OCN Review Hemato GI Skin 2015 hopsons.org/.../2016/09/OCN-Review-Hemato_GI_Skin-2015-ho.pdfNational...
Transcript of OCN Review Hemato GI Skin 2015 hopsons.org/.../2016/09/OCN-Review-Hemato_GI_Skin-2015-ho.pdfNational...

1
Symptom Management Part I
Lenise Taylor, MN, RN, AOCNS, BMTCN
Symptom Management: 22%
A. Etiology and patterns of symptoms (acute, chronic, late)B. Toxicity and grading scalesC. Anatomical and surgical alterationsD. Complementary and integrative modalities (e.g.
massage, acupuncture, herbal supplements)E. Alterations in:
1. Hematologic function 8. Cardiovascular function2. Immune function 9. Neurological function3. Gastrointestinal function 10. Musculoskeletal function4. Nutrition status 11. Comfort (e.g. pain) 5. Integumentary function 6. Genitourinary function7. Respiratory function
Myelosuppression
• Definition:• Reduction in production & maturation of all blood cell lines• Resulting in:• Neutropenia and leukopenia,• Thrombocytopenia• Anemia
• One of most common & potentially life-‐threatening clinical complications experienced by patients with cancer
Shelton, B. In Holmes Gobel, B., et al eds . Advanced Oncology Nursing Cer tification: Review & Resource Manual. 2009: 405-442.
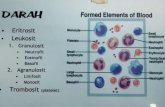
Platelets 7-8 DaysNeutrophil 7-12 HoursEosinophil 3-8 HoursBasophil/mast cell 7-12 Hours
Monocyte/macrophage 3 Days
B Lymphocyte Type dependT Lymphocyte Type depend
Erythrocyte 120 DaysBlood Cell Life Span in Blood
CIRCULATING BLOOD CELLS LIFE SPAN

2
Neutropenia
Decreased number of circulating neutrophils Associated with increased risk of potentially life-‐
threatening infection§Neutrophils 1st line of defense against bacterial infection (localize & neutralize bacteria)
§Normal rage (neutrophils)§ 2,500 to 6,000 cells/mm3
50% to 60% of total number of WBC’s
More than 50% of patients with neutropenia can be expected to develop infection.
https://www.ons.org/practice-resources/pep/prevention-infection, accessed June 10, 2014
White Blood Cell (WBC) Count Differential
WBC Type Relative Value
Absolute Value uL (mm3)
Neutrophils (total) 50-70% 2,500 – 7,000segmented (polys) 50-65% 2,500 – 6,500bands 0-5% 0 – 500
Eosinophils 1-3% 100 - 300Basophils 0.4-1.0% 40-100Monocytes 4-6% 200-600Lymphocytes 25-35% 1,700-3,500
Kee, J .L. Laborator & Diagnos itc Tes ts with Nursing Implications . 1999.

3
NeutropeniaRisk Factors
Pre-‐existing neutropenia– Comorbidities, previous treatment Myelosuppressive chemotherapy Bone marrow involvement Immune system degeneration Hepatic and renal dysfunction Malnutrition Combined modality treatment
Assessing Neutrophils:The Absolute Neutrophil Count (ANC)
ANC = Total WBC X % of neutrophils (segs + bands)
Example:WBC = 2,500/mm3
Segmented neutrophils = 35% Band neutrophils = 10%
ANC = 2,500 X (.35 + .10) = ANC = 2,500 X .45 = 1,125/mm3
Absolute Neutrophil Count Calculation
WBC = 3,000/mm3• Segmented neutrophils = 20%• Band neutrophils = 5%• Eosinophils = 3%• Basophils = 1%• Lymphocytes = 71%
What is the ANC?ANC = 3,000/mm3 X .25 = 750
The ANC Predicts the Risk for Infection
Absolute Neutrophil Count Grade Risk of InfectionWithin normal limits 0 No Risk
> 1,500 to <2,000 1 No significant risk> 1,000 to < 1,500 2 Minimal risk> 500 to <1,000 3 Moderate risk< 500 4 Severe risk
Camp-Sorrell, D. In Itano, J . & Taoka, K. eds . Core Curriculum for Oncology Nursing, 4th Ed. 2005: 259-274.

4
Potential Consequences of Neutropenia
• Delay in administering treatment on time or dose delay; dose reductions
• Infection• Sepsis and septic shock• Death
Camp-Sorrell, D. In Itano, J . & Taoka, K. eds . Core Curriculum for Oncology Nursing, 4th Ed. 2005: 259-274
Preventing Infection
• Frequent hand washing• Daily bathing• Frequent mouth care• Limit invasive procedures• Rectal temps, catheters, etc.
• Inspect IV sites• Visitor hygiene
Camp-Sorrell, D. In Itano, J . & Taoka, K. eds . Core Curriculum for Oncology Nursing, 4th Ed. 2005: 259-274.
Nursing Management:Continual Assessment for Infection
• Signs of infection MAY NOT be present
• Redness, inflammation, and drainage may be minimal or absent
Shelton, B.K. (2009). In Gobel, B.H. et al (eds .). Advanced Oncology Nurs ing Certification: Review and Resource Manual, ONS, pgs . 405-442.
Nursing Assessment:Every 4 hours (inpatient) & at each clinic visit (outpatient)
Vital signs every 4 hours Level of consciousness Intake and output Respiratory status & auscultate
lungs Presence, character and amount
of sputum, cough Skin integrity, especially at
catheter or tube insertion sites, incisions or perirectal area
Oral cavity for plaque, thrush, ulcers, redness, dryness
Character, amount and frequency of stool
Character, amount and frequency of urine
Assess peri-‐anal area daily for signs of infection for patients who are severely neutropenic
Monitor CBC and other labs for changes, if ordered
Assess central venous access site for signs of infection
Consider MD order for GCSF

