Iranian Pharmacovigilance Center Research & Development Office Undersecretary for Food & Drug...
Transcript of Iranian Pharmacovigilance Center Research & Development Office Undersecretary for Food & Drug...

Iranian Pharmacovigilance Center
Research & Development OfficeUndersecretary for Food & Drug
Ministry of Health & Medical Education



Are drugs safer today ? • During 1960-1999 there were 121 safety related withdrawals
Worldwide
– Market life was known for 87 of those
– Market life less than 2 years 31%
– Market life less than 5 years 50%
Fung et al. Drug Information Journal, 2001; 35:293-317
• During 1972-1994 in 583 new active substances were approved
– Of these 59 were withdrawn later
– During 1990-2001 in UK 24 drugs were withdrawn due to safety reasons

Limitations of Clinical Trials

The goal of drug therapy is the achievement of defined therapeutic outcomes that improve a patient’s
quality of life while Minimizing patient risk
SAFETY EFFICACY

History of drug safety after thalidomide eradication
1961 :
Dr William McBride (Australia)( thalidomide 4000 cases)
1964 :
UK started “yellow cards” system
1968 :
start of WHO Programme for International Drug Monitoring

Official member countriesAssociate member countries
WHO Drug Monitoring ProgrammeParticipating countries 1999
58 countries have joined the programme

Official member countriesAssociate member countries
WHO Drug Monitoring ProgrammeParticipating countries 1999
58 countries have joined the programme
Iranian Pharmacovigilance Center

Adverse Drug ReactionAdverse Drug Reaction

Adverse Drug Reaction
WHO definition:
Any response to a drug which is Noxious and Unintended, and which occurs at doses used in men of prophylaxis, diagnosis or treatment.

Why Should We Learn about Adverse Drug Reactions (ADR)?
Over 2 MILLION serious ADRs yearly
100,000 DEATHS yearly
6.7% of hospitalized patients have an ADR
with a fatality of 0.32,
Ref: U.S. Food and Drug Administration . Center for Drug Evaluation and Research

Annual death rates in USA
• AIDS 16,516
• Breast cancer 42,297
• Highway accidents 43,458
•ADR 100,000

percentage of hospital admissions due to adverse drug reactions:
USA 28%UK 15.6%France 12.6%Norway 11.5%

Costs Associated with ADRs
$ 136 BILLION yearly (related to morbidity and mortality)
Greater than total costs of cardiovascular or diabetic care.
Mean length of stay, cost and mortality ADR patients are DOUBLE that for control group of patients without ADR.
ADRs cause 1 out of 5 injuries or deaths per year to hospitalized patients.
Ref: U.S. Food and Drug Administration . Center for Drug Evaluation and Research

The total cost of drug-related morbidity and mortality
exceeds
the cost of the medications themselves
Ref: Ernst Frank R, Grizzle Amy J. J Am Pharm Assoc. 2001; 41: 192-9

The cost of adverse drug events: estimated lost patient activity days per year in hospitalised patients
Country Serious ADRs Lost Activity Days
US 700,000 1,218,000 Germany 206,000 358,440 UK 148,000 257,520 Australia 48,000 83,520 Sweden 22,000 38,280

ADR has financial and social effects:
1- Unreliability on manufacturer
2- Unreliability on health system (Physician, Pharmacist & Nurse)
3- Unreliability on governments in saving the social safety
4- Causing mortality & morbidity


So many prescriptions!

Tow-thirds of patients visits result in a prescription
2.8 BILLION outpatients prescriptions were filled in the year 2000 (about 10 prescriptions per person in the U.S.)
ADRs increase exponentially with 4 or more medications
Ref: U.S. Food and Drug Administration . Center for Drug Evaluation and Research

Even more, dramatic situation with drug safety is in developing countries (IRAN)
• They often have older, cheaper drugs which may be more toxic.
• Health professional have less opportunity for post-graduate education on clinical pharmacology.
• Useful,easily available, balanced information on adverse effects and their management is absent or not enough.
Ref:World Health Organization

Assessment the quality of medications
Assessment of drug safety
Detection of occurrence rate of ADR
Decreasing the risk of occurrence of adverse events

How Knowledge About ADRs Is Created?
1- Animal experiments
2- Clinical trials
3- Epidemiological methods Spontaneous reporting Cohort studies Case-control studies

Limitations of Clinical Trials
Limited size Narrow population Narrow indications Short duration
• Ref: J. Russell May. Adverse drug Reactions and interaction, In: Pharmacotherapy, A pathophysiologic Approach. 1997, Appleton & Lange.

