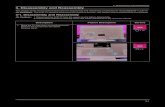
Keratins and disease at function, revealing the potential ...assembly and disassembly of filaments...
Transcript of Keratins and disease at function, revealing the potential ...assembly and disassembly of filaments...

Journ
alof
Cell
Scie
nce
Keratins and disease ata glance
Rebecca L. Haines and E. BirgitteLane*
Epithelial Biology Group, Institute of Medical Biology,Immunos, Singapore
*Author for correspondence ([email protected])
Journal of Cell Science 125, 3923–3928
� 2012. Published by The Company of Biologists Ltd
doi: 10.1242/jcs.099655
Keratins are cytoskeletal filament-forming
proteins found in skin and other epithelial
(sheet) tissues (Table 1). Keratins (type I and
II), and other highly related (types III–VI)
intermediate filament or nanofilament
proteins, used to be thought of as very inert
because they form filament networks that are
not easily disrupted, in contrast to actin and
microtubule cytoskeleton systems, which
can be rapidly and completely dismantled
by many drugs. The identification of
causative pathogenic mutations in the
keratin genes KRT5 and KRT14 in the
human blistering skin disease epidermolysis
bullosa simplex (EBS) changed this view
and demonstrated the importance of these
proteins for maintaining tissue resilience
(Bonifas et al., 1991; Coulombe et al.,
1991; Lane et al., 1992). As the first-
reported disease caused by keratin
mutations, EBS set the pattern for all the
other keratin disorders, as well as several
non-keratin genetic skin diseases. Many
‘keratinopathies’ have now been identified
with a variety of disease phenotypes,
often predicted by tissue-specific
keratin expression, with pathology usually
involving some form of tissue fragility
(Szeverenyi et al., 2008; see poster).
By reviewing the essential elements of
keratin protein structure and filament
assembly, we consider how keratin
filaments provide tissues with mechanical
resilience, and how mutations might impact
upon these processes and compromise tissue
function. The rare human keratinopathies
have provided important clues to keratin
function, revealing the potential roles for
keratins in many types of stress response,
and even effects on membrane trafficking
and cell and tissue differentiation.
This knowledge has begun to inform
development of therapeutic options for
keratin diseases, and successful first-in-man
siRNA trials have been performed. We
conclude by considering the issues and
challenges now facing the field, and how
they are or can be addressed.
Keratin protein and filamentstructureThe keratin family consists of 54 proteins
(Schweizer et al., 2006) in two families
(types I, acidic, and II, neutral or basic), each
being expressed in a defined sequence during
differentiation. The greatest expression
diversity lies in skin (see poster). Keratin
proteins can be divided into three functional
groups: ‘simple’ keratins, expressed in
embryonic and one-layered epithelia,
including liver, intestine and glandular
secretory cells; ‘barrier’ keratins, expressed
(See poster insert)
ARTICLE SERIES: Cell Biology and Disease Cell Science at a Glance 3923

Journ
alof
Cell
Scie
nce
in multilayered stratified squamous epithelia,
such as the epidermis of the skin; and the
harder ‘structural’ keratins that form hair and
nail (Table 1).
Like all intermediate filaments, a keratin
protein has three clear domains: a central
a-helical rod domain, flanked by non-
helical head and tail regions that contain
most phosphorylation sites (Geisler et al.,
1982; Omary et al., 2006). Keratins begin
assembly as heterodimers of one type I and
one type II keratin monomer (Hatzfeld
and Weber, 1990; reviewed by Herrmann
and Aebi, 2000), with rod domains aligned
in parallel and in register. These dimers
form antiparallel tetramers by overlapping
the N-terminal half of their rod domains.
Tetramers then assemble into ‘unit length
filaments’ that are 60 nm in length (see
poster). These rapidly assemble end-to-
end, within 10 seconds in vitro (Herrmann
et al., 2002), forming rope-like
nanofilaments that are ,10 nm thick. The
antiparallel nature of the tetramers results
in filaments that do not have polarity,
which is again in contrast to actin filaments
or microtubules, and implies that keratin
filaments cannot function as unidirectional
tracks for molecular motors. At either end
of the rod domain are the highly conserved
helix initiation and termination motifs,
which are hotspots for the most severe
disease mutations (Lane and McLean,
2004; Uitto et al., 2007).
Dynamic behaviour of keratinsThe cytoplasmic ropes of keratin form
branching bundles in the cell and anchor
into junctions around the cell perimeter.
Keratins link through desmoplakin into
desmosomes (Kouklis et al., 1994), which
connect neighbouring cells, and through
plectin (Andra et al., 2003) into
hemidesmosomes, which connect cells to
their attachment substrates. This filament–
junction network anchors cells in three
dimensions through the epithelium.
However, the keratin filament cytoskeleton
must be dynamic, plastic and flexible to
allow cells to proliferate during growth and
to migrate during wound healing. Dynamic
assembly and disassembly of filaments is
also needed to allow epithelial cells to
maintain an intact network while they alter
their keratin expression profile during
differentiation or in response to stress. For
example, in the epidermis, K5 and K14 are
the major keratins synthesised in basal cells
(which can proliferate), whereas K1 and
K10 are expressed in suprabasal cells (which
are ‘locked’ into differentiation). However,
keratin proteins have a long half-life, around
4 days for simple keratins (Denk et al.,
1987), and K5 and K14 can persist in
suprabasal cells (see poster) alongside K1
and K10 (Kartasova et al., 1993; Eriksson
et al., 2009; Windoffer et al., 2011). A
gradual transition, rather than complete
disassembly and re-assembly of the
network, preserves the structural integrity of
the filament network during differentiation.