5
Putting Evidence Into Practice (PEP) Resources
Yellow = CAUTION!– Benefits Balanced with Harm– Effectiveness Not Established
Green = GO!– Recommended for Practice– Likely to Be Effective– Evidence supports the consideration of these interventions
in practice
Yellow = CAUTION!– Benefits Balanced with Harm– Effectiveness Not Established– Not sufficient evidence to say whether these interventions
are effective or not
Red = STOP!– Effectiveness Unlikely– Not Recommended for Practice– Evidence indicates these interventions are ineffective or
harmful
https ://www.ons .org/practice-resources /pep, accessed June 10, 2014
Prevention of Infection (General):Recommended for Practice• Hand Hygiene with alcohol sanitizer• Contact precautions for resistant organisms• Colony-‐stimulating factors• Chemotherapy with > 20% risk of febrile neutropenia or at risk patients
• Influenza vaccine annually for all cancer patients• 2 weeks prior to or 3 months after immunosuppressive therapy
• Catheter care bundle
https ://www.ons .org/practice-resources /pep/prevention-infection/prevention-infection-general, accessed June 10, 2014.
Medical Management: Myeloid Growth Factors
• Filgrastim (Neupogen) or Tbo-‐Filgrastim (Granix)• Daily dose of 5 mcg/kg until post-‐nadir ANC recovery to normal or near-‐normal levels• Start 24-‐72 h after completion of chemotherapy and treat through post-‐nadir recovery• Administration of growth factor on same day of therapy is not recommended
• Pegfilgrastim (Neulasta)• One dose of 6 mg per cycle of treatment• Start 24-‐72 h after completion of chemotherapy
NCCN (2009). Myeloid Growth Factors : Practice Guidelines . Accessed at www.nccn.org, 08/24/09
Recommended for Practice (General)
Pneumococcal vaccine for all cancer patients
– At least 2 wks prior to chemo, if possible
Antibiotic prophylaxis with flouroquinolones for patients at high risk for infection
– Hematologic malignancies– BMT recipients– Expected neutropenia > 7 days
Antifungal & antiviral prophylaxis in high-‐risk patients
– Acute leukemia, MDS– BMT, patients with GVHD
https ://www.ons .org/practice-resources /pep/prevention-infection/prevention-infection-general, accessed June 10, 2014.

6
Likely to Be Effective (General)
• Preconstruction planning• Chlorhexidine impregnated washcloths –chlorhexidine bath• Antibiotic impregnated IV catheters in adults (short-‐term catheters only)• Antibiotic abdominal lavage in colorectal surgery
https ://www.ons .org/practice-resources /pep/prevention-infection/prevention-infection-general, accessed June 10, 2014.
Benefits Balanced with Harm (General)Benefits Balanced with Harm (General)• Intravenous Immunoglobulin (IVIG)
https ://www.ons .org/practice-resources /pep/prevention-infection/prevention-infection-general, accessed June 10, 2014.
Effectiveness Not Established (General)Effectiveness Not Established (General) Chlorhexidine sponge dressing
Protective isolation Staff training
Cranberry juice Antibiotic IV catheter lock
solutions Antibiotic coated sutures
Effectiveness Unlikely • Extended post-‐operative antibiotics• Restriction of fresh fruits and vegetables
https ://www.ons .org/practice-resources /pep/prevention-infection/prevention-infection-general, accessed June 10, 2014.
Not Recommended For Practice Live attenuated vaccines– Flumist (intranasal attenuated influenza vaccine)– Varicella (chicken pox) vaccine, oral polio vaccine, & MMR vaccine
Implantable gentamycin sponge
Detecting Signs of Infection in Patients with Neutropenia• Neutropenia: the often silent disorder• ONLY sign of an infection may be FEVER:• Take temperature every 4 hours (inpatient)• Instruct patient to take temperature QD or BID (home)• Report temperature > 1010F (38.00C) or 100.50 F (37.50 ) for > 1 hr• Tachycardia & tachypnea alone, may be developing sepsis• Hypotension with above indicates severe sepsis
Shelton, B.K. (2009). In Gobel, B.H. et al (eds .). Advanced Oncology Nurs ing Certification: Review and Resource Manual, ONS, pgs . 405-442.

7
Educate Patients & Caregivers to Recognize & Minimize Infection List measures to prevent infection– Managing environment, hygiene, diet,
activity Identify signs & symptoms of infection Emphasize when to report– Fever or other signs/symptoms of
infection– Be specific about whom and when to call Give specific oral & written instructions
Camp-Sorrell, D. In Itano, J . & Taoka, K. eds . Core Curriculum for Oncology Nursing, 4th Ed. 2005: 259-274.
Febrile Neutropenia
• ALWAYS A MEDICAL EMERGENCY• Left untreated, may be fatal• Sepsis is lethal in 47% of infected patients with neutrophil count <1000
Giamarellou, H. & Antoniadou, A. (2001). Infections complications of febrile leukopenia. Infectious Disease Clinics of Nor th Amer ica, 15: 457-482.
Febrile Neutropenia: Definition
• Febrile neutropenia• Single temperature > 38.30C orally or >38.00C over 1 hr
• Neutropenia• < 500 neutrophils/mcL or <1,000 neutrophils/mcL and a predicted decline to <500/mcL over the next 48 hrs
NCCN (2009). Myeloid Growth Factors : Practice Guidelines . Accessed at www.nccn.org, 08/24/09
Assessing/Managing Neutropenic Patients with Fever• Obtain blood cultures• Culture suspected sites of infection• Urine, sputum, stool, IV catheter sites, wounds
• Chest x-‐ray• Immediate institution of broad spectrum antibiotics• Admission to hospital (ANC<1000)

