mL A FORENSIC TOXICOLOGY ANALYSIS OF SYNTHETIC FENTANYL …€¦ · Chromatography of synthetic...
Transcript of mL A FORENSIC TOXICOLOGY ANALYSIS OF SYNTHETIC FENTANYL …€¦ · Chromatography of synthetic...
-
TO DOWNLOAD A COPY OF THIS POSTER, VISIT WWW.WATERS.COM/POSTERS ©2017 Waters Corporation For forensic toxicology use only.
Time1.20 1.40 1.60 1.80 2.00 2.20
%
0
100
1.20 1.40 1.60 1.80 2.00 2.20
%
0
100
1.20 1.40 1.60 1.80 2.00 2.20
%
0
100
1.20 1.40 1.60 1.80 2.00 2.20
%
0
100
1.20 1.40 1.60 1.80 2.00 2.20
%
0
100
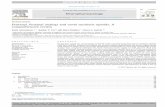
Cal_500_180418_002 13: MRM of 2 Channels ES+ 351.232 > 202.181 (3-methyl Fentanyl)
1.06e61.65
Cal_500_180418_002 22: MRM of 3 Channels ES+ TIC (Carfentanyl)
2.43e61.66
Cal_500_180418_002 12: MRM of 2 Channels ES+ 351.168 > 188.138 (Butyryl Fentanyl)
1.59e61.69
Cal_500_180418_002 17: MRM of 2 Channels ES+ TIC (PFBF)
3.12e61.76
Cal_500_180418_002 20: MRM of 2 Channels ES+ TIC (Sufentanyl)
1.39e61.80
INTRODUCTION
Overdose deaths from opiates and synthetic opioids have risen substantially over the past few years, with the largest increases attributed to synthetic opioids such as fentanyl and its analogs. Due to their high potency, the concentrations of these compounds are often in the sub ng/mL range. In addition, forensic samples are often analyzed from whole blood, presenting challenges not seen in plasma or urine samples. This work details a rapid UPLC-MS/MS method for the analysis of synthetic fentanyl compounds in whole blood employing a novel mixed-mode SPE strategy designed to minimize residual phospholipids. The resulting method minimizes ion suppression while producing linear and accurate quantification over the entire expected analytical range of these compounds.
A FORENSIC TOXICOLOGY ANALYSIS OF SYNTHETIC FENTANYL COMPOUNDS IN WHOLE BLOOD USING OASIS PRIME MCX AND WATERS TQ-S MICRO
Jonathan P. Danaceau1, Scott Freeto2, Kim Haynes1 and Lisa J. Calton3
1-Waters Corporation, Milford, MA, USA; 2-Waters Corporation, Beverly, MA, USA; 3-Waters Corporation, Wilmslow, UK
Sample Preparation
One hundred microliters (µL) of whole blood was diluted 1:1 with 100 mM ZnSO4/NH4OAc followed by precipitation with 1:1 ACN:MeOH. After centrifugation, the entire supernatant was diluted with1 mL 4% H3PO4 and loaded directly onto a Waters Oasis
® PRiME MCX µElution plate.
The sorbent was washed with 200 µL of 2% formic acid:100 mM NH4COOH followed by 200 µL of MeOH. Samples were eluted with 2 x 25 µL of 50:50 ACN:MeOH containing 5% strong ammonia and diluted with 50 µL of 97:2:1 H2O:ACN:formic acid. A graphical representation of the extraction procedure is shown in Figure 1.
RESULTS
Chromatography
UPLC System: ACQUITY UPLC® I-Class-FTN
Column: ACQUITY UPLC® BEH C18, 1.7
µm; 2.1 x 100 mm
Mobile Phase A (MPA) 0.1% Formic Acid in Water
Mobile Phase B (MPB) 0.1% Formic Acid in ACN
Flow Rate 0.6 mL/min
Column Temp: 40 ˚C
Sample Temp: 10 ˚C
Needle Wash 25:25:25:25 H2O:ACN:MeOH:IPA
Injection volume 5 µL
Instrumental Conditions
METHODS
Figure 2. Chromatography of synthetic fentanyl analogs. All samples eluted in under 2 minutes
Figure 3. Chromatogram of phospholipid removal from whole blood samples. A. Residual phospholipids remaining in a samples prepared by protein precipitation only (Top) or using Oasis PRiME MCX plates (bottom). B. Mean phospholipids as measured by peak area. Oasis PRiME MCX removed >99% of residual phospholipids compared to protein precipitation only.
Phospholipid Removal from Whole Blood
Recovery and Matrix Effects
Table 2. Quality Control results for fentanyl analogs in whole blood. In most all cases, accuracies were within 10% and %RSDs were less than 10%.