Where do keratins assemble in the cell?
Intermediate filaments do not have defined
organizing centres as microtubules do;
they can be synthesised throughout the
cell as tiny subfilamentous particles that
occur throughout the cytoplasm (Liovic
et al., 2003). The unit length filament (see
poster) might be the nucleation precursor,
but this has not been confirmed as its size
(60 nm) is below the resolution limit
of standard light microscopy. New
super-resolution microscopy techniques
(reviewed by Schermelleh et al., 2010)
could soon shed light on this. Turnover
might be accelerated at the cell periphery,
and a model of filament assembly has been
Table 1. Expression of keratin proteins in epithelial tissues
Keratin Epithelial tissue Polymerisation partner in vivo
Type ISimple K18 Simple epithelia (e.g. liver, pancreas, colon, lung) K8, K7
K20 Simple epithelia, especially gastrointestinal K8, (K7)Barrier K9 Stratified cornifying epithelia; palm, sole (K1)
K10 Stratified cornifying epithelia, suprabasal K1K12 Stratified epithelia; cornea K3K13 Stratified epithelia, non-cornifying; suprabasal K4K14 Stratified and complex epithelia; basal K5K15 Stratified epithelia (K5)K16 Stratified epithelia; induced during stress, fast turnover;
suprabasalK6a
K17 Stratified epithelia; induced during stress, fast turnover K6bK19 Simple and stratified epithelia K8K23, K24 Epithelia
Structural K25, K26, K27, K28 Stratified epithelia: hair follicle sheathK31, K32, K33a, K33b, K34, K35,
K36, K37, K38, K39, K40Stratified epithelia: hair, hard structures
Type IISimple K7, K8 Simple epithelia K18Barrier K1 Stratified epithelia, cornifying, suprabasal K10
K2 Stratified cornifying epithelia, late suprabasal (K10)K3 Stratified epithelia, cornea K12K4 Stratified epithelia, non-cornifying; suprabasal K13K5 Stratified and complex epithelia; basal cells K14, (K15)K6a Stratified epithelia; induced during stress, fast turnover K16K6b Stratified epithelia; induced during stress, fast turnover K17K6c EpitheliaK76 Stratified cornifying epithelia, oral, suprabasal (K10)K78, K79, K80 Epithelia
Structural K75 Stratified epithelia: hair follicleK71, K72, K73, K74 Stratified epithelia: hair follicle sheathK81, K82, K83, K84, K85, K86 Stratified epithelia: hair, hard structures
Journal of Cell Science 125 (17)3924

Journ
alof
Cell
Scie
nce
recently described that proposes that
filaments are nucleated in the cell
periphery at focal adhesions, before
elongating and then integrating into the
pre-existing peripheral network (Windoffer
et al., 2011).
A probable cofactor for localising the
assembling keratins is the linker protein
plectin because plectin isoforms bind actin
or intermediate filaments at different cell
locations. Plectin is known to influence
the formation and dynamics of vimentin
filaments (the intermediate filament
protein in fibroblasts) (Spurny et al.,
2008; Kostan et al., 2009), and plectin
has also been implicated in the actin-
dependent inward movement of keratins in
the cell periphery (Kolsch et al., 2009).
Keratin assembly at focal adhesions
(Windoffer et al., 2006) might also
depend on phosphorylation by the p38
mitogen-activated protein kinases, as
inhibition of these kinases prevents the
formation of filament precursors in the cell
periphery (Woll et al., 2007).
A wide range of post-translational
modifications have been described
on keratins, including phosphorylation
to ubiquitylation, sumoylation and
acetylation (Omary et al., 2006; Ku et al.,
2010; Srikanth et al., 2010; Snider et al.,
2011), which are likely to modulate the
solubility of keratins in specific situations.
The most well-documented modification is
phosphorylation, with many sites known
on simple keratins (reviewed by Omary
et al., 2006) but fewer defined on barrier
keratins. Phosphorylation of keratin
filaments by protein kinase Cf is required
for K8 and K18 filament remodelling in
response to shear stress (Flitney et al.,
2009; Sivaramakrishnan et al., 2009).
Considering all the above, even a single
amino acid change in keratins can interrupt
cell function in many ways, by altering
their post-translational modifications, their
integration into junctions or their filament
assembly kinetics (Herrmann et al., 2002;
Owens et al., 2004) to yield a less-stable
filament network and cause disease. For
example, some of the severe K14
mutations that cause Dowling–Meara
EBS, such as the hotspot mutation
p.Arg125Pro, result in the formation of
cytoplasmic keratin aggregates (Ishida-
Yamamoto et al., 1991). In vitro, this
mutation reduces the ability of
reconstituted filaments to bundle under
cross-linking conditions (Ma et al., 2001).
The impact of mutations on the mechanical
resilience of epithelial tissues is discussedbelow.
Keratins provide epithelia withmechanical resilienceThe keratin filament network can withstand
significant mechanical forces, particularly instratified epithelia such as the epidermis(Beriault et al., 2012). By conferring
mechanical continuity across an epithelialsheet, the keratin–desmosome and keratin–hemidesmosome network generates
mechanical resilience across the tissue, asthe network will dissipate mechanical stressaway from a source into the surroundingepithelium. Investigations of single-filament
mechanical properties show that keratinfilaments are flexible and tough, being lessrigid than actin filaments at low strains, and
less brittle than microtubules at high strains,where they show strain hardening (Janmeyet al., 1991; Kreplak and Fudge, 2007).
Bundling of keratin filaments appears toincrease when cells are subjected to shearstress (Flitney et al., 2009).