8
Thrombocytopenia• Decrease in circulation platelets below 100,000/mm3
• Normal platelet count 150,000 – 400,000/mm3
• Life span of platelets – 8 to 10 days
Platelet Count Grade Risk of BleedingWithin normal limits 0 No Risk< LLN – 75,000/mm3 1 No significant risk<75,000 – 50,000/mm3 2 Minimal risk< 50,000 – 25,000/mm3 3 Moderate risk< 25,000/mm3 4 Severe risk
Camp-Sorrell, D. In Itano, J . & Taoka, K. eds . Core Curriculum for Oncology Nursing, 4th Ed. 2005: 259-274.National Cancer Ins titute Cancer Therapy Evaluation Program (NCI CTEP), 2006.
Clinical Consequences of Thrombocytopenia
• Bleeding – Internal or External
• Refractory to platelet transfusions
Recommended for Practice
• Platelet thresholds: keep at• 10,000: majority of patients• 20,000
• minor procedures• bladder tumors, necrotic tumors, or highly vascular tumors likely to bleed
• 40,000 – 50,000: patients undergoing invasive procedures
• Platelet transfusions• Active bleeding with thrombocytopenia
• Mesna for prevention of hemorrhagic cystitis
http://www2.ons .org/Research/PEP/bleeding, accessed June 10, 2014
Effectiveness Not EstablishedEffectiveness Not Established
• Platelet growth factors• Recombinant Interleukin-‐11 (Neumega)
• Interventions to prevent or attenuate menstrual bleeding• Oral contraceptives, progesterone, etc.
http://www2.ons .org/Research/PEP/bleeding, accessed June 10, 2014

9
Effectiveness Not EstablishedEffectiveness Not Established
Desmopressin (DDAVP) Epsilon amino-‐caproic acid (EAC) TA alone Recombinant activated factor VI (rFVIIA) Recombinant epidermal growth hormone
(rhEGF) for urothelial protection Endoscopic procedures to attentuate bleeding Ultrasonic-‐activated surgical instruments for
endovascular embolization procedures
http://www2.ons .org/Research/PEP/bleeding, accessed June 10, 2014
Nursing Assessment:Every 4 hours (inpatient) & at each clinic visit (outpatient)
Bruises, petechiae or bleeding from orifices
Monitor for occult or frank blood in urine, stool, or emesis
Hypotension or tachycardia Monitor platelet count,
coagulation tests (if suspect Disseminated Intravascular Coagulation)
Monitor hemoglobin/hematocrit
Monitor pad count during menses
Monitor for changes in level of consciousness
Monitor fall risk, re-‐evaluate and re-‐score as necessary
Assess safety of patient environment
Reportable Concerns
• Platelet count• <50,000/mm3 or • new event < than 15,000 /mm3
• ↓ BP and ↑ pulse rate• Occult positive results from
stools, emesis or urine• Spontaneous bleeding
(increased risk when platelets are < 15,000/mm3)
• Alterations in neurologic signs• Any new or severe pain, sudden onset of pain, or a sharp exacerbation of existing pain
Safety Concerns
Platelets < 50,000/mm3
• No IM injections• No rectal temperatures• No suppositories (unless cleared by MD)• Minimize invasive procedures• Avoid medications that have potential to cause bleeding (i.e. anti-‐inflammatories, aspirin)• No straight edge razors• Consider head CT if head strike with fall or new neuro symptoms

10
Patient Education
Report signs of bleeding
Preventative and management measures
Anemia A term that indicates a low red cell countand a below normal hemoglobin or hematocrit level.
Hemoglobin (g/dl) Grade Severity of AnemiaWithin normal limits 0 Normal10 - normal 1 Mild8 - <10 2 Moderate6.5 - < 8 3 Severe< 6.5 4 Life threatening
Adapted from the Common Toxicity Criteria for adverse events . Available at:http://ctep.cancer.gov/protocolDevelopment/electronic_applications /docs /ctcaev4.pdf
Anemia Pathophysiology
Question: What is the lifespan of a red blood cell?
AnemiaRisk Factors
• Chemotherapy• Biotherapy• Bone marrow involvement• Radiation therapy• Bleeding
• Age• Nutritional deficit• Abnormal metabolism• Medications

11
Nursing Assessment:Every 8 hours (inpatient) & at each clinic visit (outpatient)
• Fatigue• Hypotension• Tinnitus• Headache• Prolonged capillary refill (monitor hemoglobin)• Dyspnea• Palpitation
• Weakness• Vertigo• Consider MD order for erythropoietic-‐stimulating agent to increase RBC (i.e., erythropoietin)
AnemiaManagement
Recognize symptoms Identify and manage underlying cause– Administer iron supplements– Consider transfusions– Consider recombinant erythropoietin*– Symptom management
Energy conservation Oxygen therapy
Monitor labs (CBC, iron, total iron binding capacity, transferrin saturation)
Case Study
• D.S. comes to the clinic for his 4th course of R-‐CHOP• Current lab values
• ANC: 950• H/H: 10.2/30.7• Platelets: 93,000
• Patient reports increasing fatigue, no fevers, no obvious bleeding
• Patient is anxious to get his treatment completed so he can get back to work full-‐time.
• What patient education is needed?
Case Study
What would the nurse educate the patient about at this point?

12
Fatigue
“Cancer-‐related fatigue is a distressing, persistent, subjective sense of physical, emotional, or cognitive tiredness or exhaustionrelated to cancer or cancertreatment that is notproportional to recentactivity and interferes with usual functioning”
NCCN, 2008
Cancer-‐Related Fatigue
• Sleep disorders• Emotional distress• Anxiety• Depression
• Anemia• Malnutrition• Decreased activity• Pain
Based on “Pharmacologic Treatment of Cancer-‐Related Fatigue” by J. Carroll et. al. 2007, Oncologis t, 12(Supp. 1) p. 44. Retrieved December 11, 2007, from http:/ /wwwtheOncologis t.com.
Cancer Related Fatigue
Non-‐cancer comorbidities• Cardiac dysfunction• Hepatic dysfunction• Hypothyroidism• Infection• Neurologic dysfunction• Pulmonary dysfunction
Based on “Pharmacologic Treatment of Cancer-‐Related Fatigue” by J. Carroll et. al. 2007, Oncologist, 12(Supp. 1), p. 44. Retrieved December 11, 2007, from http:/ /wwwtheOncologist.com.
FatigueAssessment
Fatigue scale (age appropriate) Disease status (recurrent or progression) Current medications Review of systems Onset, pattern, and duration Nutritional and metabolic evaluation Activity level Associated or alleviating factors

