Ionospheric Physics 2/4 - CASEarth’s Atmosphere • Atmospheric constituents at ground level [%]...
Transcript of Ionospheric Physics 2/4 - CASEarth’s Atmosphere • Atmospheric constituents at ground level [%]...
![Page 1: Ionospheric Physics 2/4 - CASEarth’s Atmosphere • Atmospheric constituents at ground level [%] N2 78.1 O2 20.9 Ar 0.93 CO2 0.035 He 0.00052 Kr 0.00011 H2 0.00005 CH2 0.0002 Ne](https://reader035.fdocuments.in/reader035/viewer/2022070712/5ecd9247a7eea26118002bf7/html5/thumbnails/1.jpg)
Ionospheric Physics 2/4
• Earth’s Atmosphere• Composition• Heating balance
![Page 2: Ionospheric Physics 2/4 - CASEarth’s Atmosphere • Atmospheric constituents at ground level [%] N2 78.1 O2 20.9 Ar 0.93 CO2 0.035 He 0.00052 Kr 0.00011 H2 0.00005 CH2 0.0002 Ne](https://reader035.fdocuments.in/reader035/viewer/2022070712/5ecd9247a7eea26118002bf7/html5/thumbnails/2.jpg)
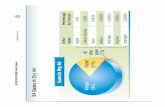
Earth’s Atmosphere• Atmospheric constituents
at ground level [%]
N2 78.1
O2 20.9
Ar 0.93
CO2 0.035
He 0.00052
Kr 0.00011
H2 0.00005
CH2 0.0002
Ne 0.0018
Xe 0.00001
Because of the Earth's gravity, atmosphere is horizontally stratified.
Its structure can be organized by using the neutral gas temperature, chemical composition, charged particle density.
Exobase
BaropauseBarosphere
Loss is significant for H, slight for He and insignificant for O Escape temperature:O … 84000KHe .. 21000KH … 5200K
![Page 3: Ionospheric Physics 2/4 - CASEarth’s Atmosphere • Atmospheric constituents at ground level [%] N2 78.1 O2 20.9 Ar 0.93 CO2 0.035 He 0.00052 Kr 0.00011 H2 0.00005 CH2 0.0002 Ne](https://reader035.fdocuments.in/reader035/viewer/2022070712/5ecd9247a7eea26118002bf7/html5/thumbnails/3.jpg)
Pressure, Density, and Temperature variations
• Description of the static atmosphere: pressure, density, temperature, composition:
• Barometric equation – relation between pressure, mass density and temperature
⎟⎟⎠
⎞⎜⎜⎝
⎛−=
⎟⎟⎠
⎞⎜⎜⎝
⎛−=
∫
∫h
h p
h
h p
HdhTT
Hdhpp
0
0
exp
exp
00
0
ρρ
Index 0 stands for values at reference level.
Above 100km (the Turbopause) each constituent obeys a separate barometric equation with its own ‘partial scale height.
Valid only in the barosphere!
∫=
=
h
h p
p
Hdhz
gmKTH
0
Pressure Scale height
Reduced height
gmKTH
ii =
nkTP =
![Page 4: Ionospheric Physics 2/4 - CASEarth’s Atmosphere • Atmospheric constituents at ground level [%] N2 78.1 O2 20.9 Ar 0.93 CO2 0.035 He 0.00052 Kr 0.00011 H2 0.00005 CH2 0.0002 Ne](https://reader035.fdocuments.in/reader035/viewer/2022070712/5ecd9247a7eea26118002bf7/html5/thumbnails/4.jpg)
At the height of the F2 layer where the peak of ionization occurs, the density of ionized atoms (almost entirelyOxygen at this altitude) is around7x105 to 106 .cm-3. Electrons have the same density. The density of non-ionized, or neutral Oxygen atoms is around 5.108 cm-3 → about 500 timesas many in any given volume. The density of Nitrogen (molecular at this altitude) is equal to that of Oxygen (again 500 times as great as the ions).
![Page 5: Ionospheric Physics 2/4 - CASEarth’s Atmosphere • Atmospheric constituents at ground level [%] N2 78.1 O2 20.9 Ar 0.93 CO2 0.035 He 0.00052 Kr 0.00011 H2 0.00005 CH2 0.0002 Ne](https://reader035.fdocuments.in/reader035/viewer/2022070712/5ecd9247a7eea26118002bf7/html5/thumbnails/5.jpg)
Composition
• Major components: oxygen, nitrogen – molecular or atomic forms, helium and hydrogen
• Minor constituents: ozone, oxides of nitrogen, alkali metals, carbon dioxide, water
• Atomic oxygen dominates at several hundreds kilometers – above that helium and hydrogen are major species
• Protonosphere starts in exosphere• Water vapour – important in the termosphere• Nitric oxide – contributes to the production of the lower ionosphere.
