Infectious porcine atrophic rhinitis: a review.
Transcript of Infectious porcine atrophic rhinitis: a review.

THE CANADIAN VETERINARY JOURNALLA REVUE VETERINAIRE CANADIENNE
November 1966
INFECTIOUS PORCINE ATROPHIC RHINITIS: A REVIEW
H. G. Pearce* and C. K. Roe*
ATROPHIC RHINITIS (AR) was initially de-scribed by Franque in 1829 (109). Sincethat time, the disease has been reportedthroughout the world (1, 9, 16, 17, 22, 27,61, 62, 115, 116). In Canada, the inci-dence in infected herds has been foundto vary from 21 to 45% (66, 92); similarfigures have been reported in Europe (7).In Ontario, up to 88% of herds may beaffected (76) and within a herd, the inci-dence may be as high as 45% (98). Allbreeds and both sexes appear to be equallysusceptible to infection (9, 28, 37, 62).Experimentally, young pigs were more
readily infected than older pigs (41).Several workers have reported that ARmay be contracted during intrauterine life(9, 41). Dietary deficiencies, helminthiasis,and poor ventilation were considered topredispose pigs to the disease (48). Pig-lets that have a light birthweight appear
to be more susceptible than their heavierlitter mates (48). It has been reportedthat AR was more common in spring littersand that litters from gilts were more
susceptible than those from older sows
(53, 54).Gendreau (36) and Goldstein (38)
stressed the economic importance of thedisease and claimed that the mortality ofthis disease may be as high as 30%. Doubthas been expressed that AR alone is ofeconomic importance, but the disease maypredispose to other infections (e.g., peri-carditis and pleurisy have been reportedto be more common in the presence ofsevere AR lesions (6)). Malocclusion of
'Ontario Veterinary College, University ofGuelph, Guelph, Ontario.
the jaws and faulty mastication may impairdigestion (48).
Pigs with AR have been reported toweigh significantly less at various ages
than their normal litter mates (66).Studies at the Ontario Veterinary Collegehave shown no significant difference inthe maturity time of normal and AR-affected litter mates from herds free ofvirus pneumonia (VPP) or from herds inwhich VPP was present and pigs were
reared under R.O.P. conditions (86).Feeding trials with a university swineherd have shown an improvement of feedefficiency and rate of gain when comparingthe old herd, which had a high level ofAR and VPP, and the present herd whichis certified free of both diseases (82).
Early investigators considered that ARwas an hereditary abnormality (defectivedevelopment of turbinates occurred first,infection was secondary) because inflam-mation was not always coincident withturbinate atrophy and the disease couldexist in clinically normal pigs (12, 28, 57,63, 64, 112). By selective breeding, thedisease was eliminated in East Prussia(64). The high incidence of AR in thebetter herds in that area was attributed totheir breeding methods. Krage later modi-fied his view that AR was an hereditarydisease but still denied that the conditionwas due to infection or to avitaminosis Abecause pigs, from both affected and pre-viously non-affected herds, had AR follow-ing breeding trials (65). Duthie was
unable to transmit the disease to pigs 40to 90 days of age and concluded that ARwas hereditary or there was an hereditarysusceptibility (28).
Scandinavian workers demonstrated that
,43
CAN. VET. JOUR., vol. 7, no. 11, November, 1966
Volume 7 Number 1 1

CANADIAN VETERINARY JOURNAL
the disease was not hereditary by raisingpigs of the same breeding from two con-secutive litters. The first litters were bornnaturally and reared by the sows. AR waspresent when the pigs were examined atmarket weight. The second litters wereremoved at birth and reared artificially; inthese litters, there was no turbinateatrophy (13, 34).AR has been ascribed to faulty nutrition
with infection playing a secondary role.Bendixen (6, 8) noted that the diseasewas more common in spring-born littersthan in other litters. Since the incidenceof AR tended to parallel the incidence ofanopthalmia, he considered that vitamin Aplayed an important role. Later, he con-cluded that AR was of complex nutritionalorigin. Bjorklund (9) considered thedisease to be of metabolic origin relatedto rapid growth, and that infection wassecondary to faulty bone development. Hewas unable to support the contention thatvitamin A deficiency was significant, sinceexamination of livers from affected pigsindicated a normal level of vitamin A, norwas he able to produce evidence of dis-turbed calcium or phosphorus metabolism.