13
FatigueCollaborative Management
• General • Energy conservation• Delegate• Energy-‐saving devices• Patient and family education
• Non-‐pharmacologic• Exercise• Complementary therapies
• Pharmacologic• Erythropoiesis-‐stimulating agents• Antidepressants• Psychostimulants• Glucocorticoids• Supplements• Complementary therapies
FatigueRecommended for Practice• Exercise• Exercise interventions in patients with cancer have been provided as • Home-‐based programs• Patient self-‐managed programs• Supervised and unsupervised individual or group exercise sessions• Varying duration and frequency and can include combinations of aerobic and resistance types of activities.
https ://www.ons .org/intervention/exercise-3, accessed June 10, 2014.
Fatigue: Likely to Be Effective
Abiraterone Acetate Cognitive behavioral
interventions/approach for sleep Energy conservation and activity
management Ginseng Management of concurrent symptoms Massage Mindfulness-‐based stress reduction Modafinil Psychoeducational interventions Yoga
https ://www.ons .org/practice-resources /pep/fatigue, accessed June 10, 2014.
FatigueBenefits Balanced with HarmFatigueBenefits Balanced with Harm
• Erythropoiesis stimulating factors (ESA’s)
https ://www.ons .org/practice-resources /pep/fatigue, accessed June 10, 2014.
Acupressure/puncture/stimulation
Animal-‐assisted therapy
Art making/art therapy
Body-‐mind-‐spirit therapy
Bupropion
Co enzyme Q 10 Cognitive Training –
group
Effectiveness Not Established (Partial List)Effectiveness Not Established (Partial List)
Cranial Stimulation Dexamphetamine
Environmental interventions
Expressive writing Meditation
Methylphenidate

14
Granulocytes collectively include:
a. Neutrophils, lymphocytes, and basophilsb. Neutrophils, basophils, and eosinophilsc. Monocytes, lymphocytes, and eosinophilsd. Lymphocytes, neutrophils, and basophils
The normal life span of platelets is:
a. 1 to 3 daysb. 4 to 5 daysc. 6 to 7 daysd. 8 to 10 days
The myeloid stem cell line produces:
a. B lymphocytes and natural killer (NK ) cellsb. Monocytes and polymorphonuclear leukocyesc. T lymphocytes and associated antigensd. Major histocompatibility complex (MHC) molecules
Radiation to which of the following areas can result in myelosuppression?
a. Iliac crests, vertebrae, ribs, skull, sternum, and long bones
b. Tibia, ribs, skull, & sternumc. Ulna, sternum, & vertebraed. Skull, ribs, patella, and metacarpals

15
A patient who completed treatment for malignant melanoma one year ago complains of still being tired. The nurse anticipates an order for:
a. Darbepoetinb. Methylphenidatec. Lorazepamd. pegfilgrastim
Gastrointestinal Function & Nutritional Alterations
GI Function & Nutritional Alterations
• Diarrhea
• Constipation
• Nausea & Vomiting
• Mucositis
• Taste Alterations
• Anorexia / Cachexia
This image cannot currently be
GI Symptoms -‐ Why does it matter?
• GI toxicity can result in dose reductions and treatment delays• Quality of Life Issues• Lead to a cascade of other symptoms

16
DiarrheaGreater than 200 g/day of fecal output with a volume of 300 ml that is 70 – 90% water and more than three stools per day.
Types
• Acute -‐ 24 – 48 hours of contact with an agent and resolves within 7 – 14 days or earlier with intervention• Chronic diarrhea -‐ late onset, lasts for 2 – 3 weeks, occurs as the result of an unidentified agent or as the result of tissue injury related to a treatment modality that interferes with normal bowel function.• Radiation induced -‐ typically occurs within 2 weeks of beginning radiation therapy
Types
Osmotic – large volume that resolved with fasting or elimination of the provoking agent
Secretory – large volume that persists despite fasting Exudative – inflammation, necrosis and sloughing of
the colonic mucosa. Occurs more than 6 times per day of variable volume
Chemotherapy induced -‐ frequent, watery to semisoild stools with abdominal pain, cramping & fecal incontinence
Radiation induced Dysmotility associated -‐ uncoordinated control of
intestinal propulsions with rapid transit of stool . Small frequent semisolid stools of variable amounts
Diarrhea: Assessment
Adverse Event 1 2 3 4 5
Diarrhea Increase of < 4 stools per day over baseline;; mild increase in ostomy output compared to baseline
Increase of 4-6 stools per day over baseline;; IV fluids indicated < 24 hrs;; moderate increase in ostomy output compared to baseline;; not interfering with ADL
Increase of >7 stools per day over baseline;; incontinence;; IV fluids > 24 hrs;; hospitalization;; severe increase in ostomy output compared to baseline;; interfering with ADL
Life-threatening consequences (e.g., hemodynamic collapse)
Death
From Common Terminology Cri teria for Adv ers e Ev ents (Vers ion 3.0), by the National Cancer Ins titu te Canc er Therapy Evaluation Program, 2006. Retriev ed Marc h 31, 2009, from http ://c tep.c anc er.gov/forms /CTCAEv3.pdf

17
Diarrhea: Risk FactorsRadiation therapy
5-‐FU + high dose leucovorin or weekly 5FU
ImmunosuppressionBowel surgery
Neutropenic sepsis C. difficile, candida
• GVHD• Dietary causes• Inflammatory conditions• Malabsorption • Anxiety and stress
Diarrhea: High-‐Risk Agents Chemotherapy:– Irinotecan– 5-‐FU
– Paclitaxel– Dactinomycin– Dacarbazine– Capecitabine
Biotherapy:– IL-‐2
– Interferons
• Targeted agents– MoAbs– Imatinib mesylate– Dasatinib– Erlotinib– Bortezomib– Lapatinib– Gefitinib– Sunitinib malate– Temsirolimus– Revlamid and thalidomide– Zolinza
Diarrhea: Clinical Manifestations Dehydration (especially children) Life-‐threatening electrolyte imbalances Cardiovascular compromise, orthostasis Impaired immune function Skin breakdown Reduced absorption of oral meds Pain Anxiety Exhaustion/decreased quality of life
Diarrhea: Management
§Monitor stool number, amount, consistency
§Consider other medications that could
contribute§Consider diet and herbal
supplements§Replace fluid and
electrolytes§BRAT diet
§ Administer antidiarrheal medication§ Diphenoxylate§ Loperamide§ Octreotide§ Anticholinergics
§ Increase clear fluid intake (with electrolytes)
§ Skin care in the peri-‐rectal area, especially if neutropenic
§ Early and aggressive patient education