The tissue fragility observed in many
keratin diseases reflects the crucial role ofkeratins in providing mechanical stability tocells and tissues (McLean and Moore, 2011).
However, studies suggest that mutations inkeratins do not reduce mechanical resiliencesimply by preventing filament formation. A
keratinocyte cell line expressing a GFP-tagged K14 carrying the p.Arg125Promutation can withstand uni-directional
stretch (greater than 100%) withoutsignificant damage or cell death as much ascells expressing wild-type filaments can(Fudge et al., 2008; Beriault et al., 2012).
However, the cells expressing mutantkeratins are much less able to survive arepetitive stretch than those expressing wild-
type keratins (Russell et al., 2004). It appearslikely that mutations alter the dynamics offilament formation, giving rise to a keratin
network that is less stable. Furthermore, celllines generated from EBS patients withmutations in K5 or K14 migrate faster intissue culture scratch wound assays than their
wild-type counterparts, perhaps because thenetwork is more dynamic, allowing the cellto re-organise its keratin filaments more
quickly for migration (Morley et al., 2003).The presence of keratin mutations, or areduction in keratin expression, also reduces
expression of desmosome components andcytoskeletal linker proteins (Long et al.,2006; Liovic et al., 2009; Wagner et al.,
2012). This suggests an alternative diseasemechanism in which keratin mutations mightresult in tissue fragility due to a reduction in
junction proteins, leading to decreasedstability of the tissue.
Keratin disease phenotypes suggestthat there are multiple functionsfor keratinsEvidence from human keratin diseases thatare not associated with tissue fragility, as
well as data from experimental models suchas knockout and transgenic mice, hassuggested that keratins are essential formany different cellular processes, including
non-mechanical stress responses, organellepositioning and tissue differentiation (Guand Coulombe, 2007; Omary et al., 2009;
Toivola et al., 2010). These are discussed inthe following sections.
Mutant keratins alter stress response
Expression of many keratins is upregulated
in response to stress, suggesting animportant role for these proteins in thestress response (reviewed by Toivola et al.,2010). In particular, the expression of
keratins K6a, K6b, K16 and K17 isinduced by inflammatory cytokines, or inresponse to wound healing, and oxidative
or UV stress (Freedberg et al., 2001;DePianto and Coulombe, 2004).
Mutations in the simple epithelialkeratins K8 and K18 have been identified
as risk factors for some patients withinflammatory bowel disease (Owens et al.,2004) or liver disease (Ku et al., 2003).
Loss of mechanical resilience in theintestine (required to withstand peristalticmovement during digestion) caused bykeratin mutations might initiate tissue
damage (Owens and Lane, 2004). Bycontrast, liver is less mechanicallystressed but is vulnerable to damage by
toxins such as alcohol, and, in liver, keratinmutations reduce the tolerance to suchassaults (Ku et al., 2007; Omary et al.,
2009). This might help explain whymutations in these keratins are silent inmost individuals, but still predisposecarriers to liver injury mediated by toxins
or viruses, which constitute situations ofcell stress (Omary et al., 2009). It has beensuggested K8 acts as a ‘phosphate sponge’,
undergoing hyperphosphorylation, whichabsorbs phosphorylated stress-activatedprotein kinase (SAPK) activity, thus
preventing apoptosis (see poster; Ku andOmary, 2006).
Mutations in K5 and K14 that causeEBS also alter the response of a cell to
stress. Cells expressing mutated K5 or K14show amplified and accelerated SAPKsignalling in response to external stresses
Journal of Cell Science 125 (17) 3925

Journ
alof
Cell
Scie
nce
(D’Alessandro et al., 2002), and the
constitutive upregulation of extracellular-signal-regulated kinase (ERK) signalling inthese cells contributes to their increased
resistance to apoptosis (Russell et al.,2010). The targeting of keratin filamentsfor degradation is increased during stressand by the presence of mutated or
misfolded keratins that cannot integrateefficiently into the keratin network(Jaitovich et al., 2008; Loffek et al.,
2010; Na et al., 2010; Rogel et al., 2010).
Keratins and organelle transport
Mutations in some keratins have revealed a
role for these proteins in certain membranetrafficking events (see poster) (Kumemuraet al., 2004; Toivola et al., 2005; Kumemura
et al., 2008). A group of rare keratindiseases, including Dowling–Degosdisease, that are caused by mutations in K5or K14 have a skin pigment phenotype
(patches of hyper- and hypo-pigmentedskin; see poster) that is not directly linkedto skin blistering (when present) (Uttam
et al., 1996; Betz et al., 2006; Lugassy et al.,2006). Melanosomes (pigment-containinggranules) are produced by melanocytes and
transferred into basal keratinocytes wherethey are arranged in a distal cap over thenucleus. A defect in the transfer of
melanosomes, or their arrangement inkeratinocytes, can lead to changes in skinpigmentation. Although the mechanism bywhich mutations in epidermal keratins alter
melanosome arrangement is not fullyunderstood, it might involve an interactionof K5 with the chaperone HSC70, which is
involved in vesicle uncoating (Planko et al.,2007).
