Midsure poster 2015
-
Upload
abigail-miller -
Category
Documents
-
view
61 -
download
0
Transcript of Midsure poster 2015

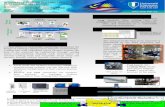
Variation of the acylsucrose biosynthesis pathway catalyzed by ASAT2 and 3 in wild tomato species
Abigail M. Miller1, Pengxiang Fan1, and Robert L. Last1,2
1Department of Biochemistry and Molecular Biology, 2Department of Plant Biology, Michigan State Univeristy, East Lansing, MI 48824, United States of America
Background: The acylsucrose biosynthesis pathway is composed of a sucrose backbone with multiple acyl-CoA donors added onto the pyranose and furanose rings. This research shows the variation and manipulation of acyl donors and acyl acceptors between S. lycopersicum, the cultivated tomato, and other wild tomato accessions. Through multiple enzyme assays, and mutagenesis, this research helps develop the framework for manipulation of a secondary pathway and illustrates the natural diversity and evolution of acylsugars in tomato plants.
1
7
4
3
2
S. lycopersicum S. pennellii
S1:5 (R4)
S2:10 (R2, R4)
S2:10 (R3,R4)
S3:22 (R2,R3,R4)
SpASAT3SlASAT2
SpASAT2
6
5 8
7
Enzyme S1:5 + iC5 S2:10 + iC5 S2:10 + nC12
SpASAT3 S2:10 X X
SlASAT3 X S3:15 S3:22
1376SpASAT3 S2:10 X X
1278SpeASAT3_1 S2:10 S3:15 S3:22
1718ShASAT3 S2:10 S3:15 S3:22
1969SchASAT3 S2:10 S3:15 S3:22
1278SpeASAT3_2 S2:10 S3:15 S3:22
1364ShuASAT3 S2:10 S3:15 S3:22
2172SaASAT3 X S3:15 X
Common acylsugar structure found in S. lycopersicum. Acyl chains added by ASAT2 on the R3 position, and by ASAT3 on the R2 position of the pyranose ring (Kim 2010).
Acylsugars are a natural pesticide for the tomato plant. Hornworm larvae on tobacco plants eat acylsugars as a first meal, and are tagged with volatile components which attract predators(Weinhold 2011).
Protein homology model used to find amino acids for mutagenesis to test ASAT2 activity. Phe408Val results in a gain of activity shown in the chromatogram below.
Pathway difference between S. lycopersicum and S. pennellii. S. pennellii acylsugars have a completely acylated pyranose ring, and no acyl chains on the furanose ring (Schilmiller 2015).
Multiple sequence alignment (MSA) of ASAT3 in wild tomatoes, G to C aligns with the activity of S. lycopersicum differing from S. pennellii when using S1:5 as a substrate.
SlASAT2
SlASAT2_C304G
1777ASAT2
Mutagenesis of C304G ASAT2 which gains activity to use S2:10 as a substrate.
iC5-CoA
aiC5-CoA
nC12-CoA
SpASAT2_HC-QY
SlASAT3_YCT-HSV
S1:5(R4) +iC5-CoA
Mutagenesis of SlASAT3 gains the activity to use S1:5(R4) as a substrate. Mutations found using the same method as demonstrated in section 4.
What about S. pennellii ASAT3 using S2:10?
SlASAT2_C304G
SpASAT2_HC-QY
SlASAT3_YCT-HSV
Activity of ASAT3 in wild tomatoes and S. lycopersicum was tested in order to find a correlation for SpASAT3 using S2:10. Species were selected based on sequence alignment resembling both S. lycopersicum and S. pennellii activity.
Future Work
References and Acknowledgements
Retention time for S3:22 is shifted for 1718ShASAT3 which indicates that the nC12-CoA is added on at a different position on the furanose ring. The original position is on the R3’, but this shows that it is somewhere new.
1969SpASAT3
1278SpeASAT3
1777ShASAT3
The NSF for funding the Trichome project, Lyman Briggs College for Spring 2015 research scholarships, and ASPB for funding Summer 2015 research.Schilmiller AL, Charbonneau AL, Last RL (2012a) Identification of a BAHD acetyltransferase that produces protective acyl sugars in tomato trichomes . Proc Natl Acad Sci USA 109:16377-82.Weinhold A, Baldwin IT (2011) Trichome-derived O-acyl sugars are a first meal for caterpillars that tags them for predation . Proc Natl Acad USA 108: 7855-7859.Kim J, Kang K, Gonzales-Vigil E. Shi F, Jones AD, Barry CS, Last RL (2012) Striking natural diversity in glandular trichome acylsugar composition is shaped by variation at the Acyltransferase2 locus in the wild tomato Solanum habrochaites. Plant Physiol 160:1854-70.Schillmiller A, Shi F, Kim J, Charbonneau AL, Holmes D, Jones AD, Last RL (2010) Mass spectrometry screening reveals widespread diversity in trichome specialized metabolites of tomato chromosomal substitution lines. Plant J 62: 391-403.Schilmiller AL, Moghe GD, Fan P, Ghosh B, Ning J, Jones AD, Last RL (2015) Functionally Divergent Alleles and Duplicated Loci Encoding an Acyltransferase Contribute to Acylsugar Metabolite Diversity in Solanum Trichomes. Plant Cell. Doi: 10.1105/tpc.15.00087Ghosh B, Westbrook T, Jones AD. 2013. Comparative structural profiling of trichome specialized metabolites in tomato (Solanum lycopersicum) and S. habrochaites: acylsugar profiles revealed by UHPLC/MS and NMR. Metabolomics: 1-12.
Alignments of the S. pennellii and the wild tomato species will help us understand the evolutionary significance of this pathway divergence.
Analyzing the retention time differences in section 8 will need to be conducted and NMR structures will also need to be developed.
Summary of the three mutations
Acyl-acceptor mutagenesis
Proposed acylsucrose pathway
Mutagenesis with three mutations
ASAT2 acyl-donor mutagenesis
What about SpASAT3 using S2:10?Mutagenesis HC135QY
S3:15
S4:17
2172ASAT2_V408F
2172ASAT2
SlASAT2_F408V
SlASAT2
-----+++++----
ASAT2ASAT3
S1:5
S1:5
S1:5 as substrate
Mutations found through a similar MSA method as in section 4.