Distribution is affected by mesospheric dynamics. Usually minimum occurs at 85-90km – deeper in summer and varies with latitude
• Ozone – strongly absorbs UV – heat source of the stratosphere; shielding/protection of life. Varies with latitude, season. Ozone holes and progressive reduction from year to year observed since early 1980s. Reduction is likely caused by the action of atomic halogens originating inclorofluorocarbons compounds (long life time ~75-150years)
• Metals – from meteors – sodium, calcium, iron and magnesium influence aeronomy of the lower ionosphere.
![Page 6: Ionospheric Physics 2/4 - CASEarth’s Atmosphere • Atmospheric constituents at ground level [%] N2 78.1 O2 20.9 Ar 0.93 CO2 0.035 He 0.00052 Kr 0.00011 H2 0.00005 CH2 0.0002 Ne](https://reader035.fdocuments.in/reader035/viewer/2022070712/5ecd9247a7eea26118002bf7/html5/thumbnails/6.jpg)
Mesosphere-Thermosphere Composition
![Page 7: Ionospheric Physics 2/4 - CASEarth’s Atmosphere • Atmospheric constituents at ground level [%] N2 78.1 O2 20.9 Ar 0.93 CO2 0.035 He 0.00052 Kr 0.00011 H2 0.00005 CH2 0.0002 Ne](https://reader035.fdocuments.in/reader035/viewer/2022070712/5ecd9247a7eea26118002bf7/html5/thumbnails/7.jpg)
• Absorption of solar radiation– > airglow,
chemiluminiscence(first noticed in 1868 by Anders
Ångström) – > chemical energy– > direct gas heat
• Magnetospheric convection• Wave dissipation
– Gravity waves (minutes –hours)
– Tidal waves (hours – days)– Planetary waves (days – 2, 5,
10, 16 days)
![Page 8: Ionospheric Physics 2/4 - CASEarth’s Atmosphere • Atmospheric constituents at ground level [%] N2 78.1 O2 20.9 Ar 0.93 CO2 0.035 He 0.00052 Kr 0.00011 H2 0.00005 CH2 0.0002 Ne](https://reader035.fdocuments.in/reader035/viewer/2022070712/5ecd9247a7eea26118002bf7/html5/thumbnails/8.jpg)
![Page 9: Ionospheric Physics 2/4 - CASEarth’s Atmosphere • Atmospheric constituents at ground level [%] N2 78.1 O2 20.9 Ar 0.93 CO2 0.035 He 0.00052 Kr 0.00011 H2 0.00005 CH2 0.0002 Ne](https://reader035.fdocuments.in/reader035/viewer/2022070712/5ecd9247a7eea26118002bf7/html5/thumbnails/9.jpg)
Absorption of solar radiation
• λ < 200nm almost all incoming radiation is absorbed above 80 km• Solar protons with λ < 102.5nm are predominantly absorbed by N2, O2, O and
consequently ionized• Ionization energy: 1) kinetic energy
2) chemical product energy1) – Coulomb collisions with thermal electrons, elastic and nonelastic collisions
with neutral particles - electronXelectron collisions – heating of ionized gas - electronXneutral collisions – heating of the neutral atmosphere
- Electrons enter ionization, excitation- new pairs electron - ion- collisions – heating- airglow
2) – Enegy Transfer due to charge transfer, ion-molecule interchange reactions- realization/deposition of the absorbed energy even in the distant regions of the origin
![Page 10: Ionospheric Physics 2/4 - CASEarth’s Atmosphere • Atmospheric constituents at ground level [%] N2 78.1 O2 20.9 Ar 0.93 CO2 0.035 He 0.00052 Kr 0.00011 H2 0.00005 CH2 0.0002 Ne](https://reader035.fdocuments.in/reader035/viewer/2022070712/5ecd9247a7eea26118002bf7/html5/thumbnails/10.jpg)
Atmospheric Cooling
Airglow
The most effective sources:• Upper atmosphere – radiation (particularly in the infrared, oxygen emission at
λ=63µm))• Middle thermosphere (120 – 200 km) – radiation (NO infrared at λ=5.3µm)• Below 120 km, mesosphere – radiation from CO2 infrared at 15µm and ozone infrared at
9.6µm
• Dayglow (entire atmosphere is illuminated by the Sun) is the brightest airglow due to the importance of RESONANT and FLUORESCENT processes but it is overwhelmed by direct and scattered sunlight
• Twilightglow (only the upper atmosphere is illuminated) is the most readily observable airglow from the ground since the observer is in darkness (and Rayleigh scattering of sunlight by the dense lower atmosphere is absent) while the airglow region of upper atmosphere is still illuminated
• Nightglow (entire atmosphere is in darkness) is not as bright as dayglow since CHEMILUMINESCENCE is the dominant process; however contributes more light than starlight to the total luminosity of the night sky
![Page 11: Ionospheric Physics 2/4 - CASEarth’s Atmosphere • Atmospheric constituents at ground level [%] N2 78.1 O2 20.9 Ar 0.93 CO2 0.035 He 0.00052 Kr 0.00011 H2 0.00005 CH2 0.0002 Ne](https://reader035.fdocuments.in/reader035/viewer/2022070712/5ecd9247a7eea26118002bf7/html5/thumbnails/11.jpg)
Airglow
The brightest region of airglow is about 10 to 20 km thick zone at an altitude of about 100 km.