18
ONS PEP Resource:Chemotherapy-‐Induced Diarrhea Likely to be Effective– Octreotide Treatment Benefits Balanced with Harm– Amifostine– Neomycin Effectiveness Not Established– AG1004 -‐Probiotics– Budesonide -‐Prophylactic Octreotide– Charcoal– Glutamine– Levofloxacin and Cholestyramine– Oral Alkalinization
ONS PEP Resource:Radiation-‐Induced Diarrhea
Likely to be Effective– Psyllium Fiber Effectiveness Not Established– Dietary Restrictions (fiber & lactulose)– Elemental diet– Glutamine– Probiotics– Vitamin E & C Effectiveness Unlikely– Octreotide -‐Pentosan Polysulfate Not Recommended for Practice– Sucralfate
Constipation
(Reprinted with permission from Lenz Marketing, Decatur, GA)
Causes:•Presenting symptom of cancer•Side effect of treatment•Result of tumor progression•Unrelated to cancer or treatment
Affects 40% - 70% of cancer patients
Constipation Assessment
• Patterns of elimination• Dietary intake• Activity level• Abdominal pain or cramping• Characteristics of last BM• Current medications• Laboratory values• Abdominal/rectal exam• Radiographic studies

19
Agents that decrease motility of the large intestine
§ Vinca alkaloids§ Agents that increase nausea
and vomiting (thereby decreasing oral intake)
§ Opioids§ Anti-‐depressants§ Iron supplements§ Diuretics§ OTC analgesics (Tylenol, non
steroidals)
• Incidence• Vinblastine: 20-‐35%• Vinorelbine: 35%• Thalidomide: 55%• Bortezomib: 41%
Constipation: Clinical Consequences• Abdominal or rectal discomfort• Nausea/vomiting• Anorexia• Impaction• Ileus• Anal fissures• Hemorrhoids• Ruptured bowel and life-‐threatening sepsis
Constipation: Laxative Options• Bulk forming• Lubricants and
emollients• Saline laxatives• Osmotic laxatives• Polyethylene glycol
(with or without electrolytes)
Detergent laxatives Stimulant laxatives Suppositories Prokinetic agents Methylnaltrexone Combination laxative-‐
stool softener prophylactically for patients receiving vinca alkaloids.
Constipation: Non-‐pharmacologic interventions
• Increase physical activity or passive exercise
• Maintain usual bowel habits during hospitalization
• Increase fluid and fiber intake• Do not perform rectal exams, use
suppositories or enemas if myelosuppressed
• Consider rotating opioids

20
ONS PEP Resource:Constipation
Recommended for Practice– Methylnaltrexone– Oxycodone/Naloxone– Transdermal Fentanyl
Likely to Be Effective– Alvimopan– Amidotrizoate– Polyethylene Gylcol (PEG)– Prophylactic laxatives for patients on opioids– Senna and Docusate
ONS PEP Resource:Constipation
Effectiveness Not Established– Baker’s Yeast -‐Massage/Aromatherapy– Biofeedback -‐Naloxone– Biscodyl -‐Opioid Switching– Colchicine -‐Probiotics– Lactulose -‐Sorbitol
Therapy-‐Related Emesis Patterns
• Anticipatory: Occurs before or during treatment from associated stimuli; a conditioned response• 25% incidence
• Acute: Occurs within 24 hours• Incidence determined by agents
• Delayed: Occurs at least 24 hours after therapy and may persist up to 6 days• Cisplatin associated with highest incidence
Risk Factors for CINV: Patient Characteristics
• Female• Age < 50 years• History of low alcohol intake (<1.5 oz/day)• History of motion sickness• History of morning sickness during pregnancy• History of prior CINV• Extreme anxiety
• Other factors• pain, constipation, medications
Navari RM. J Support Oncol. 2003;;1:89–103.

21
Risk Factors for CINV: Chemotherapy-Specific Factors
• Use of moderately or highly emetogenic regimens, such as:• Cisplatin-‐based regimens• Cyclophosphamide-‐based regimens (e.g. CHOP)• AC (anthracycline + cyclophosphamide)• Carboplatin-‐based regimens• ABVD (doxorubicin + bleomycin + vinblastine + dacarbazine)• FOLFOX/FOLFIRI (oxaliplatin + leucovorin + 5FU/irinotecan + leucovorin + 5FU)
• Short IV infusion time• Repeated cycles of chemotherapy
Hes k eth PJ . Oncologist. 1999;;4:191–196. Nav ari RM. J Support Oncol. 2003;;1:89–103.NCCN Guidel ines. v.3.2008: antiemesis.
NCCN Guidel ines. v.2.2008: Hodgkin diseas e/lymphoma.NCCN Guidel ines. v.1.2008: c olon cancer.NCCN Guidel ines. v.1.2008: rectal cancer.
Both Peripheral and Central Pathways Play a Role in CINV
1. Tav orath R et a l. Drugs . 1996;;52:639–648.2. Grunberg SM, Hes k eth PJ . N Engl J Med . 1993;;329(24):1790–1796.
Il lus tra tion by Ki rk Moldoff. 83
bNeurok in in-1
Nausea and Vomiting Pathophysiology
(Polovich et. al., 2005, reprinted with permission)

22
Nausea & VomitingPharmacologic Management
• Prevention of nausea and vomiting is the goal• Select appropriate antiemetic based on treatment regimen• Consider cumulative effects• Administer through entire anticipated period of nausea & vomiting • Oral and IV antiemetic have equivalent effectiveness• Consider other potential causes of emesis
Serotonin Antagonists
•Indications:•High & moderate to high emetogenic chemotherapy
•Common side effects:•Headache, diarrhea, constipation, fever•Examples:•Ondansetron, granisetron, dolasteron, palonosetron
NK-‐1 Antagonist
Indications: – Acute and delayed nausea / vomiting– Highly emetogenic chemotherapy Common side effects:– Diarrhea, hiccups, fatigue Examples:– Aprepitant (oral)– Fosaprepitant (IV)
Other agents
Corticosteroids -‐ used in combination with other meds. Mechanism of action is unknown Cannabinoids -‐ used for refractory CINV when other agents are ineffective. Many side effects Dopamine antagonists -‐ used for low potential and for breakthrough Benzodiazepines -‐ used for anticipatory CINV and breakthrough

