MBio Newsletter Winter-Spring 2016 - MBio Diagnosticsmbiodx.com › ... › uploads › 2016 › 03...
Transcript of MBio Newsletter Winter-Spring 2016 - MBio Diagnosticsmbiodx.com › ... › uploads › 2016 › 03...

In this issue (2) Collaboration with Queens University Belfast (3) Pilot Production & Medical Diagnostics (4) Working with MBio
Winter/Spring 2016
MBio POINT OF CARE PLATFORM
1
MBio Diagnostics has developed a
compact instrument and disposable
cartridge system that performs dozens
of independent tests on a single drop of
a sample, and delivers parts-per-trillion
sensitivity. We will fundamentally
change today’s slow paradigm for
bioanalysis: collect a sample and send it
to the lab. In a clinic, emergency room,
or in the field, our platform rapidly
delivers results wherever they are
needed. The platform enables
applications across multiple industries.
We are licensing the technology for
2
various applications and forging
partnerships with both academic and
commercial groups. Our first
commercial partnership was recently
announced, a collaboration and license
deal with Roka Biosciences covering
food safety testing.
Our technology is based on novel
planar waveguides and is covered by a
portfolio of patents. The waveguide
technology enables a simple “load-n-go”
cartridge—new users learn the system
in a matter of minutes. The company
has adapted tests for over 100
3
biomarkers to the platform,
encompassing all key analytes: protein,
cells, and DNA. Our comprehensive
platform has been evaluated at 12 sites
on 5 continents. It has been repeatedly
selected as the simplest, most sensitive
and accurate multiplexed point-of-care
system available. Through strategic
partnerships, we are developing
applications for emergency room use,
global health, environmental
monitoring, and food safety testing.
MBio Diagnostics
MBio’s technology will enable a new era of instant-answers in clinical diagnostics and sample testing. Our technology enables on-the-spot testing, wherever and whenever it is needed.

MBio diagnostics
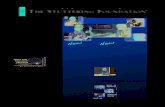
Performance
The MBio LightDeck® technology is a
high performance point-of-care diagnostic
platform: fast, high multiplexing, low
cost, and sensitive. The core patented
waveguide technology enables simple, no-
wash “load-n-go” assay workflow without
compromising performance. Assays can
be performed in as few as 2 or 3 minutes.
For simple multiplexing, MBio has few
competitors. The image shown is an 88-
feature array delivering quantitative results
on 57 different serological assays.
Compared to Luminex and MSD, MBio
can deliver comparable multiplexing in a
simple field portable system. The MBio
sensitivity results rival those of highly
complex, lab-based solutions. The data
below shows a comparison between an
MBio IL-6 assay and a commercial ELISA
kit, with sensitivity down to a few
pg/ml—ideal for markers like IL-6,
cardiac troponin, or HIV p24.
1
Several years ago, MBio was
approached by researchers from
Queen’s University Belfast (QUB),
who were interested in developing
tests related to food safety, specifically
multiplex detection of harmful marine
and freshwater toxins. This is a
leading problem in water resources
and shellfish beds, as exemplified by
the shutdown of the Toledo municipal
water supply in 2014, and the recent
outbreak of multi-toxin algal blooms
in the US Pacific Northwest in 2015.
In the case of mixed toxin algal
blooms, the analytical challenge for
field-screening assays is high.
Conventional technology requires
multiple test strips. Ideally, a
screening tool would comprehensively
2
cover the likely algal toxins for that
body of water.
We worked with Professor Chris
Elliott and his team to define a panel
of five toxins that are commonly found
in hazardous algal blooms. Each of
these toxins could severely sicken a
consumer, with a range of
neurotoxins, hepatotoxins, and
vomittoxins. We set about developing
the custom 5-plex panel, which was
the basis for a series of publications to
date by the QUB group.
Professor Elliott and his colleagues
were impressed, noting “The highly
innovative assay was proven to
accurately detect toxin … at an
unprecedented cell density of 10
MBIo Partners with
Queen’s University Belfast

3
3
cells/L.” [1] In fact, they took on
assay development and manufacturing
of cartridges using MBio components.
They have forged ahead in a number of
applications, and even demonstrated a
multiplex milk assay with a
commercial partner. [2]
Professor Elliott introduced the MBio
technology to the EAGER consortium,
4
and now under NSF funding the MBio
system is being integrated into an
autonomous underwater vehicle, in
collaboration with the Monterey Bay
Aquarium Research Institute.
In summary, the QUB group has been
a wonderful collaborator with MBio,
resulting in great science, multiple
publications, and follow-on funding.
Pilot production
The MBio platform has been designed for
manufacturability from day one. The
disposables are constructed from simple
injection molded plastic, with a highly
scalable print-and-assembly operation.
The automated print process, developed
using Scienion automation, deposits tiny
quantities of reagents. The manufacturing
process has been thoroughly audited by
partners, and the plans have been set to
produce millions of disposable cartridges
per year. Our pilot production plant for
cartridges in Boulder Colorado can
produce up to 2,000 disposables per week
under our ISO13485 quality system, and
we can scale to meet the needs of our
partners. The instrument is built on
CMOS imaging technology and diode
lasers, the same technologies found in your
camera phone and DVD player,
respectively. These design choices make
for robust, affordable instrumentation,
without compromising on performance.
Medical diagnostics Our mission in medical diagnostics is simple: to become the “go-to” platform for
instant diagnosis at the point of care. The MBio platform is ideal for delivering
multiple results in a clinic, ICU, or doctor’s office, whether in New York or
Nairobi. Just a single drop of blood is applied to an MBio cartridge, where it flows
by capillary action, and multiple results are reported in a matter of minutes. Tests
for HIV, hepatitis, and influenza have been demonstrated in diverse settings ranging
from San Diego and Seattle, to Kenya and Brazil. Stay tuned to hear about our
upcoming work on malaria and sepsis.

Mbio diagnostics Newsletter Winter/spring 2016
Collaborating with MBio MBio scientists and engineers are able to help you translate an assay or set of assays onto our
platform. A typical commercial engagement follows a common process: (1) A prototype assay
is developed using your reagents and qualified samples (positives/negatives, or quantitated
samples), (2) the assay is validated against a blinded set of samples and the data shared, and (3)
the assay cartridges are shipped to you for use in your application. We charge a nominal fee to
perform these development activities, and we invest the time necessary to ensure an attractive
result. Once an assay or panel is validated, MBio can produce and ship additional cartridges as
needed. When a product is ready for commercialization, we transfer to manufacturing, and
then set up a supply agreement and license to ensure your ability to commercialize. We can
tailor a set of terms that meet your business needs. With academic collaborators, we have
structured multiple collaborations and together won extensive funding for the academic labs
and research centers that work with MBio technology. How can we serve you?
MBio Diagnostics, Inc. 5603 Arapahoe, Suite 1 Boulder, CO80303
Selected Articles by the Queen’s University Belfast Group: 1. Meneely, J.P., et. al., Biosens. Bioelectron. (2012), http://dx.doi.org/10.1016/j.bios.2012.09.043 2. McGrath, T.F., et. al., Anal. Bioanal. Chem. (2015), http://dx.doi.org/10.1007/s00216-015-8526-4
Meet us at the 2016 Molecular Medicine Tri-Conference in San Francisco Chris Myatt Speaking at the Point of Care Symposium on March 11