Tissue differentiation is affected bykeratin expression
The tissue-specific expression pattern ofkeratins is set early in development and
differentiation and is therefore tightlycontrolled. Histopathologists havehistorically used keratin expression inepithelial tumours to identify the tissue of
origin (Lane and Alexander, 1990), andmore recently as prognostic markers(reviewed by Karantza, 2011). Changing
the keratins that are expressed in a cell canhave wide-ranging effects; these might berelated to a change in cell fate that is
reflected in the altered keratin profile andmight lead to a subsequent alteration inphysical properties (discussed in Owens and
Lane, 2003). For example, mice expressingK1 in pancreatic b cells develop diabetescharacterised by a reduction of insulin-
secretory vesicles (Blessing et al., 1993).Deletion of K17 in a mouse model leads to
defects in hair follicle cycling, wound repair(through a decrease in cell size and proteinsynthesis), and an altered inflammatorycytokine profile (Kim et al., 2006; Tong
and Coulombe, 2006; DePianto et al., 2010).Although interactions of K17 with theadaptor protein 14-3-3s and TRADD
(tumour necrosis factor receptor type 1-associated death domain) suggest potentialmechanisms by which loss of K17 could
give rise to some of these phenotypes, itseems likely that deletion of K17 has a moregeneral effect on tissue differentiation.Studies of ‘unnatural’ keratin pairs, such as
K5 paired with K16 or K18 rather than itsnormal partner, K14, suggest that theassembly and mechanical properties of the
filaments can be drastically altered bychanging the type I or type II keratinpresent (Yamada et al., 2002; Lee and
Coulombe, 2009). This is reflected inthe mouse knockout of K14, in whichexpression of K16 or K18 only leads to
partial rescue of the phenotype, to differingdegrees (Hutton et al., 1998; Paladini andCoulombe, 1999), indicating that expressionof the correct keratins is crucial for normal
tissue function. The correct expressionof keratins is also important in thedevelopment and maintenance of
epidermal appendages (hair follicles andsebaceous glands). Mutations in the keratinsexpressed in these appendages lead to
various defects including the hair fragilitysyndrome monilethrix, or pachyonychiacongenita, a disorder characterised bynail dystrophy, painful palmoplantar
keratoderma, and either hair-follicle-associated cysts or cell fragility in mucoussurfaces (McLean et al., 2005; Schweizer
et al., 2007) (see poster).
Understanding the precise role of keratinsin these processes, and how mutations give
rise to the defects described will be crucialto the development of therapies for thekeratin diseases.
Opportunities for therapySo far, therapeutic approaches to keratindiseases have focussed on two different
areas, (i) genetic ablation of the mutantprotein, and (ii) small-molecule therapies tostabilise the keratin network in some way. A
successful trial of small interfering RNA(siRNA) against mutant K6a in apachyonychia congenita patient recently
demonstrated the feasibility of the firsttherapeutic route (Leachman et al., 2010),and without doubt further siRNA-based
approaches will follow (Atkinson et al.,2011; Liao et al., 2011). However,
significant concerns have been expressedregarding potential off-target effects ofsiRNA (Jackson and Linsley, 2010). Aschanges in keratin expression can have
dramatic effects on tissue differentiation(see above), attempts to alter keratinexpression for therapeutic purposes must
be approached with caution. Nevertheless,small-molecule therapies are also beinginvestigated to manipulate keratin levels in
the cell. Several small molecules have nowbeen shown to modulate keratin expression,and they either alter the expression ofseveral keratins or target a specific keratin.
They include statins, which moderatelydownregulate the activity of the promoterfor K6a (Kerns et al., 2007; Torma, 2011;
Zhao et al., 2011). In severe keratindisorders, where aggregates are observed,it might be possible to ameliorate the
symptoms by reducing the amount ofaggregation with the use of chemicalchaperones, which could ‘clear the way’
for the keratin filament cytoskeleton toreform correctly (Loffek et al., 2010;Chamcheu et al., 2011).
PerspectivesKeratin filaments have a crucial functionin providing mechanical resilience to
epithelial tissues. Associated with thisfunction, keratins are involved in the cellstress response, tissue differentiation and
organelle transport. Despite significanteffort, the details of the substructure ofkeratin filaments, and in particular the roleof the head and tail domains, are poorly
understood (Strelkov et al., 2003), as is thecontrol of filament assembly and dynamicswithin the cell. One model of filament
dynamics, in which filaments are nucleatedin the cell periphery and elongate andmature as they move towards the centre of
the cell, was presented above. However,filament dynamics are likely to be differentin a migrating or dividing cell, where thekeratin cytoskeleton is highly dynamic,
compared with cells in an intact tissue,where filaments appear to be stable and areanchored at cell junctions. A combination
of localised highly dynamic filament re-organisation [as has been observed forvimentin (Helfand et al., 2011)] and some
form of subunit exchange within thenetwork seems more likely, but themechanisms by which keratin dynamics
are controlled are not yet understood.Disease-causing keratin mutations mightprovide essential information in this area,
Journal of Cell Science 125 (17)3926

Journ
alof
Cell
Scie
nce
particularly in explaining how changing
filament formation and dynamics give rise
to fragile epithelial tissues. In order to
address these issues, it will be necessary to
improve laboratory models of keratin
diseases to better reflect the physiological
condition. Understanding the connection
between the role of keratin filaments in
providing mechanical resilience and the
apparently unrelated phenotypes that are
observed in certain keratin diseases is a
priority, and further investigations in this
area will hopefully yield new therapeutic
interventions for keratin diseases.
Acknowledgements
The authors would like to thank John
Common for critical reading of the
manuscript, and John Common, Declan
Lunny and Graham Wright (IMB
Microscopy Unit) for assistance in
producing images.
Funding
Work in the authors’ laboratory is supported
by the Biomedical Research Council,
Agency for Science, Technology and
Research (A*STAR), Singapore.
A high-resolution version of the poster is available for
downloading in the online version of this article at
jcs.biologists.org. Individual poster panels are available
as JPEG files at http://jcs.biologists.org/lookup/suppl/
doi:10.1242/jcs.099655/-/DC1
ReferencesAndra, K., Kornacker, I., Jorgl, A., Zorer, M.,
Spazierer, D., Fuchs, P., Fischer, I. and Wiche, G.
