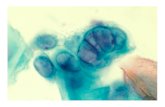
Neonatal Herpes Simplex Infection
-
Upload
elton-vincent -
Category
Documents
-
view
84 -
download
7
description
Transcript of Neonatal Herpes Simplex Infection

Neonatal Herpes Simplex Neonatal Herpes Simplex InfectionInfection
Jesus Peinado M.D.Jesus Peinado M.D.Dept. of Pediatrics: PGY2Dept. of Pediatrics: PGY2
July 24, 2008July 24, 2008

INTRODUCTIONINTRODUCTION
Neonatal HSV infections were first described Neonatal HSV infections were first described in the mid-1930s.in the mid-1930s.
The earliest antiviral agents proved too toxic The earliest antiviral agents proved too toxic in humans to be useful.in humans to be useful.
Vidarabine was the first systemically Vidarabine was the first systemically administered antiviral medication.administered antiviral medication.

VIRAL STRUCTUREVIRAL STRUCTURE
Large and enveloped virionsLarge and enveloped virions
Icosahedral nucleocapsid consisting of 162 Icosahedral nucleocapsid consisting of 162 capsomerescapsomeres
Arranged around a linear, double stranded Arranged around a linear, double stranded DNA coreDNA core
The genome consists of 2 covalently linked The genome consists of 2 covalently linked componentscomponents

VIRAL STRUCTUREVIRAL STRUCTURE
Core: Consists of a single linear molecule of dsDNa in the form of a torus
Capsid: Surrounding the core w/ a 100 nm diameter and 162 capsomeres
Tegument: Consists of viral enzymes
Envelope: Outer layer composed of altered host membrane and a dozen
of viral glycoproteins

VIRAL STRUCTUREVIRAL STRUCTURE

VIRAL STRUCTUREVIRAL STRUCTURE
There is considerable cross-reactivity between There is considerable cross-reactivity between HSV-1 and HSV-2 glycoproteinsHSV-1 and HSV-2 glycoproteins
Unique antigenic determinants exist for each Unique antigenic determinants exist for each virusvirus
Eleven glycoproteins have been identifiedEleven glycoproteins have been identified
They mediate attachment, penetration and They mediate attachment, penetration and provoke immune responsesprovoke immune responses

VIRAL STRUCTUREVIRAL STRUCTURE
gD is the most potent inducer in neutralizing gD is the most potent inducer in neutralizing antibodiesantibodies
gD is related to viral entry into the cellgD is related to viral entry into the cell
gB is related to infectivitygB is related to infectivity
gG provides w/ antigenic specificity w/ the gG provides w/ antigenic specificity w/ the resulting antibody response allowing resulting antibody response allowing distinction between HSV-1 and HSV-2distinction between HSV-1 and HSV-2

BIOLOGYBIOLOGY
Latency and neurovirulence directly influence Latency and neurovirulence directly influence on human diseaseon human disease
During HSV infection , virions are transported During HSV infection , virions are transported by by retrograde flow along axonsby by retrograde flow along axons
Vioral multiplication occurs in a small number Vioral multiplication occurs in a small number of sensory neuronsof sensory neurons
Viral genome remains in a latent state for the Viral genome remains in a latent state for the life of the hostlife of the host

BIOLOGYBIOLOGY
Periodic reactivation brought on by events of Periodic reactivation brought on by events of physical or emotional stressphysical or emotional stress
The virus is transported back down the axon to The virus is transported back down the axon to replicate again at or near the point of entryreplicate again at or near the point of entry
Reactivation can result in apparent disease Reactivation can result in apparent disease (lesions) or clinically inaparent (subclinical) (lesions) or clinically inaparent (subclinical) infectioninfection
Latency is incompletely understood Latency is incompletely understood

BIOLOGYBIOLOGY
Neurovirulence is the affinity with which HSV Neurovirulence is the affinity with which HSV is drawn to and propagated in neuronal tissueis drawn to and propagated in neuronal tissueThis can result in profound disease with severe This can result in profound disease with severe neurologic sequelaneurologic sequelaSites for neuurovirulence have been mapped to Sites for neuurovirulence have been mapped to the thymidine kinase genethe thymidine kinase geneThe gene identified as YThe gene identified as Y1134.5 is required for 34.5 is required for replication in the CNS and prevents apoptosis replication in the CNS and prevents apoptosis of infected cellsof infected cells