CONCLUSIONS
Rapid and efficient analysis of fentanyl analogs from
whole blood
No evaporation or reconstitution steps needed
Removal of residual phospholipids from whole blood
extracts
Highly efficient and consistent recovery with negligible
matrix effects for all analytes
Sensitivity appropriate for sub lethal doses and minor
metabolite quantification
Linear dynamic range to accommodate overdose cases
Excellent correlation with results from external reference
lab
R.T. Name M+H+ MRM CV CE
0.68 Acetyl Norfentanyl 219.1 84.1
136.1 4 4
18 20
0.91 Norfentanyl 233.1 84.1
150.1 5 5
15 15
1.03 Norcafentanil 291.1
231.2 259.2
5 5
14 10
1.06 Remifentanil acid 363.2 214.1 113.0
5 5
30 18
1.18 Remifentanil 377.2 113.0 228.1
5 5
30 20
1.24 Methoxyacetyl Fentanyl
353.2 188.2 105.1
10 10
22 37
1.29 Acetyl Fentanyl 323.1 188.1 105.0
5 5
34 22
1.33 β-OH Fentanyl 353.2 186.1 204.2
10 10
26 25
1.48 4-ANPP 281.2 188.1 105.0
8 8
16 30
1.50 Fentanyl 337.2 188.1 105.0
5 5
22 35
1.55 Furanyl Fentanyl 375.2 188.1 105.0
5 5
22 38
1.59 Cyclopropyl Fentanyl 349.2 105.0 188.1
10 10
35 25
1.65 (±)-cis-3-Methylfentanyl
351.2 202.2 105.0
5 5
22 35
1.66 Carfentanyl 395.2 355.2 113.1
10 10
16 32
1.69 Butyryl Fentanyl 351.2 188.1 105.0
5 5
22 38
1.76 para-Fluorobutyryl Fentanyl (PFBF)
369.2 188.1 105.0
5 5
24 40
1.80 Sufentanyl 387.2 238.1 355.2
10 10
18 18
Solid Phase Extraction
Figure 1. Solid phase extraction (SPE) procedures for the extraction of synthetic fentanyl anlogs from whole blood samples. No evaporation or reconstitution steps were required.
Time0.50 0.60 0.70 0.80 0.90 1.00 1.10 1.20 1.30
%
0
100
0.50 0.60 0.70 0.80 0.90 1.00 1.10 1.20 1.30
%
0
100
0.50 0.60 0.70 0.80 0.90 1.00 1.10 1.20 1.30
%
0
100
0.50 0.60 0.70 0.80 0.90 1.00 1.10 1.20 1.30
%
0
100
0.50 0.60 0.70 0.80 0.90 1.00 1.10 1.20 1.30
%
0
100
0.50 0.60 0.70 0.80 0.90 1.00 1.10 1.20 1.30
%
0
100
Cal_500_180418_002 1: MRM of 2 Channels ES+ TIC (Acetyl Norfentanyl)
1.09e60.68
Cal_500_180418_002 3: MRM of 3 Channels ES+ TIC (Norfentanyl)
1.66e60.91
Cal_500_180418_002 6: MRM of 2 Channels ES+ TIC (Norcarfentanil)
7.14e51.03
Cal_500_180418_002 16: MRM of 2 Channels ES+ TIC (Remifentanyl Acid)
4.70e51.06
Cal_500_180418_002 19: MRM of 2 Channels ES+ TIC (Remifentanil)
1.44e61.18
Cal_500_180418_002 14: MRM of 2 Channels ES+ TIC (Methoxyacetyl Fentanyl)
3.04e6
1.33
Acetyl Norfentanyl
Norcarfentanyl
Norfentanyl
Remifentanil Acid
Remifentanil
Methoxyacetyl Fentanyl
Time1.20 1.40 1.60 1.80 2.00 2.20
%
0
100
1.20 1.40 1.60 1.80 2.00 2.20
%
0
100
1.20 1.40 1.60 1.80 2.00 2.20
%
0
100
1.20 1.40 1.60 1.80 2.00 2.20
%
0
100
1.20 1.40 1.60 1.80 2.00 2.20
%
0
100
1.20 1.40 1.60 1.80 2.00 2.20
%
0
100
Cal_500_180418_002 7: MRM of 2 Channels ES+ TIC (Acetyl Fentanyl)
3.15e61.29
Cal_500_180418_002 15: MRM of 2 Channels ES+ TIC (Beta-OH Fentanyl)
5.10e51.33
Cal_500_180418_002 5: MRM of 2 Channels ES+ TIC (4-ANPP)
2.57e61.48
Cal_500_180418_002 9: MRM of 2 Channels ES+ TIC (Fentanyl)
3.31e61.50
Cal_500_180418_002 18: MRM of 2 Channels ES+ TIC (Furanyl Fentanyl)
3.60e61.55
Cal_500_180418_002 11: MRM of 2 Channels ES+ TIC (Cyclopropyl Fentanyl)
3.41e61.59
Acetyl Fentanyl