(2003). Plectin-isoform-specific rescue of
hemidesmosomal defects in plectin (-/-) keratinocytes. J.
Invest. Dermatol. 120, 189-197.
Atkinson, S. D., McGilligan, V. E., Liao, H.,
Szeverenyi, I., Smith, F. J. D., Moore, C. B. T. and
McLean, W. H. I. (2011). Development of allele-specific
therapeutic siRNA for keratin 5 mutations in
epidermolysis bullosa simplex. J. Invest. Dermatol. 131,
2079-2086.
Beriault, D. R., Haddad, O., McCuaig, J. V., Robinson,
Z. J., Russell, D., Lane, E. B. and Fudge, D. S. (2012).
The mechanical behavior of mutant K14-R125P keratin
bundles and networks in NEB-1 keratinocytes. PLoS ONE
7, e31320.
Betz, R. C., Planko, L., Eigelshoven, S., Hanneken, S.,
Pasternack, S. M., Bussow, H., Van Den Bogaert, K.,
Wenzel, J., Braun-Falco, M., Rutten, A. et al. (2006).
Loss-of-function mutations in the keratin 5 gene lead to
Dowling-Degos disease. Am. J. Hum. Genet. 78, 510-519.
Blessing, M., Ruther, U. and Franke, W. W. (1993).
Ectopic synthesis of epidermal cytokeratins in pancreatic
islet cells of transgenic mice interferes with cytoskeletal
order and insulin production. J. Cell Biol. 120, 743-755.
Bonifas, J. M., Rothman, A. L. and Epstein, E. H., Jr
(1991). Epidermolysis bullosa simplex: evidence in two
families for keratin gene abnormalities. Science 254,
1202-1205.
Chamcheu, J. C., Navsaria, H., Pihl-Lundin, I., Liovic,
M., Vahlquist, A. and Torma, H. (2011). Chemical
chaperones protect epidermolysis bullosa simplex
keratinocytes from heat stress-induced keratin
aggregation: involvement of heat shock proteins and
MAP kinases. J. Invest. Dermatol. 131, 1684-1691.
Coulombe, P. A., Hutton, M. E., Letai, A., Hebert, A.,
Paller, A. S. and Fuchs, E. (1991). Point mutations in
human keratin 14 genes of epidermolysis bullosa simplex
patients: genetic and functional analyses. Cell 66, 1301-
1311.
D’Alessandro, M., Russell, D., Morley, S. M., Davies,
A. M. and Lane, E. B. (2002). Keratin mutations of
epidermolysis bullosa simplex alter the kinetics of stress
response to osmotic shock. J. Cell Sci. 115, 4341-4351.
Denk, H., Lackinger, E., Zatloukal, K. and Franke,
W. W. (1987). Turnover of cytokeratin polypeptides in
mouse hepatocytes. Exp. Cell Res. 173, 137-143.
DePianto, D. and Coulombe, P. A. (2004). Intermediate
filaments and tissue repair. Exp. Cell Res. 301, 68-76.
DePianto, D., Kerns, M. L., Dlugosz, A. A. and
Coulombe, P. A. (2010). Keratin 17 promotes epithelial
proliferation and tumor growth by polarizing the immuneresponse in skin. Nat. Genet. 42, 910-914.
Eriksson, J. E., Dechat, T., Grin, B., Helfand, B.,
Mendez, M., Pallari, H.-M. and Goldman, R. D. (2009).
Introducing intermediate filaments: from discovery to
disease. J. Clin. Invest. 119, 1763-1771.
Flitney, E. W., Kuczmarski, E. R., Adam, S. A. and
Goldman, R. D. (2009). Insights into the mechanical
properties of epithelial cells: the effects of shear stress on
the assembly and remodeling of keratin intermediate
filaments. FASEB J. 23, 2110-2119.
Freedberg, I. M., Tomic-Canic, M., Komine, M. and
Blumenberg, M. (2001). Keratins and the keratinocyteactivation cycle. J. Invest. Dermatol. 116, 633-640.
Fudge, D., Russell, D., Beriault, D., Moore, W., Lane,
E. B. and Vogl, A. W. (2008). The intermediate filament
network in cultured human keratinocytes is remarkably
extensible and resilient. PLoS ONE 3, e2327.
Geisler, N., Kaufmann, E. and Weber, K. (1982).
Proteinchemical characterization of three structurally
distinct domains along the protofilament unit of desmin
10 nm filaments. Cell 30, 277-286.
Gu, L.-H. and Coulombe, P. A. (2007). Keratin function
in skin epithelia: a broadening palette with surprising
shades. Curr. Opin. Cell Biol. 19, 13-23.
Hatzfeld, M. and Weber, K. (1990). The coiled coil of in
vitro assembled keratin filaments is a heterodimer of type
I and II keratins: use of site-specific mutagenesis and
recombinant protein expression. J. Cell Biol. 110, 1199-
1210.
Helfand, B. T., Mendez, M. G., Murthy, S. N. P.,
Shumaker, D. K., Grin, B., Mahammad, S., Aebi, U.,
Wedig, T., Wu, Y. I., Hahn, K. M. et al. (2011).
Vimentin organization modulates the formation of
lamellipodia. Mol. Biol. Cell 22, 1274-1289.
Herrmann, H. and Aebi, U. (2000). Intermediate
filaments and their associates: multi-talented structuralelements specifying cytoarchitecture and cytodynamics.