MATERNAL GENITAL MATERNAL GENITAL INFECTIONSINFECTIONS
Most common form of genital infection during Most common form of genital infection during gestationgestation
10% of HSV-2 seronegative pregnant women 10% of HSV-2 seronegative pregnant women have an HSV-2 seropositive partnerhave an HSV-2 seropositive partner
2/3 of women who acquire genital herpes 2/3 of women who acquire genital herpes during pregnancy have no symptomsduring pregnancy have no symptoms
60 to 80% of women who deliver an HSV-60 to 80% of women who deliver an HSV-infected infant have no evidence of infectioninfected infant have no evidence of infection

NEONATAL TRANSMISSIONNEONATAL TRANSMISSION
Gravid woman must be shedding virus w/ or Gravid woman must be shedding virus w/ or w/out symptomsw/out symptomsIn non-pregnant HSV-seropositive women In non-pregnant HSV-seropositive women HSV as detected by PCR is shed w/out HSV as detected by PCR is shed w/out symptoms 1 of every 3 dayssymptoms 1 of every 3 daysAmong pregnant women the viral excretion Among pregnant women the viral excretion proximate to delivery from 0.20 to 0.39%proximate to delivery from 0.20 to 0.39%Pregnant women w/ recurrent genital HSV the Pregnant women w/ recurrent genital HSV the incidence is from 0.70 to 1.4%incidence is from 0.70 to 1.4%

FACTORS INFLUENCING FACTORS INFLUENCING TRANSMISSIONTRANSMISSION
Type of maternal infection (primary/recurrent)Type of maternal infection (primary/recurrent)
Maternal antibody statusMaternal antibody status
Duration of rupture of membranesDuration of rupture of membranes
Integrity of mucocutaneous barriersIntegrity of mucocutaneous barriers
Mode of delivery (C-section/vaginal)Mode of delivery (C-section/vaginal)

INCIDENCE OF NEONATAL INCIDENCE OF NEONATAL DISEASEDISEASE
2/1000 mothers are HSV culture + at delivery 2/1000 mothers are HSV culture + at delivery asymptomaticasymptomatic50-70% affected infants born to women 50-70% affected infants born to women asymptomatic at the time of deliveryasymptomatic at the time of deliveryAntepartum cultures are not useful in assessing Antepartum cultures are not useful in assessing risk of neonatal infectionrisk of neonatal infectionIncreased risk w/ primary vs recurrent Increased risk w/ primary vs recurrent infectioninfectionIncidence from 1/2000 to 1/5000 live births Incidence from 1/2000 to 1/5000 live births and increasingand increasing

RISK OF NEONATAL HSV RISK OF NEONATAL HSV INFECTIONINFECTION
50% risk: Infants born to women w/ primary 50% risk: Infants born to women w/ primary infection near the time of deliveryinfection near the time of delivery30% risk: Infants born to mothers with first 30% risk: Infants born to mothers with first episode, non-primary infection (antibody to episode, non-primary infection (antibody to type 1, new acquisition type 2 and vice versa)type 1, new acquisition type 2 and vice versa)1 to 3% of infants born to mothers w/ recurrent 1 to 3% of infants born to mothers w/ recurrent infectioninfectionPassive immunity protects against infection , Passive immunity protects against infection , but has little effect on the severity of diseasebut has little effect on the severity of disease

RISK FACTORSRISK FACTORS
Associated w/ primary maternal HSV:Associated w/ primary maternal HSV:
1.1. Genital symptoms, UTI symptoms not Genital symptoms, UTI symptoms not responsive to therapyresponsive to therapy
2.2. Positive HSV cultures from both cervix and Positive HSV cultures from both cervix and vaginavagina
3.3. New sexual partner immediately prior to or New sexual partner immediately prior to or during pregnancyduring pregnancy

RISK FACTORSRISK FACTORS
Reasons for increased risk w/ primary Reasons for increased risk w/ primary maternal HSV infectionmaternal HSV infection
1.1. Prolonged shedding of virus up to 3 wk vs 2 Prolonged shedding of virus up to 3 wk vs 2 to 5 daysto 5 days
2.2. Increased number of viral particles excretedIncreased number of viral particles excreted
3.3. Less passive immunity to HSV, i.e. less Less passive immunity to HSV, i.e. less maternal-fetal transfer of HSV neutralizing maternal-fetal transfer of HSV neutralizing antibodiesantibodies