Other workers noted that the diseaseoccurred on farms on which breedingstock was reared and when boars wereintroduced from these breeding farms toother farms, the disease appeared. Forthis reason, they considered the disease tobe infectious (113). Clinical and experi-mental investigation has tended to sup-port this view (22, 59, 84). Bacilluspyocyaneus was the first organism to beinvestigated as a possible cause of thedisease. Broth cultures of this organismwere instilled intranasally into two pigs;the two experimental pigs died and thecontrol pig survived. From this experi-ment, it was concluded that AR wascaused by B. pyocyaneus (63). Jensen wasunable to confirm this work (51, 63).Pigs were injected with cultures of Pseudo-monas aeruginosa and filtrates of Ps.aeruginosa broth cultures obtained frompigs with AR. The pigs thus injected diedand it was considered that the toxin ofthis organism was the etiologic agent.These workers also prepared an auto-genous Ps. aeruginosa vaccine which theyclaimed was effective in preventing thedisease (31). Radtke stated that AR wasa manifestation of "Ferkelgrippe" (suck-
ling pig influenza) since he observed nasallesions only where this condition occurred;he was the first person to transmit thedisease (91). Crude nasal material, nasalexudate, or washings, have been used toreproduce AR in young pigs (41, 42, 59,60, 89).
Bacteriological surveys of the nasalpassages of affected pigs have consistentlydemonstrated that P. multocida is present(52, 76). The incidence of this organismin specific pathogen free (S.P.F.) andknown infected herds has been found tobe 1% and 2%, respectively (85). Trans-mission experiments using cultures of P.multocida have been inconsistent (44, 45,76, 97). P. multocida bacterin or anti-serum had no protective value when ad-ministered prior to intranasal instillationof crude infectious material (46, 60). Byusing a rabbit passage technique, it hasbeen possible to produce AR in pigs byinstilling intranasally, mixed cultures ofP. multocida and Actinomyces necrophorusof swine nasal origin. McKay inoculatedyoung pigs with rabbit passage materialand reproduced the disease in 82% of theexperimental animals (76). Other workershave been unable to reproduce these re-sults (42). Schofield and Robertson wereable to reproduce AR in piglets givenmultiple instillations of pure cultures ofPseudomonas aeruginosa and P. multocida(97). AR in young pigs was reproducedfollowing intranasal instillation of culturescontaining P. multocida and Haemophilussuis and streptococci (34). Van der Schaafwas unable to reproduce AR in month oldpiglets using intranasal instillation of cul-tures of H. suis (115). On the basis ofclinical observation it has been suggestedthat Erysipelas and AR may be related(79, 80), but Borgman was unable todemonstrate Erysipelothrix rhusiopathiae inground up nasal material from affectedpigs (11).
Moynihan was unable to reproduce ARwith mixed cultures of Alcaligenes bronchi-septica, Staphylococcus albus and S. aureus(81). McKay observed an incidence of16% of Bordetella bronchiseptica in thenasal passages of 31 normal pigs (76).More recently, workers were able to pro-duce turbinate atrophy experimentally inyoung pigs (24). Initially, they reproducedthe disease in eight out of nine specificpathogen free (SPF) piglets using a
244

INFECTIOUS PORCINE A.R.
turbinate homogenate in saline. From thepigs thus infected, they were able to isolateB. bronchiseptica. Using 48-hour nutrientbroth cultures of B. bronchiseptica, turbin-ate atrophy was produced in four out ofsix pigs. It was found that nine out of tenpigs, all from different herds, harbouredB. bronchiseptica in their nasal passages.
Ross et al. reported an incidence of B.bronchiseptica of 54% on a herd basis,when they examined the nasal passages offour pigs, from each of 87 swine herds(93). Using egg-passaged B. bronchisep-tica cultures turbinate atrophy was pro-duced in 66% of four-week-old pigs, and in95% of pigs aged one to three days (94).In Canada, it was demonstrated that B.bronchiseptica was present in the nasalpassages of pigs from herds free of thedisease and AR affected herds (85). Cul-tures of this organism were not capableof producing turbinate atrophy in naturallyborn SPF piglets but did so when instilledintranasally into caesarean derived, colos-trum-deprived pigs (86).