Curr. Opin. Cell Biol. 12, 79-90.
Herrmann, H., Wedig, T., Porter, R. M., Lane, E. B.
and Aebi, U. (2002). Characterization of early assembly
intermediates of recombinant human keratins. J. Struct.
Biol. 137, 82-96.
Hutton, E., Paladini, R. D., Yu, Q. C., Yen, M.,
Coulombe, P. A. and Fuchs, E. (1998). Functional
differences between keratins of stratified and simple
epithelia. J. Cell Biol. 143, 487-499.
Ishida-Yamamoto, A., McGrath, J. A., Chapman, S. J.,
Leigh, I. M., Lane, E. B. and Eady, R. A. (1991).
Epidermolysis bullosa simplex (Dowling-Meara type) is a
genetic disease characterized by an abnormal keratin-
filament network involving keratins K5 and K14. J. Invest.
Dermatol. 97, 959-968.
Jackson, A. L. and Linsley, P. S. (2010). Recognizing
and avoiding siRNA off-target effects for target
identification and therapeutic application. Nat. Rev.
Drug Discov. 9, 57-67.
Jaitovich, A., Mehta, S., Na, N., Ciechanover, A.,
Goldman, R. D. and Ridge, K. M. (2008). Ubiquitin-
proteasome-mediated degradation of keratin intermediate
filaments in mechanically stimulated A549 cells. J. Biol.
Chem. 283, 25348-25355.
Janmey, P. A., Euteneuer, U., Traub, P. and Schliwa,
M. (1991). Viscoelastic properties of vimentin compared
with other filamentous biopolymer networks. J. Cell Biol.
113, 155-160.
Karantza, V. (2011). Keratins in health and cancer: morethan mere epithelial cell markers. Oncogene 30, 127-138.
Kartasova, T., Roop, D. R., Holbrook, K. A. and
Yuspa, S. H. (1993). Mouse differentiation-specifickeratins 1 and 10 require a preexisting keratin scaffoldto form a filament network. J. Cell Biol. 120, 1251-1261.
Kerns, M. L., DePianto, D., Dinkova-Kostova, A. T.,
Talalay, P. and Coulombe, P. A. (2007).Reprogramming of keratin biosynthesis by sulforaphanerestores skin integrity in epidermolysis bullosa simplex.Proc. Natl. Acad. Sci. USA 104, 14460-14465.
Kim, S., Wong, P. and Coulombe, P. A. (2006). Akeratin cytoskeletal protein regulates protein synthesis andepithelial cell growth. Nature 441, 362-365.
Kolsch, A., Windoffer, R. and Leube, R. E. (2009).Actin-dependent dynamics of keratin filament precursors.Cell Motil. Cytoskeleton 66, 976-985.
Kostan, J., Gregor, M., Walko, G. and Wiche, G.
(2009). Plectin isoform-dependent regulation of keratin-integrin alpha6beta4 anchorage via Ca2+/calmodulin. J.
Biol. Chem. 284, 18525-18536.
Kouklis, P. D., Hutton, E. and Fuchs, E. (1994). Makinga connection: direct binding between keratin intermediatefilaments and desmosomal proteins. J. Cell Biol. 127,1049-1060.
Kreplak, L. and Fudge, D. (2007). Biomechanicalproperties of intermediate filaments: from tissues tosingle filaments and back. Bioessays 29, 26-35.
Ku, N.-O. and Omary, M. B. (2006). A disease- andphosphorylation-related nonmechanical function forkeratin 8. J. Cell Biol. 174, 115-125.
Ku, N. O., Darling, J. M., Krams, S. M., Esquivel,C. O., Keeffe, E. B., Sibley, R. K., Lee, Y. M., Wright,
T. L. and Omary, M. B. (2003). Keratin 8 and 18mutations are risk factors for developing liver disease ofmultiple etiologies. Proc. Natl. Acad. Sci. USA 100, 6063-6068.
Ku, N.-O., Strnad, P., Zhong, B.-H., Tao, G.-Z. and
Omary, M. B. (2007). Keratins let liver live: Mutationspredispose to liver disease and crosslinking generatesMallory-Denk bodies. Hepatology 46, 1639-1649.
Ku, N.-O., Toivola, D. M., Strnad, P. and Omary, M. B.
(2010). Cytoskeletal keratin glycosylation protectsepithelial tissue from injury. Nat. Cell Biol. 12, 876-885.
Kumemura, H., Harada, M., Omary, M. B., Sakisaka,
S., Suganuma, T., Namba, M. and Sata, M. (2004).Aggregation and loss of cytokeratin filament networksinhibit golgi organization in liver-derived epithelial celllines. Cell Motil. Cytoskeleton 57, 37-52.
Kumemura, H., Harada, M., Yanagimoto, C., Koga,H., Kawaguchi, T., Hanada, S., Taniguchi, E., Ueno, T.
and Sata, M. (2008). Mutation in keratin 18 inducesmitochondrial fragmentation in liver-derived epithelialcells. Biochem. Biophys. Res. Commun. 367, 33-40.
Lane, E. B. and Alexander, C. M. (1990). Use of keratinantibodies in tumor diagnosis. Semin. Cancer Biol. 1, 165-179.
Lane, E. B. and McLean, W. H. I. (2004). Keratins andskin disorders. J. Pathol. 204, 355-366.
Lane, E. B., Rugg, E. L., Navsaria, H., Leigh, I. M.,
Heagerty, A. H. M., Ishida-Yamamoto, A. and Eady,R. A. J. (1992). A mutation in the conserved helixtermination peptide of keratin 5 in hereditary skinblistering. Nature 356, 244-246.