و دارو،وزارت بهداشت درمان دفتر تحقيق و توسعه-معاونت غذا ومركز ثبت و بررسي عوارض ناخواسته داروها، آموزش پزشكي
Incidence of ADRs to be detected
Spontaneous background incidence
Minimum number of patients to be exposed
1 in100 0 360
1 in 10000 520
1 in 1000 730
1 in 100 2000
1 in 1000 0 3600
1 in 10000 7300
1 in 1000 20300
1 in 100 136400
1 in 5000 0 18200
1 in 10000 67400
1 in 1000 363000
1 in 100 3255000

Some drugs cause serious ADRs at very low frequencies
bromfenac hepatotoxicity (1 in 20,000)
removed from the market in 1998, less than 1 year after it was introduced.
• Ref: U.S. Food and Drug Administration . Center for Drug Evaluation and Research

Adverse reaction Drug Time lag(yr)
Pulmonary embolism Oral contraceptive 3 Myocardial infarction Oral contraceptive 5 Death fro asthma Sympathomimetic 4 Jaundice Halothane 7 Colitis Lincomycin 6 Colitis Clindamycin 5 Aplastic anemia Phenylbutazone 6
Ref: J.Russel May.Adverse Drug Reactions and interactions,In:Pharmacotherapy, a pathophysiologic approach.1997, Appleton & Lange.

“yellow cards”

Spontaneous Reporting
Large populationAll medicinesHospital and out-patient careLong perspectivePatient analysis possibleNon-interventionalCheap

Detection, Assessment & Prevention of ADRs in Human.
Ref: World Health Organization.

• Drugs• Vaccines• Blood products• Traditional and complementary medicines• herbals• Food additives• Medical devices• Biologicals• Biotechnology products• cosmetics
Pharmacovigilance concerns have been
widened to include:

Pharmacovigilance Major Aims
Early detection of unknown reactions and interactions
Detection of increase in frequency
Identification of risk factors
Quantifying risks
Preventing patients from being affected unnecessarily
RATIONAL AND SAFE USE OF DRUGS
Ref: World Health organization.

The ultimate goal of pharmacovigilance is
improving pharmacotherapy
Ref:World Health Organization

04/21/23 35

Comparison Type A and Type B
A B
Pharmacologically predictable
Yes No
Dose-dependent Yes No
Incidence and morbidity High Low
Mortality Low High
Treatment Adjust dose
Stop

Drug Classes Responsible for ADRsDrug Class FrequencyAntibiotics Most frequent
Antitumor agents
Anticoagulants
Cardiovascular agents
Anticonvulsant agents
Antihypertensives
Analgesics
Antiasthmatics
Sedative/hypnotics
Antidepressants
Antipsychotics
Peptic ulcer therapy Least frequent
Ref: J. Russell May. Adverse Drug Reactions and Interactions, In: Pharmacotherapy, A pathophysiologic Approach. 1997,
Appleton & Lange.

Types of Drug-Related Effects by Frequency
Type of adverse event Frequency Marrow suppression Most frequentBleeding Central nervous systemAllergic/cutaneousMetabolicCardiacGastrointestinalRenalRespiratory Least frequent
Ref: J. Russell May. Adverse Drug Reactions and Interactions, In: Pharmacotherapy, A pathophysiologic Approach. 1997, Appleton & Lange.