β-OH Fentanyl
4-ANPP
Fentanyl
Furanyl Fentanyl
Cyclopropyl Fentanyl
Time2.00 4.00 6.00 8.00 10.00
%
0
100
2.00 4.00 6.00 8.00 10.00
%
0
100
Post_MCX_PL_180103_003 Parents of 184ES+ TIC
4.11e9
3.53
3.41
3.19
3.65
Post_PRiME_PL_180103_003 Parents of 184ES+ TIC
4.11e9
3.53
0.00E+00
2.00E+08
4.00E+08
6.00E+08
8.00E+08
1.00E+09
1.20E+09
1.40E+09
1.60E+09
1.80E+09
2.00E+09
PRiME MCX Area PPT Area
PL
Pea
k ar
ea
Residual Phospholipids
0.7%
A B
LowQC 75 pg/mL
Mid QC 2500 pg/mL
High QC 15,000 pg/mL
Name Bias %CV Bias %CV Bias %CV
Acetyl Norfentanyl -2.0% 10.1% -1.6% 0.8% -2.0% 2.1%
Norfentanyl 1.0% 4.4% -0.6% 0.4% -3.4% 1.6%
Norcarfentanil -6.7% 12.5% 1.3% 3.1% -3.4% 4.0%
Remifentanil Acid -8.0% 21.1% -7.6% 6.7% -3.2% 3.5%
Remifentanil -8.3% 6.3% 0.5% 1.0% -3.5% 1.7%
Methoxyacetyl Fentanyl -11.7% 6.6% 0.0% 1.3% -3.4% 1.1%
Acetyl Fentanyl -5.3% 4.7% 0.6% 1.7% -2.9% 1.2%
β-OH Fentanyl -7.3% 8.8% -1.2% 2.9% -5.8% 0.9%
4-ANPP 29.3% 1.5% 5.0% 4.1% -5.8% 4.4%
Fentanyl -4.0% 7.4% 0.1% 3.6% -6.4% 1.5%
Furanyl Fentanyl -1.7% 3.7% -0.8% 1.7% -6.1% 0.8%
Cyclopropyl Fentanyl -3.6% 8.3% -2.6% 1.8% -7.2% 2.2%
3-methyl Fentanyl 2.3% 5.5% -3.5% 3.4% -7.3% 2.6%
Carfentanyl -8.0% 9.7% -0.9% 3.1% -7.7% 2.4%
Butyryl Fentanyl 1.3% 5.2% -2.2% 1.9% -7.3% 2.6%
PFBF -9.7% 10.5% -2.1% 1.1% -7.0% 2.3%
Sufentanyl -7.3% 7.7% -1.9% 2.3% -6.1% 2.0%
Figure 4. Recovery and matrix effects from fentanyl analogs extracted from 4 lots of whole blood, including 3 post-mortem samples. 4 lots of matrix were used with 4 replicates each. Bars and error bars represent the means and standard deviations of 4 lots of whole blood.
Compound name: Furanyl Fentanyl
Correlation coefficient: r = 0.997982, r^2 = 0.995968
Calibration curve: 0.00125373 * x + -0.00900191
Response type: Internal Std ( Ref 12 ), Area * ( IS Conc. / IS Area )
Curve type: Linear, Origin: Exclude, Weighting: 1/x, Axis trans: None
Conc-0 2500 5000 7500 10000 12500 15000 17500 20000
Re
sp
on
se
-0.0
20.0
Conc
Re
sid
ua
l
-0.0
20.0
-20%
0%
20%
40%
60%
80%
100%
Recovery and Matrix Effects Mean Rec Mean ME
Compound name: Fentanyl
Correlation coefficient: r = 0.999474, r^2 = 0.998948
Calibration curve: 0.00126271 * x + -0.00596527
Response type: Internal Std ( Ref 14 ), Area * ( IS Conc. / IS Area )
Curve type: Linear, Origin: Exclude, Weighting: 1/x, Axis trans: None
pg/mL-0 2500 5000 7500 10000 12500 15000 17500 20000
Re
sp
on
se
-0.0
20.0
pg/mL
Re
sid
ua
l
-0.0
10.0
Figure 5. Calibration curves and residuals for Fentanyl and Furanyl Fentanyl. Calibration curves ranged from 20-20,000 pg mL. LLOQs were 50 ng/mL for most analytes with the exception of Remifentanil acid and 4-ANPP at 100 pg/mL.
y = 1.0936x - 0.7216
R² = 0.9919
0.0
2.0
4.0
6.0
8.0
10.0
12.0
14.0
16.0
18.0
0.0 5.0 10.0 15.0 20.0Wa
ters/
Re
f. R
esu
lt (
ng
/m
L)
Reference Result (ng/mL)
Furanyl Fentanyl
y = 0.9545x - 0.1293R² = 0.9761
-5.0
0.0
5.0
10.0
15.0
20.0
25.0
0.0 5.0 10.0 15.0 20.0 25.0
Wa
ters/
Re
f. R
esu
lt (
ng
/m
L)
Reference Result (ng/mL)
4-ANPP
Figure 6. Comparison of the current method with results from a reference laboratory. 40 authentic samples were analyzed. R
2 values were > 0.95 with biases