Leachman, S. A., Hickerson, R. P., Schwartz, M. E.,
Bullough, E. E., Hutcherson, S. L., Boucher, K. M.,Hansen, C. D., Eliason, M. J., Srivatsa, G. S.,
Kornbrust, D. J. et al. (2010). First-in-human mutation-targeted siRNA phase Ib trial of an inherited skin disorder.Mol. Ther. 18, 442-446.
Lee, C.-H. and Coulombe, P. A. (2009). Self-organization of keratin intermediate filaments into cross-linked networks. J. Cell Biol. 186, 409-421.
Liao, H., Irvine, A. D., Macewen, C. J., Weed, K. H.,
Porter, L., Corden, L. D., Gibson, A. B., Moore, J. E.,Smith, F. J. D., McLean, W. H. I. et al. (2011).Development of allele-specific therapeutic siRNA inMeesmann epithelial corneal dystrophy. PLoS ONE 6,e28582.
Liovic, M., Mogensen, M. M., Prescott, A. R. and Lane,
E. B. (2003). Observation of keratin particles showing fastbidirectional movement colocalized with microtubules. J.
Cell Sci. 116, 1417-1427.
Journal of Cell Science 125 (17) 3927

Journ
alof
Cell
Scie
nce
Liovic, M., D’Alessandro, M., Tomic-Canic, M.,
Bolshakov, V. N., Coats, S. E. and Lane, E. B. (2009).Severe keratin 5 and 14 mutations induce down-regulationof junction proteins in keratinocytes. Exp. Cell Res. 315,2995-3003.
Loffek, S., Woll, S., Hohfeld, J., Leube, R. E., Has, C.,
Bruckner-Tuderman, L. and Magin, T. M. (2010). Theubiquitin ligase CHIP/STUB1 targets mutant keratins fordegradation. Hum. Mutat. 31, 466-476.
Long, H. A., Boczonadi, V., McInroy, L., Goldberg, M.
and Maatta, A. (2006). Periplakin-dependent re-organisation of keratin cytoskeleton and loss ofcollective migration in keratin-8-downregulatedepithelial sheets. J. Cell Sci. 119, 5147-5159.
Lugassy, J., Itin, P., Ishida-Yamamoto, A., Holland, K.,
Huson, S., Geiger, D., Hennies, H. C., Indelman, M.,
Bercovich, D., Uitto, J. et al. (2006). Naegeli-Franceschetti-Jadassohn syndrome and dermatopathiapigmentosa reticularis: two allelic ectodermal dysplasiascaused by dominant mutations in KRT14. Am. J. Hum.
Genet. 79, 724-730.
Ma, L., Yamada, S., Wirtz, D. and Coulombe, P. A.
(2001). A ‘hot-spot’ mutation alters the mechanicalproperties of keratin filament networks. Nat. Cell Biol.
3, 503-506.
McLean, W. H. I. and Moore, C. B. T. (2011). Keratindisorders: from gene to therapy. Hum. Mol. Genet. 20,R189-R197.
McLean, W. H. I., Smith, F. J. D. and Cassidy, A. J.
(2005). Insights into genotype-phenotype correlation in
pachyonychia congenita from the human intermediatefilament mutation database. J. Investig. Dermatol. Symp.
Proc. 10, 31-36.
Morley, S. M., D’Alessandro, M., Sexton, C., Rugg,
E. L., Navsaria, H., Shemanko, C. S., Huber, M., Hohl,
D., Heagerty, A. I., Leigh, I. M. et al. (2003). Generationand characterization of epidermolysis bullosa simplex celllines: scratch assays show faster migration with disruptivekeratin mutations. Br. J. Dermatol. 149, 46-58.
Na, N., Chandel, N. S., Litvan, J. and Ridge, K. M.
(2010). Mitochondrial reactive oxygen species are
required for hypoxia-induced degradation of keratinintermediate filaments. FASEB J. 24, 799-809.
Omary, M. B., Ku, N.-O., Tao, G.-Z., Toivola, D. M.
and Liao, J. (2006). ‘‘Heads and tails’’ of intermediatefilament phosphorylation: multiple sites and functional
insights. Trends Biochem. Sci. 31, 383-394.
Omary, M. B., Ku, N.-O., Strnad, P. and Hanada, S.
(2009). Toward unraveling the complexity of simpleepithelial keratins in human disease. J. Clin. Invest. 119,1794-1805.
Owens, D. W. and Lane, E. B. (2003). The quest for thefunction of simple epithelial keratins. Bioessays 25, 748-758.
Owens, D. W. and Lane, E. B. (2004). Keratin mutationsand intestinal pathology. J. Pathol. 204, 377-385.Owens, D. W., Wilson, N. J., Hill, A. J. M., Rugg, E. L.,Porter, R. M., Hutcheson, A. M., Quinlan, R. A., van
Heel, D., Parkes, M., Jewell, D. P. et al. (2004). Humankeratin 8 mutations that disturb filament assemblyobserved in inflammatory bowel disease patients. J. Cell
Sci. 117, 1989-1999.Paladini, R. D. and Coulombe, P. A. (1999). Thefunctional diversity of epidermal keratins revealed by thepartial rescue of the keratin 14 null phenotype by keratin16. J. Cell Biol. 146, 1185-1201.Planko, L., Bohse, K., Hohfeld, J., Betz, R. C.,Hanneken, S., Eigelshoven, S., Kruse, R., Nothen,
M. M. and Magin, T. M. (2007). Identification of akeratin-associated protein with a putative role in vesicletransport. Eur. J. Cell Biol. 86, 827-839.Rogel, M. R., Jaitovich, A. and Ridge, K. M. (2010).The role of the ubiquitin proteasome pathway in keratinintermediate filament protein degradation. Proc. Am.