Patient’s specification
Patient’s drug history
Pharmacology of prescribed drugs
Prescription of minimum effective dosage
Important factors to decreases ADR

Preventing ADR
Over 75% of all ADR are dose-dependentMany ADR arise from failure to tailor the
dosage of drugs to widely different individual needs.
Ref:World Health Organization

Essential factors causing ADRs:
Route of administration Dosage Duration of treatment Problems with drug:
1-Formulation
2-Problems with preparing of drug
Factors related to drug:

Sex Age (weight) Genetic (PHARMACOGENOMICS) Drug allergy Lack of knowledge in patients Concomitant drugs
Factors related to patient:
Essential factors causing ADRs:

Drugs cause hospitalization
Digoxin 41 Aspirin 25
Aspirin 24 Digoxin 24
Prednisone 15 Warfarin 12
Warfarin n 9 HCTZ 11
Guanethidine 5 Prednisone 8

Type of Alerting Order One time stat dose
PRN orders
Short course therapy
Abrupt decrease in dose Followed by a stat
Serum level
State laboratory tests
Example Sub-cutaneous epinephrine, corticosteroids, dextrose
50%, sodium polystyrene sulfate
Antihistamins, topical corticosteroids Oral corticosteroids
(eg.20 mg prednisone p.o 7 days) aminoglycosides, antiarrhythmic agents,
anticonvulsants Theophylline, phenytoin, aminoglycosides, Drug interactions (eg. Digoxin-verapamil,
cimetidine-theophylline)
Stool guiac, prothrombin time

فوايد وجود ADR در بيمارستان برنامه
افزايش كيفيت درمان -1جلوگيري از شكايات حقوقي -2
ارزيابي مشكالت دارويي -3ارزيابي مشاهدات پزشكان و ديگر حرف -4
پزشكيارتقاء دانش دارويي دست اندركاران -5
درمان

ADRداليلي كه باعث كاهش گزارشات ميگردد:
عدم اطالع از مكانيزم موجود براي ارسال گزارش -1
عدم دسترسي به فرم مربوطه -2عدم اهميت عارضه از نظر گزارشگر -3
نداشتن وقت -4در رابطه با فرم مربوطه -5
اجتناب از درگيري در كارهاي اداري -6ترس از شكايات حقوقي, كيفري -7عدم اطمينان از -ADR 8توسط دارو
بوجود آمدن

Misconceptions about ADR Reporting
All serious ADRs are documented by the time a drug is marketed.
About patient receiving multiple medications,it is difficult to determine if a drug is responsible for the ADR.
ADRs should only be reported if absolutely certain.
One reported case can’t make a different.
Ref: U.S. Food and Drug Administration . Center for Drug Evaluation and Research

PREGNANCY & LACTATION

Drugs In ElderlyDrugs In Elderly



Spontaneous Reporting

Case I
آقاي اورژانس 72بيمار توسط كه باشند مي اي با 115ساله ،آورده بيمارستان اورژانس بخش به هوشياري سطح كاهش . فشار سابقه پدرش كه كند مي ذكر بيمار همراه است شده . پزشك نمايد مي استفاده استنشاقي اپيوم و داشته خونسطح كاهش با بيمار با برخورد پروتكل طبق اورژانس كشيك
گلوكز وي براي عرض cc 25، %50هوشياري و 5در دقيقهنالوكسان تزريق . 0.4mgآمپول از پس نمايد مي عدد 5شروع
بيمار ولي شده بهتر كمي هوشياري سطح ، نالوكسان آمپولپرسنل تالش عليرغم كه شده قلبي ايست دچار بالفاصله
. نمايد مي فوت بيمار احيا، جهت اورژانس
چيست؟ بيمار فوت علت شما نظر به

Case II
با 35خانم درمان تحت خون فشار و افسردگي سابقه با اي ساله . به ايشان باشد مي هيدروكلرتيازيد و اناالپريل سيپرومين، ترانيلقرارمي پتيدين و هالوتان با بيهوشي تحت اورژانس، جراحي دليل
اطاق. در بيمار براي جراحي از پس خون Recoveryگيرد فشار210/120 . عدم دليل به بيمار شود مي ثبت ميوكلونيك انقباضات و
به اورژانس خون فشار دليل به و كامل تحت ICUهوشياري و منتقل . ويا كاهش به اقدام از پس گيرد مي قرار پروسايد نيترو با درمان . كليه يابد مي افزايش بيمارمجددا خون فشار نيتروپروسايد، قطع
. باشد مي طبيعي بيمار پاراكلينيك آزمايشات
چيست؟ : بيمار خون فشار افزايش علت شما نظر به سوال

