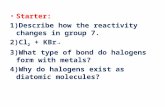I’ve highlighted the group that halogens are in. Write what group number it is _____________.
-
Upload
junior-price -
Category
Documents
-
view
217 -
download
3
Transcript of I’ve highlighted the group that halogens are in. Write what group number it is _____________.

I’ve highlighted the group that halogens are in.
Write what group number it is _____________

One of the Halogens is used in swimming pools as a disinfectant (to kill germs).
Circle the halogen you think it is from the list on the right.
Fluorine (F)
Chlorine (Cl)
Bromine (Br)
Iodine (I)
Astatine (At)

Halogens are non metals. They have the typical properties of non metals.
Circle the two properties below that are typical property of a non-metal
Can be bent and re-shaped
Can be bent and re-shaped
Conducts electricity well
Conducts electricity well
Conducts heat and electricity
badly
Conducts heat and electricity
badly
Low melting point
Low melting point

Chlorine =Pale yellow
The halogens all form coloured gases
Bromine =Orange
Iodine =Dark purple
Underline the correct word…
The gases get darker/lighter with increasing atomic number.

These are all the halogens (group 7 elements).
Write the atomic number of Fluorine (F) ____________

Halogen Melting point (oC)
Boiling point(oC)
Solid/liquid/gas at
room temperature
Chlorine -110 -34Bromine -7 59 Liquid
Iodine 114 184
If the melting point (the temperature it melts at) is below (colder than) 25oC it is a liquid at room temperature
If the boiling point (the temperature it evaporates at) is below (colder than) 25oC it is a gas at room temperature.
Fill in the last column in the table below:

The atomic number of Chlorine is 17 – that means it has 17 electrons in its atom
Please draw the electrons onto the diagram of a chlorine atom to the right
• There can be a maximum of 2 electrons in shell 1
• And a maximum of 8 electrons in shell 2

Halogens Iodine
Group 7 Darker 7
Chlorine Toxic (kills things)