RISK FACTORSRISK FACTORS
Neonatal risk factors:Neonatal risk factors:
1.1. Rupture of membranes > 6 hrsRupture of membranes > 6 hrs
2.2. Scalp electrodes or other internal monitoringScalp electrodes or other internal monitoring
3.3. ChorioamnionitisChorioamnionitis
4.4. CervicitisCervicitis
5.5. Vaginal deliveryVaginal delivery

TIMES OF TRANSMISSIONTIMES OF TRANSMISSION
HSV of the newborn is acquired during one HSV of the newborn is acquired during one of three distinct time intervals:of three distinct time intervals:
1.1. Intrauterine (in utero 5%)Intrauterine (in utero 5%)
2.2. Peripartum (perinatal 85%)Peripartum (perinatal 85%)
3.3. Psotpartum (postnatal 10%)Psotpartum (postnatal 10%)

DISEASE CLASSIFICATIONDISEASE CLASSIFICATION
Disease localized to the skin, eyes and mouth Disease localized to the skin, eyes and mouth SEM disease accounting for 45% of casesSEM disease accounting for 45% of cases
Encephalitis w/ or w/out CNS involvement Encephalitis w/ or w/out CNS involvement accounting for 30% accounting for 30%
Disseminated infection including CNC, lungs, Disseminated infection including CNC, lungs, etc. accounting for 25% etc. accounting for 25%
This classification is predictive of morbidity This classification is predictive of morbidity and mortalityand mortality

SEM DISEASE SEM DISEASE

CUTANEOUS LESIONSCUTANEOUS LESIONS

VESICULAR LESIONS HSVVESICULAR LESIONS HSV

HERPES PNEUMONITISHERPES PNEUMONITIS

INTRAUTERINE INFECTIONINTRAUTERINE INFECTION
Occurs in 1/300,000 deliveriesOccurs in 1/300,000 deliveries
Cutaneous manifestations: scarring, active Cutaneous manifestations: scarring, active lesions, hypo and hyperpigmentation, aplasia lesions, hypo and hyperpigmentation, aplasia cutis and an erythematous macular exanthemcutis and an erythematous macular exanthem
Ophthalmologic: microopthalmia, retinal Ophthalmologic: microopthalmia, retinal dysplasia, optic atrophy, chorioretinitisdysplasia, optic atrophy, chorioretinitis
Neurologic: microcephaly, encephalomalacia, Neurologic: microcephaly, encephalomalacia, hydranencephaly, intracranial calcificationhydranencephaly, intracranial calcification

DISSEMINATED DISEASEDISSEMINATED DISEASE
1/2 to 2/3 of all children w/ neonatal HSV1/2 to 2/3 of all children w/ neonatal HSV
Encephalitis is a common component Encephalitis is a common component occurring in about 60% to 75% occurring in about 60% to 75%
20% w/ disseminated disease do not develop 20% w/ disseminated disease do not develop vesicular rashvesicular rash
Death relate to severe coagulopathy, liver Death relate to severe coagulopathy, liver dysfunction and pulmonary involvementdysfunction and pulmonary involvement

CNS DISEASECNS DISEASE
1/3 of all neonates w/ HSV infection w/ or 1/3 of all neonates w/ HSV infection w/ or w/out SEM involvementw/out SEM involvement
Manifestations include: seizures (focal and Manifestations include: seizures (focal and generalized), lethargy, irritability, tremors, generalized), lethargy, irritability, tremors, poor feeding and bulging fontanellepoor feeding and bulging fontanelle
60 to 70% have associated skin vesicles60 to 70% have associated skin vesicles
Mortality is cause by brain destruction w/ Mortality is cause by brain destruction w/ acute neurologic and autonomic dysfunctionacute neurologic and autonomic dysfunction