Ccase III
بچه پسر با 4 بيمار درمان تحت حاد مدياي اوتيت تشخيص با كه است اي ساله . تب بيمار اوليه شكايت است گرفته قرار سيلين بوده –آموكسي گوش درد و قراري بي
. 2است. است شده بهتر عمومي حال و شده قطع بيمار تب دارو شروع از بعد 4روزو بوده خوب عمومي حال ولي است كرده تب دوباره كودك درمان، شروع از بعد روز
Toxic . و است داده موقع به را سيلين آموكسي كه نمايد مي ذكر بيمار مادر باشد نمي. دارد ادامه درمان هنوز
نداشته را سيلين آموكسي به مقاوم اوتيت تجربه كند مي طبابت كه اي منطقه در پزشك. دهد نمي را مكرر اوتيت و اخير ماه يك در بيوتيك آنتي مصرف سابقه نيز بيمار و
. در شود مي مشاهده تنه روي بر ماكولوپاپوالر راشهاي اخير معاينه بيمار :CBCدر اخيرWBC = 9000PMN = 57%L= 30%E=10%M=2%B= 1%
پزش�ك تص�ميم ب�ه قط�ع داروي آموكس�ي س�يلين و ادام�ه درم�ان ب�ا اريترومايس�ين مي گيرد.
س�اعت بع�د از قط�ع آموكس�ي س�يلين تب بيم�ار قط�ع ش�ده و راش�هاي جل�دي مح�و 48 مي شود.
لطفا در مورد اين بيمار به سواالت زير پاسخ دهيد:
تشخيص شما در مورد مشكل بيمار چيست؟ -1چه نكات مثبتي به تشخيص شما كمك مي كند؟ -2تشخيص هاي افتراقي در اين بيمار كدامند؟ -3درمان مشكل اخير بيمار چيست؟ -4

Report of Iranian ADR Monitoring Center

www.fdo.ir
دارو و غذا معاونت

ضروری اطالعات
ايران داروهای الفبايی فهرست •(PDF يا Excel فرمت)
غيردولتی آزمايشگاههای برداری بهره و تاسيس •ضوابطآرايشی و بهداشتی غذايی، مواد كنترل
غذا • كل اداره به مربوط ضوابطايران • اسالمی جمهوری دارويی ملی سياست
ماده 226•فهرست مشمول بهداشتی آرايشی اوليه مواد 16قلمدارويی ارتباطی پروتكلهای و اطالعاتی آيتمهای •
آالت ماشين سازنده وشركتهای فنی دانش دهنده انتقال راهنمای •ها داروخانه امور به مربوط هاي بخشنامه •
داروخانه ها • جديد نامه آييننسخه • بدون داروهایدارو • و غذا معاونت خبرنامه
بهداشتی و آرايشی مجوزهای صدور راهنمای •دارويی • ناخواسته عوارض بررسی و ثبت مركز
داروسازی شركتهای تلفن و آدرس •
www.fdo.ir

كاربردی تحقيقات دبيرخانه
دارويی ناخواسته عوارض بررسی و ثبت مركزIranian Adverse Drug Reaction Monitoring
Center
• مركز ADRمعرفي • ADR فرم
عضويت• ها • اطالعيهخبرنامه• مركز • ADRگزارشاتمفيد • لينكهای

The numbers of reports, registered in ADR center of Iran
The numbers of reports, registered in ADR center of Iran
From the year 1377 to Mordad 83 , 5861 cases of Adverse Drug Reaction have been sent to Iranian ADR Center

-
International Vigilance
Every healthcare professional in the world should be constantly alert for adverse effects or potentional new
hazards and reporting them to their National Centers.

Countries with the best reporting rates generate:
• Over 200 reports per 1,000,000 inhibitants per year.
• Over 150 reports per 1000 physicians per year.