23
Nausea & VomitingNon-‐pharmacologic Management
• Music therapy• Moderate exercise• Acupressure• Acupuncture• Behavioral interventions• Dietary interventions• Small frequent meals, room temperature food (↓ odors)
• Patient education• Notify if N/V > 24 hrs or unable to maintain fluid intake• Take antiemetics before arriving • Follow-‐up in 24-‐48 hours
ONS PEP Resource:Chemotherapy-‐Induced Nausea & Vomiting
Recommended for Practice– Cannabis/Cannabinoids– Neurokinin 1 Receptor Antagonist (NK1)– Serotonin 5HT3 Receptor Antagonist (5HT3)– Transdermal Granisetron– Triple Drug Regimen Likely to Be Effective– Gabapentin– Hypnosis for Anticipatory CINV– Managing Patient Expectations– Olanzapine for breakthrough CINV– Progesins– Progressive Muscle Relaxation– Single Agent Dexamethasone
ONS PEP Resource:Chemotherapy-‐Induced Nausea & Vomiting
Benefits Balanced with Harm: Virtual Reality Effectiveness Not Established– Acupressure -‐Massage/Aromatherapy Massage– Acupuncture -‐Metaclopramide (prophylactic)l– Acstimulation -‐Mirtazapine– Electroacupuncture -‐Olanzapine– Exercise -‐Ondansetron as rescue medication– Ginger -‐Prochlorperazine for breakthrough N/V– Grape Juice -‐Progressive Muscle Relaxation (PMR)– Guided Imagery -‐Psychoeducation– Haloperidol -‐Thalidomide– Herbal Medicine -‐Yoga Effectiveness Unlikely: Cocculine Not Recommended for Practice: Metopimazine
Mucositis: Defined
•Mucositis -‐ Inflammatory process involving the mucous membranes of the oral cavity and gastrointestinal tract
• Stomatitis – inflammatory disease of the mouth.
•Mucosal membranes proliferate with a high turnover rate every 7 – 14 days

24
Patient Related Assessment Areas for Mucositis
Age (very young and very old)– Young recover more quickly
Gender (females > males) Poor oral health and hygiene Low body mass index Renal Toxicity (increased creatinine, increased toxicity) Smoking History Previous cancer treatment Hyposalivation (or increased viscosity) Ill-‐fitting prostheses Hematologic malignancy (to some extent, related to
treatment regimen)Beck, 2004, Eilers & Million, 2007 Jaroneski, 2006
Risk Factors for MucositisRegimen-‐related
• Cytotoxic agent used• Prolonged or repetitive administration (vs. bolus)• Radiation therapy to the head/neck region in combination with chemotherapy• Cumulative radiation dose• Number of cycles and intensity of treatment• History of previous episodes of mucositis• Blood/stem cell transplantation
Agents Most CommonlyAssociated with Mucositis
• actinomycin D• amsacrine• bleomycin• cytarabine• daunorubicin• docetaxel• doxorubicin• etoposide
• floxuridine• 5-‐fluorouracil• methotrexate• mitoxantrone• plicamycin• thioguanine• vinblastine• vindesine
Mucositis: Consequences
• Pain -‐ *** The hallmark of oral mucositis• Difficulty swallowing• Difficulty in communication• Infection• Bleeding• Dose reduction and dose delays• Increased fatigue• Increased need nutritional support

25
Mucositis: Assessment• Perform a thorough oral assessment• Use a penlight• Use a gloved finger to gently manipulate tongue and cheek• Inspect under the tongue and along inner cheeks and gums, inspect hard and soft palate
• Ask the right questions
Mucositis: Assessment• Subjective:• pain, burning, increased sensitivity, altered taste, dry mouth
• Objective: • erythema, ulceration, saliva, bleeding, cracked lips, hoarse voice
• Functional:• Ability to chew, difficulty swallowing or speaking
“Systematic oral assessment at least daily or at each patient visit”
ONS Putting Evidence Into Practice, 2009
Mucositis: Management• Prevention• Collaborate with a multidisciplinary team• Oral care products• Patient education (written, verbal, demonstration)• Treat dental problems before cytotoxic therapy• High protein diet• Fluid intake > 1500 ml/day• Cryotherapy for bolus 5-‐FU
• Treatment• Oral agents & hygiene• Systemic pain medications• Culture lesions
Mucositis: Oral Hygiene Program
• Keep oral cavity clean and moist• Daily oral self-‐exam, report signs of mucositis• Oral hygiene after each meal and at bedtime, increase to q 2 hours as needed• Floss daily with dental tape• Brush with soft toothbrush, 90 seconds bid• Swish after each meal, at bedtime, at other times with water or mouth rinse (Normal Saline, sodium bicarbonate)
• Avoid oral irritants including tobacco and alcohol• Maintain adequate hydration• Use water based moisturizers to protect the lips
ONS Putting Evidence Into Practice, 2009