HERPES ENCEPHALITISHERPES ENCEPHALITIS

HERPES ENCEPHALITISHERPES ENCEPHALITIS

SIGNS AND SYMPTOMSSIGNS AND SYMPTOMS
Sings/symptomsSings/symptoms SEM diseaseSEM disease CNS diseaseCNS disease Disseminated Disseminated diseasedisease
TotalTotal
Skin vesiclesSkin vesicles
LethargyLethargy
83%83%
19%19%
63%63%
49%49%
58%58%
47%47%
68%68%
38%38%
FeverFever
ConjunctivitisConjunctivitis
SeizureSeizure
17%17%
25%25%
2%2%
44%44%
16%16%
57%57%
56%56%
17%17%
22%22%
39%39%
19%19%
27%27%
DICDIC
PneumoniaPneumonia
00
00
00
3%3%
20%20%
34%34%
11%11%
13%13%

LABORATORY ASSESSMENTLABORATORY ASSESSMENT
Type-specific antibody assays have been Type-specific antibody assays have been approved by FDAapproved by FDA
Serologic testing identifies only past infectionSerologic testing identifies only past infection
Can not identify the site of HSV infectionCan not identify the site of HSV infection
Serological diagnosis is not of clinical valueSerological diagnosis is not of clinical value
Transplacentally acquired IGg confounds the Transplacentally acquired IGg confounds the assessment of neonatal antibody statusassessment of neonatal antibody status
Serologic studies play no role in diagnosis Serologic studies play no role in diagnosis

VIRAL CULTUREVIRAL CULTURE
Remains the definitive diagnostic methodRemains the definitive diagnostic method
If skin lesions If skin lesions scraping of the vesiclesscraping of the vesicles
Other sites include: CSF, urine, blood, stool or Other sites include: CSF, urine, blood, stool or rectum, oropharynx and conjunctivarectum, oropharynx and conjunctiva
Duodenal aspirates if hepatitis/NEC/GI diseaseDuodenal aspirates if hepatitis/NEC/GI disease
Of the sites cultured for HSV skin, eye and Of the sites cultured for HSV skin, eye and conjunctiva provides the greatest yieldsconjunctiva provides the greatest yields

PCR AMPLIFICATION
PCR results from CSF of infected neonates No. (%) of patients with:
PCR result SEM (n29) CNS (n34) Disseminated (n14)
Positive 7 (24) 26 (76) 13 (93)
Negative 22 (76) 8 (24) 1 (7)

TREATMENT & MANAGEMENTTREATMENT & MANAGEMENT
Mortality in preantiviral era by 1 y of age was Mortality in preantiviral era by 1 y of age was 85% disseminated and 50% CNS disease85% disseminated and 50% CNS disease
W/ high dose acyclovir 60mg/kg/day 12mo W/ high dose acyclovir 60mg/kg/day 12mo mortality for disseminated 29% and 4% for mortality for disseminated 29% and 4% for CNS diseaseCNS disease
Lethargy/hepatitis are associated w/ mortality Lethargy/hepatitis are associated w/ mortality in disseminated diseasein disseminated disease
Prematurity/seizures in CNS diseasePrematurity/seizures in CNS disease

TREATMENTTREATMENT
Topical agents (trifluridine) are recommended Topical agents (trifluridine) are recommended for use along w/ parenteral acyclovir for ocular for use along w/ parenteral acyclovir for ocular diseasedisease
IVIG has no value in the treatment of HSVIVIG has no value in the treatment of HSV
Higher doses of acyclovir are associated w/ Higher doses of acyclovir are associated w/ neutropenia neutropenia
Adequate hydration reduces risk of Adequate hydration reduces risk of nephrotoxicitynephrotoxicity

SUMMARY OF CURRENT SUMMARY OF CURRENT TREATMENTTREATMENT
Acyclovir 60mg/kg/day improves morbidity Acyclovir 60mg/kg/day improves morbidity and mortalityand mortality
In preterm dosing interval based on CrClIn preterm dosing interval based on CrCl
Disseminated and CNS disease 21 daysDisseminated and CNS disease 21 days
SEM 14 daysSEM 14 days
All patients w/ CNS disease should have a All patients w/ CNS disease should have a repeat LP at the end of therapy repeat LP at the end of therapy
CSF w/ + PCR should continue treatment until CSF w/ + PCR should continue treatment until PCR negativePCR negative