Country Profile
Countries Population Physicians Pharmacists Dentists N.Drugs
Iran 60,000,000 53,927 7,327 10,076 1,300
India 900,000,000 450,000 300,000 200,000 >5,000
Thailand 59,000,000 21,105 11,058 5,590 25,511
S.Africa 38,700,000 28,381 9,450 4,235 7,308
Australia 7,720,000 18,737 3,745 3,002 7,490
France 58,000,000 190,000 59,800 42,000 30,986
Germany 81,800,000 274,000 43,700 60,600 50,500
Italy 57,000,000 308,440 56,700 33,805 5,285
UK 57,700,000 36,049 40,117 28,000 15,974
WHO. National Pharmacovigilance System. Second Edition 1999

Country ProfileCountries Year of
establishment Physicians Pharmacist/
ScientistAdministrative/Secretariat
Average Number of ADR Reports/year
Iran 1997 - 6 1 1,600
India 1997 2 2 1 -
Thailand 1983 - 2 2 3,500
S.Africa 1987 1 2 1 250
Australia 1987 - 4 3 500
France 1975 7 6 2 15,000
Germany 1978 13 9 29 19,000
Italy 1980 6 1 2 3,000
UK 1964 7 30 22 18,000
WHO. National Pharmacovigilance System. Second Edition 1999

1837 1793
734671
446
17 7
276
730
200
400
600
800
1000
1200
1400
1600
1800
2000
Pharmac
ist
Gen
eral p
ractit
ioner
Special
ist
Nurse
Patie
nts
Dentis
t
Clinic
al p
harm
acist
Oth
ers
Unknown
Reporters

1720
1502
601
469 448
281 235 205112
63 61 57 49 45
0
200
400
600
800
1000
1200
1400
1600
1800
Adverse Drug Reaction vs Drug classes
From:
1377
To
Mordad 83

691
352
223 212
96 90 88 8252 49 40 40 25
0
100
200
300
400
500
600
700
CNS
GI
Body as a whole
Skin & apprndages
Application site
Cardiovascular
Psychiatric
Respiratory
Autonomic
Musculoskeletal
Urinary
Vision
Heart rate & rhythm
Site of Reaction (CNS agents )
From:
77
To
Mordad 83

Site of Reaction (Antibiotics)
596
347
179 167
71 6126 21 18
0
100
200
300
400
500
600
Skin &
Appen
dages G
I
Body as
a w
holeCNS
Respira
tory
Liver
& b
iliar
y
Visio
n
Coagula
ting S
ys
Repro
ductiv
e
From:
77
To
Mordad 83

87
140 137
109
145
127
162171
83
136
121 120
0
20
40
60
80
100
120
140
160
180
ردينفرو
شتيبه
ارددادخر تير داد
مر
ورهريش هر
مانآب ذر
آدي
منبه
سفندا
Reports of 1382 year

189
150 149
79
143
100
118
159
133
155
98
41
0
20
40
60
80
100
120
140
160
180
200
ردينفرو
شتيبه
ارددادخر تير داد
مر
ورهريش هر
مانآب ذر
آدي
منبه
سفندا
Reports of 1381 year

Diclofenac Na Above 160 Cases of Foot drop
Withdrawal from Iranian Pharmacopoeia
Streptokinase

Bupivacaine
4 Cases of Para-plegia following IT injection
2 of them led to Death

Tramadol?
• From 04.81 to 05.83, 289 cases of adverse effects of Tramadol have reported to ADR center
• Among them :
81 cases have been in Male
&
208 cases have been in Female

Tehean 70 Khorasan 29 Yazd 27 Esfahan 24 Markazi 16 Mazandaran 13 Kerman 12 Fars 9 Cha va bakh 9 Lorestan 8 Azar e gharbi 7 Hamedan 7 Kermanshah 7 Ardebil 7
Ilam 7 Khoozestan 5 Azar e sharghi 5 Gilan 5 Koh va boyer 5 Semnan 4 Ghom 3 Sistan va baloo 3 Booshahr 2 Ghazvin 2 Zanjan 1 Golestan 1 Unknown 1
States

0-10 3 11-20 7 21-30 86 31-40 61 41-50 51 51-60 29 61-70 18 71-80 11 >80 3 Unknown 20
Age groups Age groups (Reaction of Tramadol):(Reaction of Tramadol):

84.78%
9.34%5.19%
0.35%
0.35%
IM PO IV ID Unknown
Rout of administration(In patients with Tramadol adverse effects)

Adverse Reaction of Tramadol from 04.81 to 03.83
6 Major Adverse Effects of Tramadol:
Nausea 125 Vomiting 116 Vertigo 109 Asthenia 57 Dyspnoea 42 Hypotension 41