26
Cryotherapy for Bolus Mucotoxic Chemotherapy with Short Half Life
• Bolus 5-‐fluorouracil (5-‐FU) & Melphalan• Instruct patients to hold ice chips in their mouth starting 5 minutes prior and for 30 minutes after.• The effectiveness of this intervention is based on vasoconstriciton of the circulation in the oral cavity and the short half life of these agents. • Evidence is lacking to support the benefit with other chemotherapy agents.• Do not use in patients receiving oxaliplatin
ONS Putting Evidence Into Practice, 2009
ONS PEP Resource:Mucositis Recommended for Practice– Cryotherapy– Low Level Laser Therapy– Oral Care Protocol– Palifermin– Sodium Bicarbonate– Viscous Lidocaine Likely to Be Effective– Benzydamine– Lactobacillus Lozenges– Prophylactic Chlorhexidine
ONS PEP Resource:Mucositis
Effectiveness Not EstablishedAllopurinol Mouthwash Flurbiprofen tooth patch Mauka & Kanuka Tetracaine
Aloe Vera Folinic Acid Misoprostol Oral Rinse Tricolsan Mouth Rinse
Amifostine Glutamine Payayor Turmeric
ATL-104 Hangeshashinto Phenylbutyrate rinse Vitamin EBethanechol Herbal Medicine Pilocarpine Zinc/Zinc supplements
Ca++ Phosphate Rinse High dose laser therapy Povidone Iodine
Calendula Offic inalis Honey Professional Oral Care
Camellia/Wheat Extract Human Intestinal Trefoil Prophylactic CSF
Caphosol Hylauronic Acid Propolis (Bee Glue)
Colchic ine Mouthwash Indigowood Root RhEGF
CSF Mouth Rinses Infrared Phototherapy Repifermin
Doxepin Mouthwash Irsogladine Maleate Rhodiola Algida
Fluoride gum Light Therapy Salivary stimulation
ONS PEP Resource:Mucositis
Effectiveness Unlikely– Iseganan– Traumeel S– Wobe-‐Mugos Not Recommended for Practice– Chlorhexidine (not prophylactic)– Sucralfate– Magic Mouthwash

27
Taste Alterations: Causes• Disease related• Invasion of the tumor• Oral infections• Excretion of amino acid-‐like substances from the tumor cells
• Treatment related• Specific surgical sites• Radiation• Chemotherapy:• Lowered threshold for bitter taste• Increased threshold for sweet, sour and salty taste• Aversion to meats• Metallic taste
Taste Alterations: Consequences
• Anorexia
• Decreased intake
• Altered or perverted sense of taste for certain foods
• * Can persist for up to 1 year *
Taste Alterations: Management
Experiment with spices and flavorings Use the aroma of foods to stimulate taste Encourage oral hygiene before and after meals Add increased sweeteners Substitute other sources of protein Marinate meats in sweet marinades Avoid the sight and smell of foods causing unpleasantness Avoid alcohol, commercial mouthwashes, smoking Consume hard candies and / or chew gum to change taste
before meals and before chemotherapy treatment to reduce metallic taste
Refer to dietitians for nutritional counseling Assess for weight loss
Common Nutritional Challenges
• Anorexia• Malnutrition• Weight Loss • Muscle Mass Loss• Cachexia
• At diagnosis: 50% of patients present with nutritional issues• Malnutrition is the most common secondary diagnosis to cancer

28
Patients with Weight Loss have worse outcomes
Chemotherapy dose reductions Increase dose limiting toxicity Decreased Treatment response Decreased Quality of Life and performance status Shorter survival
Andreyev HJN, Eur J Cancer, 1998/34(4) 503-‐509
Malnutrition’s Effect on Oncology Patients
Just a small loss of weight may be a sign of a nutritional decline that leads to:
• Treatment Delays• Complications• More frequent hospitalizations• Reduced key outcomes such as quality of life
Dewys, WD et al. Am J Med, 1980, 69: 491-497
In addition to decreasing inflammation, corticosteroids:
a)Improve muscle toneb)Stimulate weight lossc)Stimulate the appetited)Reduce anxiety
Nursing interventions for the management of nausea include encouraging patients to:
a) Use sauces and graviesb) Eat foods that are cold or at room temperaturec) Eat high protein / high potassium foodsd) Avoid brushing their teeth when they are nauseated

29
Cachexia in the patient with cancer may result in increased:
a) Bone densityb) Infection ratesc) Glucose turnoverd) Lipoprotein lipase activity
Symptom ManagementAlterations in:• Integumentary Function
Juanita Madison, MN, RN, AOCN
/
Skin Reactions
• Alopecia• Rash• Palmar-‐Plantar Erythrodysesthesia (PPE)–Also called “Hand-‐Foot Syndrome”• Xerosis• Pruritus• Paronychia• Radiation dermatitis• Radiation recall
1 1 5 /
Alopecia
• Chemotherapy-‐induced most commonly occurs on the scalp• Extent depends on:–Mechanism of action of the drug–Administration route–Drug dose, serum half-‐life–Duration (bolus versus continuous infusion)– Response of the patient– Condition of the hair prior to treatment
Chemotherapy-‐Induced
1 1 6

30
/
Risk Factors
• Combination chemotherapy – higher risk• Epidermal growth factor receptor (EGFR) inhibitors• High-‐dose chemotherapy• Non-‐cytotoxic medications• Hypothyroidism• Aging• Poor hair condition before cytotoxic treatment• Concomitant or previous radiation therapy to head
1 1 7 /
Chemotherapy Risk of Chemotherapy-‐Induced Alopecia
Classification High Risk Moderate Risk Low Risk
Alkylating Agent CyclophosphamideIfosfamide
MechlorethamineMethotrexate
CarboplatinCisplatin
Antimetabolite -‐ AmsacrineBusulfanCytarabineGemcitabine
Capecitabine5-‐FUFludarabineHydroxyureaThiotepa
Antitumor Antibiotic DaunorubucinDoxorubicinEpirubicin
-‐ BleomycinMitoxantrone
Camptothecins IrinotecanTopecan
-‐ -‐
Epiopdophyllotoxins Etoposide -‐ -‐
Targeted Therapies -‐ Tyrosine Kinase Inhibitors
Taxanes DocetaxelPaclitaxel
-‐ -‐
VincaAlkaloids Vinorelbine -‐ VincristineVinblastine
/
Degrees of Alopecia
• Grade 1– Hair loss of <50% of normal for that individual that is obvious only on close inspection– A different hair style may be required to cover the hair loss but it does not require a wig
• Grade 2– Hair loss of 50% or greater compared to normal for that individual that is readily apparent to others– A wig or hair-‐piece is necessary if the patient desires to completely camouflage the hair loss– Associated with psychosocial impact
1 1 9 /
Expected Time and Pattern of Hair Loss
• Timing:– Hair shedding begins approximately 1-‐3 weeks after administration of chemotherapy
– May last 1-‐2 months after initiation of therapy• Pattern– Hair loss tends to occur first on the crown and sides of head above ears
– Generally reversible• Regrowth– Begins 1-‐3 months after discontinuation of therapy– Regrown hair may change in color, structure, or texture– Permanent alopecia after chemotherapy rare • Can occur with high-‐dose busulfan and cyclophosphamide
1 2 0