PCR results following completion of antiviral therapy
No. (%) with PCR resultInfant characteristic
Negative Positive PDisease classification
CNS disease 4 (36.4) 14 (73.7) < 0.001 Disseminated disease 0 (0.0) 5 (26.3) SEM disease 7 (63.6) 0 (0.0)
CSF indices Normal 6 (54.5) 1 (5.3) Abnormal 3 (27.3) 17 (89.4)
Morbidity/mortality >12 mo Normal 6 (54.5) 1 (5.3) <0.001 Mild 0 (0.0) 0 (0.0) Moderate 1 (9.1) 3 (15.8) Severe 2 (18.2) 10 (52.6) Dead 0 (0.0) 5 (26.3) Unknown 2 (18.2) 0 (0.0)

ANTIBODY THERAPYANTIBODY THERAPY
Utilization of passive immunotherapy as Utilization of passive immunotherapy as adjuvant to active antiviral interventionsadjuvant to active antiviral interventions
Human and humanized monoclonal Ab against Human and humanized monoclonal Ab against gB or gD are benefical in animal modelsgB or gD are benefical in animal models
Studies w/ humans have documented Studies w/ humans have documented protective effectsprotective effects

VACCINE DEVELOPMENTVACCINE DEVELOPMENT
HSV-2 gD adjuvanted w/ alum combined w/ HSV-2 gD adjuvanted w/ alum combined w/ 3-deacylated monmophosphoryl lipid A has 3-deacylated monmophosphoryl lipid A has demostrated promising resultsdemostrated promising results
75% efficacy in preventing HSV-1 HSV-2 75% efficacy in preventing HSV-1 HSV-2 genital diseasegenital disease
40% efficacy in preventing HSV-2 infection40% efficacy in preventing HSV-2 infection

RECOMMENDATIONSRECOMMENDATIONS
Pregnant women w/ primary or first episode: Pregnant women w/ primary or first episode: Acyclovir therapy at 36 wk 400mg tidAcyclovir therapy at 36 wk 400mg tid
Primary HSV in 3Primary HSV in 3rdrd trimester: C-section should trimester: C-section should be offered where lesions can occur within 6 be offered where lesions can occur within 6 wk of anticipated delivery and seroconversion wk of anticipated delivery and seroconversion has not occurred yethas not occurred yet
Recurrent HSV: Acyclovir at 36 wk 400 mg Recurrent HSV: Acyclovir at 36 wk 400 mg tidtid

RECOMMENDATIONSRECOMMENDATIONS
Women in labor: HSV lesionsWomen in labor: HSV lesions C-section C-section may reduce the risk if performed 4-6 hrs ROMmay reduce the risk if performed 4-6 hrs ROM
Many recommend C-section even if ROM >6 Many recommend C-section even if ROM >6 hrshrs
If term and active lesions If term and active lesions C-section C-section
If preterm If preterm Acyclovir 15mg/kg/day Acyclovir 15mg/kg/day
If no lesions only history If no lesions only history VD VD
If genital lesionsIf genital lesions No invasive procedures No invasive procedures (scalp sampling or monitors or early ROM)(scalp sampling or monitors or early ROM)

INFANTS BORN BY NSVDINFANTS BORN BY NSVD
CULTURESCULTURES
Asymptomatic infants exposed to HSVAsymptomatic infants exposed to HSV
Mother had HSV but no lesions at deliveryMother had HSV but no lesions at delivery
Should be taken from urine, stool, rectum, Should be taken from urine, stool, rectum, mouth, eyes and nasopharynxmouth, eyes and nasopharynx
If therapy is initiated a CSF sample should be If therapy is initiated a CSF sample should be obtained prior to treatmentobtained prior to treatment
Duration of therapy is 14 to 21 daysDuration of therapy is 14 to 21 days

INFANTS BORN BY NSVDINFANTS BORN BY NSVD
Acyclovir therapy is not recommended for the Acyclovir therapy is not recommended for the asymptomatic infantasymptomatic infant
Symptomatic infants PCR testing of CSF and Symptomatic infants PCR testing of CSF and bloodblood
The index of suspicion should be maintained The index of suspicion should be maintained for 6 wkfor 6 wk
HSV infection may occur as late as 4-6 wk HSV infection may occur as late as 4-6 wk after deliveryafter delivery