![Page 12: Ionospheric Physics 2/4 - CASEarth’s Atmosphere • Atmospheric constituents at ground level [%] N2 78.1 O2 20.9 Ar 0.93 CO2 0.035 He 0.00052 Kr 0.00011 H2 0.00005 CH2 0.0002 Ne](https://reader035.fdocuments.in/reader035/viewer/2022070712/5ecd9247a7eea26118002bf7/html5/thumbnails/12.jpg)
• RESONANCE: emitted light is at same color as that absorbed resulting from excitation by the absorption of solar radiation
• FLUORESCENCE: emitted light is at lower frenquency, i.e. a different color, resulting from excitation by the absorption of solar radiation
• PHOTOIONIZATION: emitted light is from the excited states of ionized fragments caused by solar radiation
• PHOTODISSOCIATION: emitted light is from the excited states of neutral fragments caused by solar radiation
• INELASTIC COLLISIONS: emitted light results from excitation caused by the impact of high energy ("hot") electrons that are produced inphotoionization [The same process that causes the luminous aurora but in that case the "hot" electrons come from the magnetosphere through interactions with the solar wind.]
• CHEMILUMINESCENCE: emission results from chemical reactions mainly between oxygen and nitrogen atoms and molecules and hydroxyl molecules at a height between 100 and 300 kilometers. Solar radiation energy breaks molecules apart during the day, and it is their recombination, which is accompanied by the emission of light, that generates the nightglow.
• EXCITATION by COSMIC RAYS (high energy radiation and particles from outside the solar system) make a small contribution to airglow as well.
![Page 13: Ionospheric Physics 2/4 - CASEarth’s Atmosphere • Atmospheric constituents at ground level [%] N2 78.1 O2 20.9 Ar 0.93 CO2 0.035 He 0.00052 Kr 0.00011 H2 0.00005 CH2 0.0002 Ne](https://reader035.fdocuments.in/reader035/viewer/2022070712/5ecd9247a7eea26118002bf7/html5/thumbnails/13.jpg)
Airglow
![Page 14: Ionospheric Physics 2/4 - CASEarth’s Atmosphere • Atmospheric constituents at ground level [%] N2 78.1 O2 20.9 Ar 0.93 CO2 0.035 He 0.00052 Kr 0.00011 H2 0.00005 CH2 0.0002 Ne](https://reader035.fdocuments.in/reader035/viewer/2022070712/5ecd9247a7eea26118002bf7/html5/thumbnails/14.jpg)
Heating is effective up to 200-300km
Dominant heat region – 100-150 km Solar radiation absorbed by O2
Temperature maximum at the stratopause –absorption of 200-300nm radiation by ozone
Pressure gradients leads to circulation/turbulence
Wind system
Influence of the wave systems – GW, Tides, PW
![Page 15: Ionospheric Physics 2/4 - CASEarth’s Atmosphere • Atmospheric constituents at ground level [%] N2 78.1 O2 20.9 Ar 0.93 CO2 0.035 He 0.00052 Kr 0.00011 H2 0.00005 CH2 0.0002 Ne](https://reader035.fdocuments.in/reader035/viewer/2022070712/5ecd9247a7eea26118002bf7/html5/thumbnails/15.jpg)
Heat transport
• Radiation – most effecient at lowest levels. The radiative equilibrium between 30 and 90 km.
• Molecular conduction – most efficient in the thermosphere (above 150km), because of low pressure and free electrons. Thermosphere is isothermal above 300 or 400km (though T is varying with time)
• Eddy diffusion or convection – more efficient below the turbopause. Below ~ 100 km eddy diffusion carries heat from termosphere to mesosphere. (Major loss for thermosphere but minor contribution for mesosphere)
• Transport by large scale winds – affects the horizontal distribution of the thermosphere
• Chemical transport – ionized or dissociated species is created in one place and recombines in another. The mesosphere is heated in part by the recombination of atomic oxygen created at a higher level.
Result → two hot regions: Stratopause and Thermosphere
![Page 16: Ionospheric Physics 2/4 - CASEarth’s Atmosphere • Atmospheric constituents at ground level [%] N2 78.1 O2 20.9 Ar 0.93 CO2 0.035 He 0.00052 Kr 0.00011 H2 0.00005 CH2 0.0002 Ne](https://reader035.fdocuments.in/reader035/viewer/2022070712/5ecd9247a7eea26118002bf7/html5/thumbnails/16.jpg)
Wind system
![Page 17: Ionospheric Physics 2/4 - CASEarth’s Atmosphere • Atmospheric constituents at ground level [%] N2 78.1 O2 20.9 Ar 0.93 CO2 0.035 He 0.00052 Kr 0.00011 H2 0.00005 CH2 0.0002 Ne](https://reader035.fdocuments.in/reader035/viewer/2022070712/5ecd9247a7eea26118002bf7/html5/thumbnails/17.jpg)