31
/
Alopecia ManagementPrevention
• Scalp hypothermia– It is practiced and has shown benefit, however, is not FDA approved– Safety concerns exist for patients with hematologic malignancies– Further research continues regarding safety and efficacy
• Pharmacologic management– Little data exists– Some drugs that may prove beneficial but require further research include topical calcitriol and amifostine
1 2 1 /
Alopecia Management
• For Grade 1 and 2 toxicities– Consider minoxidil 5% twice daily during chemotherapy; biotin 2.5 mg daily, and orthascilic acid 10 mg daily after chemotherapy is completed– Counsel the patient on the use of hats, scarves, and wigs
1 2 2
/
Patient and Family Education
• Cause of alopecia• Expected timeframe for hair loss and regrowth• Strategies to manage hair loss and regrowth–Most strategies have not been tested in clinical trials–Use shampoos without detergents, menthol, salicylic acid, alcohol, or heavy perfumes–Avoid permanents, bleach, coloring agents, vigorous brushing, hot rollers, excessive heating with dryer– Protect scalp from cold and sun (hats, scarves, wigs)
Presen tatio n Title 1 2 3 /
Local Resources for Support
• The American Cancer Society's program "Look Good...Feel Better“–Materials available coveringcranial prosthesis (wig) information and pointers on head coverings.• Website: www.lookgoodfeelbetter.org• Call 1-‐800-‐395-‐LOOK to get a free copy of the catalog
• The American Cancer Society’s Tender Loving Care® publication (both magazine and catalog) – Combines helpful articles and information on any cancer treatment that causes hair loss.• Website: www.tlcdirect.org• Call 1-‐800-‐850-‐9445 to get a free copy of the catalog
1 2 4

32
/
Epidermal Growth Factor Receptor (EGFR) Rash
• EGFR is normally expressed in the epidermis, sebaceous glands, and hair follicular epithelium• Plays important role in maintenance of normal skin health• Inhibition of EGFR believed to cause cutaneous injury and skin rash
1 2 5 /
Examples of Epidermal Growth Factor Receptor (EGFR) Inhibitors
• Small molecule targeted therapy-Erlotinib (Tarceva)-Lapatinib (Tykerb)-Vandetanib (Caprelsa)
• Monoclonal antibodies -Cetuximab (Erbitux)-Panitumumab (Vectibix)
1 2 6
/
Epidermal Growth Factor Receptor (EGFR) Inhibitor Rash
• Resembles acne• Characterized by skin eruption consisting of:– Papules (small, raised pimples)– Pustules (small pus-‐filled blister)• Typically appears on face, scalp, upper chest, and back• Unlike acne, does not present with whiteheads or blackheads• Can be symptomatic (itchy or tender lesions)
1 2 7 /
Management: Acneiform Rash
• Alcohol-‐free OTC moisturizing creams or ointments twice daily• Sunscreen SPF >=15 applied to exposed areas of body and reapply every 2 hours when outside• Topical low/moderate potency steroid to the face and chest twice daily• Topical/oral antibiotics or oral low-‐dose steroids as indicated
Low Grade Toxicity Management
1 2 8

33
/
Management: Acneiform Rash
• Stop topical antibiotic if being used• Begin oral antibiotic for 6 weeks (tetracycline)
AND• Topical low/moderate potency steroid• Topical low/moderate potency steroid +/-‐• Isotretinoin at low doses (20-‐30mg/day)
Severe Toxicity Management
(Ro sen , et al. , 2 0 1 4 ; Memo rial S lo an Ketterin g Can cer Cen ter; 2 0 1 4 a)1 2 9 /
Recommendations for Prevention and Management of EGFR-‐Inhibitor Rash
MASCCPreventive/Prophylactic• Systemic
• Minocycline 100 mg daily or doxycycline 100 mg BID
• Topical• Hydrocortisone 1% creamwith moisturizer
and sunscreen BIDTreatment• Topical
• Alclometasone0.05% cream• Fluocinonide0.05% cream BID• Clindamycin 1%
• Systemic• Doxycycline 100 mg BID• Minocycline 100 mg daily• Isotretinoin at low doses 20-‐30 mg/day
NCCNPreventive/Prophylactic• Systemic
• Oral semisynthetic tetracycline agents (doxycycline or minocycline)
• Topical• Hydrocortisone 1%, skin moisturizer and
sunscreenTreatment• Topical
• Topical steroids and antibiotics, such as clindamycin and erythromycin
• Systemic• Oral antibiotics includedoxycycline or
minocycline• Systemic steroids are typically not used• Isotretinoin reactively (based on anecdotal
or nonrandomized studies)
Not Recommended: Pimecrolimus 1% cream, tazarotene0.05% cream, sunscreen as a single agent, tetracycline 500 mg BID, vitamin K, cream, acitretin, oil-‐ in water topical trolamineemulsion
1 3 0
MASCC: Multinational Association of Supportive Care in CancerNCCN: National Comprehensive Cancer Network. Note: Based on information from Burtness, et al,
2009; Eaby-Sandy et al, 2012; Lacouture, 2011
/
What is the mainstay of treatment for a patient experiencing maculopapular rash related to epidermal growth factor receptor inhibitors?
a. Oral steroids, moisturizers, antifungalb. Topical steroids, antibacterials, and moisturizersc. Topical benzyl peroxided. Discontinuation of the offending agent
Presen tatio n Title 1 3 1 /
A patient is experiencing grade 1 alopecia secondary to chemotherapy. Which of the following treatments is most appropriate to recommend?
a. Scalp hyperthermiab. Topical calcitriolc. Amifostined. Minoxidil
Presen tatio n Title 1 3 2