Thorac. Soc. 7, 71-76.Russell, D., Andrews, P. D., James, J. and Lane, E. B.
(2004). Mechanical stress induces profound remodellingof keratin filaments and cell junctions in epidermolysisbullosa simplex keratinocytes. J. Cell Sci. 117, 5233-5243.Russell, D., Ross, H. and Lane, E. B. (2010). ERKinvolvement in resistance to apoptosis in keratinocyteswith mutant keratin. J. Invest. Dermatol. 130, 671-681.Schermelleh, L., Heintzmann, R. and Leonhardt, H.
(2010). A guide to super-resolution fluorescencemicroscopy. J. Cell Biol. 190, 165-175.Schweizer, J., Bowden, P. E., Coulombe, P. A.,Langbein, L., Lane, E. B., Magin, T. M., Maltais, L.,
Omary, M. B., Parry, D. A. D., Rogers, M. A. et al.
(2006). New consensus nomenclature for mammaliankeratins. J. Cell Biol. 174, 169-174.Schweizer, J., Langbein, L., Rogers, M. A. and Winter,H. (2007). Hair follicle-specific keratins and theirdiseases. Exp. Cell Res. 313, 2010-2020.Sivaramakrishnan, S., Schneider, J. L., Sitikov, A.,Goldman, R. D. and Ridge, K. M. (2009). Shear stressinduced reorganization of the keratin intermediatefilament network requires phosphorylation by proteinkinase C zeta. Mol. Biol. Cell 20, 2755-2765.Snider, N. T., Weerasinghe, S. V. W., Iniguez-Lluhı,
J. A., Herrmann, H. and Omary, M. B. (2011). Keratinhypersumoylation alters filament dynamics and is amarker for human liver disease and keratin mutation. J.
Biol. Chem. 286, 2273-2284.Spurny, R., Gregor, M., Castanon, M. J. and Wiche, G.
(2008). Plectin deficiency affects precursor formation anddynamics of vimentin networks. Exp. Cell Res. 314, 3570-3580.Srikanth, B., Vaidya, M. M. and Kalraiya, R. D. (2010).O-GlcNAcylation determines the solubility, filament
organization, and stability of keratins 8 and 18. J. Biol.
Chem. 285, 34062-34071.Strelkov, S. V., Herrmann, H. and Aebi, U. (2003).Molecular architecture of intermediate filaments.Bioessays 25, 243-251.Szeverenyi, I., Cassidy, A. J., Chung, C. W., Lee, B. T. K.,Common, J. E. A., Ogg, S. C., Chen, H., Sim, S. Y., Goh,
W. L. P., Ng, K. W. et al. (2008). The Human IntermediateFilament Database: comprehensive information on a genefamily involved in many human diseases. Hum. Mutat. 29,351-360.Toivola, D. M., Tao, G.-Z., Habtezion, A., Liao, J. and
Omary, M. B. (2005). Cellular integrity plus: organelle-related and protein-targeting functions of intermediatefilaments. Trends Cell Biol. 15, 608-617.Toivola, D. M., Strnad, P., Habtezion, A. and Omary,
M. B. (2010). Intermediate filaments take the heat asstress proteins. Trends Cell Biol. 20, 79-91.Tong, X. and Coulombe, P. A. (2006). Keratin 17modulates hair follicle cycling in a TNFalpha-dependentfashion. Genes Dev. 20, 1353-1364.Torma, H. (2011). Regulation of keratin expression byretinoids. Dermatoendocrinol. 3, 136-140.Uitto, J., Richard, G. and McGrath, J. A. (2007).Diseases of epidermal keratins and their linker proteins.Exp. Cell Res. 313, 1995-2009.Uttam, J., Hutton, E., Coulombe, P. A., Anton-Lamprecht, I., Yu, Q. C., Gedde-Dahl, T., Jr, Fine,
J. D. and Fuchs, E. (1996). The genetic basis ofepidermolysis bullosa simplex with mottledpigmentation. Proc. Natl. Acad. Sci. USA 93, 9079-9084.Wagner, M., Hintner, H., Bauer, J. W. and Onder, K.(2012). Gene expression analysis of an epidermolysisbullosa simplex Dowling-Meara cell line by subtractivehybridization: recapitulation of cellular differentiation,migration and wound healing. Exp. Dermatol. 21, 111-117.Windoffer, R., Kolsch, A., Woll, S. and Leube, R. E.
(2006). Focal adhesions are hotspots for keratin filamentprecursor formation. J. Cell Biol. 173, 341-348.Windoffer, R., Beil, M., Magin, T. M. and Leube, R. E.
(2011). Cytoskeleton in motion: the dynamics of keratinintermediate filaments in epithelia. J. Cell Biol. 194, 669-678.Woll, S., Windoffer, R. and Leube, R. E. (2007). p38MAPK-dependent shaping of the keratin cytoskeleton incultured cells. J. Cell Biol. 177, 795-807.Yamada, S., Wirtz, D. and Coulombe, P. A. (2002).Pairwise assembly determines the intrinsic potential forself-organization and mechanical properties of keratinfilaments. Mol. Biol. Cell 13, 382-391.Zhao, Y., Gartner, U., Smith, F. J. D. and McLean,W. H. I. (2011). Statins downregulate K6a promoteractivity: a possible therapeutic avenue for pachyonychiacongenita. J. Invest. Dermatol. 131, 1045-1052.
Journal of Cell Science 125 (17)3928