INFECTION CONTROL INFECTION CONTROL MESASURESMESASURES
Contact precautionsContact precautions
Hand washing before and after care of infantsHand washing before and after care of infants
Mother w/ lesions on hands hand hygiene and Mother w/ lesions on hands hand hygiene and gloves gloves
BF is allowed if no lesions on the breast and if BF is allowed if no lesions on the breast and if active lesions are coveredactive lesions are covered
Mother w/ herpes labialis or stomatitis should Mother w/ herpes labialis or stomatitis should wear disposable maskswear disposable masks

DIAGNOSTIC TESTSDIAGNOSTIC TESTS
Cultures from skin lesions, mouth, Cultures from skin lesions, mouth, nasopharynx, conjunctiva, urine, stool/ano-nasopharynx, conjunctiva, urine, stool/ano-rectum and CSF.rectum and CSF.
Positive cultures at more than 48 hrs are Positive cultures at more than 48 hrs are consistent w/ viral replication as opposed to consistent w/ viral replication as opposed to colonization colonization
Serologic tests should not be relied onSerologic tests should not be relied on
PCR testing for CSF HSV DNA is the PCR testing for CSF HSV DNA is the diagnostic method of choice for HSV diagnostic method of choice for HSV encephalitisencephalitis

TREATMENT AND FOLLOW UPTREATMENT AND FOLLOW UP
Acyclovir is the treatment of choiceAcyclovir is the treatment of choice
SEM 14 days of treatmentsSEM 14 days of treatments
CNS and disseminated disease 21 daysCNS and disseminated disease 21 days
Oral acyclovir contraindicated in neonates for Oral acyclovir contraindicated in neonates for HSV treatmentHSV treatment
Ocular involvement requires trifluridine Ocular involvement requires trifluridine

FUTURE RESEARCH ISSUESFUTURE RESEARCH ISSUES
The optimal management of pregnant women The optimal management of pregnant women w/ genital HSV as this relates to antenatal w/ genital HSV as this relates to antenatal acycloviracyclovir
The management of women w/ known or The management of women w/ known or primary HSV who present w/ PROMprimary HSV who present w/ PROM
The pharmacokinetics of acyclovir in VLBWThe pharmacokinetics of acyclovir in VLBW
The significance of acyclovir-resistant HSV The significance of acyclovir-resistant HSV from neonates w/ prolonged exposurefrom neonates w/ prolonged exposure

FUTURE RESEARCH ISSUESFUTURE RESEARCH ISSUES
The role of new agents such as famciclovir The role of new agents such as famciclovir and valacyclovir where oral therapy is desiredand valacyclovir where oral therapy is desiredThe impact of long-term suppressive therapy The impact of long-term suppressive therapy on neurological outcomes and immune on neurological outcomes and immune responsesresponses Development/standardization of PCR on Development/standardization of PCR on different body fluidsdifferent body fluidsRole of combination antiviral therapy w/ Role of combination antiviral therapy w/ acyclovir plus monoclonal HSV antibodiesacyclovir plus monoclonal HSV antibodiesDevelopment of vaccines against HSVDevelopment of vaccines against HSV

REFERENCESREFERENCES
Neonatal Herpes Simplex Infection, Kimberlin Neonatal Herpes Simplex Infection, Kimberlin David; Clinical Microbiology reviews, Jan David; Clinical Microbiology reviews, Jan 2004 p 1-13.2004 p 1-13.AAP 2005, Herpes Simplex, p 309-318. In AAP 2005, Herpes Simplex, p 309-318. In L.K. Pickering (ed.) 2005 Red Book.L.K. Pickering (ed.) 2005 Red Book.Current management of HSV infection in Current management of HSV infection in pregnant women and their newborn infants, pregnant women and their newborn infants, Canadian Paediatric Society, Paediatrics and Canadian Paediatric Society, Paediatrics and Child health 2006;11:363-5Child health 2006;11:363-5

![Immunology of Herpes Simplex Virus Infection: …...[CANCER RESEARCH 36, 836-844, February 1976] Immunology of Herpes Simplex Virus Infection: Relevance to Herpes Simplex Virus Vaccines](https://static.fdocuments.in/doc/165x107/5e3c207dedbcb80872726a41/immunology-of-herpes-simplex-virus-infection-cancer-research-36-836-844.jpg)