Adverse effects of Tramadol from 04.81 to 03.83
Reaction Number Reaction Number
Sweating 24 Myalgia 7
Headache 21 Pale 7
Agitation 20 Ataxia 6
Somnolence 17 Vision disorders 6
Pruritus 16 Paraesthesia 6
Rigors 15 Injection site reaction 6
Flushing 11 Delusion 6
Urticaria 11 Tachycardia 6
Bronchospasm 10 Respiratory depression 6
Hallucination 9 Palpitation 5
Convulsion 9 Rash 4
Hypertension 8 Cold extremity 4
Confusion 8 Apnoea 3
Abdominal pain 8 Anxiety 3
Dry mouth 7 Stupor 3

Reaction Number Reaction Number
Cardiac arrest 3 Back pain 1
Anorexia 3 Arrhythmia 1
Shock 3 Bradycardia 1
Allergic reaction 2 Lacrimation abnormal 1
Cyanosis 2 Myocardial Ischemia 1
Constipation 2 Diarrhea 1
GI disorders 2 Depression 1
Leg pain 2 Erythem 1
Dysphagia 2 Coma 1
Speech disorders 2 Edema 1
Urinary retention 2 Hearing decrease 1
Chest pain 2 Facial pain 1
Fever 2 Withdrawal syndrome 1
Syncope 2 Foot drop 1
Insomnia 2 Tremor 1
Adverse effects of Tramadol from 04.81 to 03.83

Sildenafil
• Sildenafil has cardiac related side effect.
• Some cases of myocardial infarction were reported to ADR center due to this drug.

The following tips must be reminded when using Sildenafil:
• Cardiovascular adverse effects such as atrial fibrillation, cardiomyopathy, flushing, hypotension, myocardial infarction, thrombosis, ventricular tachycardia have been reported with Sildenafil.
• Concomitant use of Sildenafi with following drugs are forbidden:
Organic nitrates (eg. Nitroglycerin) Nitrates & Nitric donors (eg. Nitroproside)

Lamotrigine
Common adverse effects:
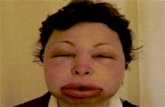
Skin reaction: rash ,Steven's Johnson
syndrome, TEN
Women more than men Onset Caution Adverse events causing hospitalization Weight limitation

Age Limitation
• Not effective & safe in children under 16 years old
• Person younger than 16 years old:
• Risk factor for severe skin reactions


Lindane
This drug has entered to the world drug
market since 1901.
Since the year 1990 Lindane has been
introduced as a second line treatment.

Suggestion:
Single dose of use Second line of treatment Labeling Contraindication Precaution

Systemic adverse effects of Lindane 70% of adverse effects have been the CNS
adverse events, including: Seizure,Vertigo, Headache, Parasthesia
17 cases of death have been reported to FDA, IN 3 cases an established relationship between
the events and using of drug were found
FDA alert (2003)FDA alert (2003)

CelecoxibLabelling Changes
• Celecoxib Long-term Arthritis Safety Study
(Class) did not show a safety advantage of
upper GI events for celecoxib compared with
diclofenac or ibuprofen.

Risperidone
Extrapyramidal Reactions:
Rabbit Syndrome
1 Case in the USA 2 Cases in the English- Language Literature 4 Cases reported to IADRMC

.
IV IG
3 Cases of Death3 Cases of Death
following Administration of Vials with Unusual Colorfollowing Administration of Vials with Unusual Color

Benzyl benzoate
.
5 Cases of Sever Systemic Side Effects 5 Cases of Sever Systemic Side Effects following Topical Administration following Topical Administration
3 of them led to Death3 of them led to Death

Drugs of current Interest
• Strptokinase• Tramadol• Risperidone• Celecoxib • Sildenafil• Clozapin• Cisapride
• Diclofenac Na• Anti -TB Agents • Hypiran• Co-trimoxazol
All New AgentsAll New Agents

The FDA Safety Information and Adverse Event Reporting Program:
Safety alerts Recalls Withdrawals Important labeling changes Biologicals, Drugs, Dietary supplements
MedWatch
www.fda.gov/medwatch/www.fda.gov/medwatch/

Journals of special interest

Reference books

WHO Publications








04/21/23 105