total Organic Carbon 2.064 1.820 mg/l as C
Table 1 – Typical Water Quality Data

12
Figure 4 – Injector - Degassing Separator ozone Mass Transfer Test System

13
Figure 5 – Ozone Transfer Efficiency
Figure 6 – Ozone Transfer Efficiency
OZONE TRANSFER EFFICIENCYRELATIVE TO GAS PHASE OZONE CONCENTRATION
2 4 6 8 10 12 14 16
GAS PHASE OZONE CONCENTRATION, (% wt) IN
85
90
95
100
TR
AN
SF
ER
EF
FIC
IEN
CY
, %
0
0.05
0.1
0.15
Vg
/Vl (G
AS
/LIQU
ID R
AT
IO)Transfer Efficiency
Gas/Liquid Ratio
Applied Ozone Dose: 5 mg/l, Injector Outlet Pressure : 25 PSIG
Temperature 64 F (18 C)Detention Time 12 Seconds
OZONE TRANSFER EFFICIENCYRELATIVE TO GAS PHASE OZONE CONCENTRATION
2 4 6 8 10 12 14 16GAS PHASE OZONE CONCENTRATION, (% wt) IN
75
80
85
90
95
TR
AN
SF
ER
EF
FIC
IEN
CY
, %
0
0.05
0.1
0.15
0.2
0.25
0.3
0.35
0.4
Vg/V
l (GA
S/LIQ
UID
RA
TIO
)
Transfer Efficiency
Gas/Liquid Ratio
Applied Ozone Dose: 10 mg/l, Injector Outlet Pressure :25 PSIG
Temperature 63 F (17 C)Detention Time 12 Seconds

14
Figure 7 – Ozone Transfer Efficiency
Figure 8 – Transfer Efficiency vs. Injector Outlet Pressure
OZONE TRANSFER EFFICIENCYRELATIVE TO GAS PHASE OZONE CONCENTRATION
2 4 6 8 10 12 14 16
GAS PHASE OZONE CONCENTRATION, (% wt) IN
70
75
80
85
90
TR
AN
SF
ER
EF
FIC
IEN
CY
, %
0
0.05
0.1
0.15
0.2
0.25
0.3
0.35
0.4
Vg/V
l (GA
S/LIQ
UID
RA
TIO
)
Transfer Efficiency
Gas/Liquid Ratio
Applied Ozone Dose: 14-16 mg/l, Injector Outlet Pressure: 25 PSIG
Temperature 57 F (14 C)Detention Time 12 Seconds
TRANSFER EFFICIENCY vs INJECTOR OUTLET PRESSUREVg/Vl: 0.1, Applied Ozone Dose: 15 mg/l, Ozone Concentration: 10% wt
15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 3586
88
90
92
94
96
Detention Time 12 Seconds
Temperature 46 F (8 C )

15
Figure 9 – Mass Transfer Efficiency vs. Gas/Liquid Ratio
Figure 10 – Transfer Efficiency vs. Detention Time
MASS TRANSFER EFFICIENCY vs GAS/LIQUID RATIOApplied Ozone Dose: 10-14 mg/l, Gas Concentration: 11 % wt
0.06 0.07 0.08 0.09 0.1 0.1188
89
90
91
92
93
94
95
Injector Outlet Pressure10 PSIG 20 PSIG 25 PSIG
Detention Time 12 secondsTemperature 43 F (6 C)
TRANSFER EFFICIENCY vs DETENTION TIMEApplied Ozone Dosage: 15-18 mg/l, Injector Outlet Pressure: 25 PSIG
0 10 20 30 40 50 60 70 80 90 100 110 12086
88
90
92
94
96
98
100
Temperature 53 F (12 C)Ozone Conc.: 9.8 - 12.2% wt

16
Figure 11 – Ozone Transfer Efficiency
Figure 12 - Side Stream Contacting
OZONE TRANSFER EFFICIENCYRELATIVE TO OZONE FEED
0 5 10 15 20 25
OZONE FEED, mg/l
94
96
98
100
TR
AN
SF
ER
EF
FIC
IEN
CY
, %
Ozone Concentration: 12% wt, Injector Outlet Pressure: 25 PSIG
Temperature 54 F (12 C)Detention Time 55 Seconds
Side Stream Contacting
9680 gpmat 15 psi
GDT Degas Vessel& Relief Valve
30” dia pipe (4.0 fps)
Pipe LineContactor84” diameter(0.5 fps)
17.4 mg/l dissolvedozone less demand
Back PressureControl Valve
1586 gpm@ 40 psi
Injector
350 lb/day Ozone
at 10% wt (149.0 Ng/m3)
29.4 scfm
Vg/Vl: 0.12
Design Transfer: 95%
Applied Dosage: 3.0 mg/l
Side Stream Ratio: 16.4%
Side Stream Dosage: 18.3 mg/l
Side Stream Pump: 23.1 WHP
Mixing Energy: 17.2 KW
Contact Vessel
GDT Corporation

17
Figure 13 - Side Stream Contacting
Side Stream Contacting
2200 m3/hat 1.0 bar
GDT Degas Vessel& Relief Valve
DN 1000 pipe (0.8 m/s)
Pipe LineContactorDN 2000(0.2 m/s)
17.4 mg/l dissolvedozone less demand
Back PressureControl Valve
360 m3/hat 2.75 barVg/Vl: 0.12
Design Transfer: 95%
Applied Dosage: 3.0 mg/l
Side Stream Ratio: 16.3%
Side Stream Dosage: 18.4 mg/l
Side Stream Pump: 23.1 WHP
Contact VesselInjector
6.6 Kg/hr Ozone
at 10% wt (149.0 Ng/m3)
44.3 m3/h GDT Corporation













![SOLUBILITY AND SOLUBILITY PRODUCT - Instruct · SOLUBILITY AND SOLUBILITY PRODUCT [MH 5; 16.1 & 16.2] • In this section we are going to consider the solubility of ionic solids in](https://static.fdocuments.in/doc/165x107/5ae1efb27f8b9a90138bbc8f/solubility-and-solubility-product-instruct-and-solubility-product-mh-5-161.jpg)