It has been reported that 80% of affectedswine harbored trichomonads in theirnasal passages, whereas the figure for nor-mal pigs was 2.8% (104). The incidenceof nasal trichomonads in pigs has beenreported by other workers as 35.2%, 39.6%,56.3%, and 75-90%, respectively (16, 49,50, 72). The successful transmission of ARto piglets with trichomonads has been re-ported (103) but this has not been con-firmed (50, 69). Trichomonads werefound, in the absence of bacteria, in thenasal passages of pigs on farms where ARoccurred but not on AR-free farms (91).Lyubimoya (72) was able to establishnasal Trichomonas suis in the nasal pas-sages of swine but not trichomonads iso-lated from feces. He noted that while acatarrhal rhinitis occurred, this was notfollowed by turbinate atrophy.From observations of the clinical disease
and initial experiments, Phillips considereda filterable agent to be important becausehe was able to reproduce sneezing in pigswith the supematant of centrifuged nasalmaterial obtained from pigs with AR(88). Switzer (105) isolated a filterableagent from the nasal passages of pigs withAR and was able to reproduce mild lesionsof AR in piglets following intranasal instil-lation of this agent. When reisolated fromexperimental piglets, the agent induced an
acute inflammatory disease in chick em-bryos but these changes were not inducedby filtrates of nasal material obtained froma herd known to have AR. Later, Switzerfound that this filterable agent producedserositis when administered to pigs by in-traperitoneal injection (103). Subsequentexperiments illustrated that nasal filtratescontained at least one virus and twotypes of pleuro-pneumonia-like organism(PPLO) which could be established inthe nasal cavity of swine, and produceda mild cellular reaction in the upper respi-ratory tract (110). A filterable agent,capable of producing AR in pigs, hasbeen grown in chick embryos (117). Thisagent passed Berkfield V and N filters andwas capable of producing the disease inexperimental pigs even after 20 eggpassages. Using filtrates of nasal mucosafrom affected animals instilled intranasallyinto young pigs, Tsarev et al. (114) wereable to produce AR but other workershave been unable to reproduce the diseasein young pigs with bacteria-free filtratesof either nasal washings or nasal materialfrom affected pigs (13, 24, 34, 41, 42,46, 76).A pleuro-pneumonia-like organism (PP-
LO) associated with AR has been demon-strated (18, 107). It was later reportedthat this organism was probably identicalwith the agent which caused the serositisin pigs reported by Switzer (106). Gwat-kin et al. were unable to reproduce AR inyoung pigs using nasal instillations ofPPLO (46).Done described a previously unreported
disease and suggested that it might berelated to AR (25). Since this time, inclu-sion body rhinitis has been reported in anumber of countries (17, 32, 39, 51).Gwatkin et al. were unable to show anyrelationship between the presence of in-tranuclear inclusions and the incidence ofAR (47). Intranuclear inclusions in pigsaffected with AR have been described andit was stated that inclusions could be foundin pigs inoculated with nasal materialderived from pigs affected with AR.Whether the pigs from which the inoculumwas derived had inclusions or not, did notappear to matter; inclusions were foundin the nasal mucosa of experimental pigs(10). Obel considers that inclusion bodyrhinitis, VPP and AR may have a commonetiology (83).
245

CANADIAN VETERINARY JOURNAL
It has been suggested that AR may be asubacute manifestation of swine influenza(26); this was also the view of Radtkewho was able to isolate influenza virusfrom the ethmoid sinus and nasopharynxof pigs with AR (91). French workershave demonstrated neorickettsial bodies insections of nasal turbinates, with Machia-vello stain (14, 15). Later studies ledthem to believe that the disease was pri-marily due to nervous damage whichcaused vascular obliteration; non-inflam-matory turbinate atrophy resulted. Theywere able to demonstrate antibodiesagainst the psittacosis-lymphogranulomagroup in the sera of the sows, and alsointra- and extracellular bodies which pos-sessed the morphological and stainingcharacteristics of neorickettsiae.
PATHOLOGY
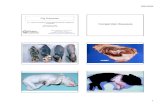
In the late stages of the disease themaxillary and nasal bones may be dis-torted, giving the nose a wrinkled appear-ance, or there may be lateral twisting ofthe snout. Longitudinal section of thenasal cavity, or cross-section at the levelof the first premolar cheek teeth, mayreveal atrophy of the turbinates. Thismore commonly affects the ventral turbi-nates but may be so extensive as to reducethe turbinates to budlike projections fromthe maxillae. Bjorklund described an hy-pertrophy of the facial bones whichoccurs when gross nasal distortion ispresent (9). There may be a purulent ormuco-purulent exudate in the nasal pas-sage covering the ethmoturbinates or con-tained within the paranasal sinuses; thisexudate may become inspissated. Thesinuses themselves may be irregular inshape and volume. The nasal septum maybe normal or moderately, to severely, dis-torted. Grossly, the mucosa may appearnormal or be reddened and congestedwith a mottled or eroded appearance(95). Changes of the osseous bulla withloss of its porous nature resulting invariable consolidated areas have been re-ported. Microscopically, the mucosa maybe normal in type but eroded in parts toform shallow necrotic ulcers. In otherinstances, the mucosa undergoes meta-plasia and may become cuboidal or squa-mous instead of the uniform tall columnar
cells. The mucosa may have a ragged orvacuolated appearance.The submucosa may be infiltrated with
leucocytes, usually lymphocytes. Neutro-philes may be present in the acini of thesubmucous glands. Schofield and Jones(95) noted that the lymphocytes did notpenetrate further than the outer layer ofthe periosteum. Submucous glands may beengorged with mucus and microabscessesmay be formed. In the later stages, theremay be extensive fibrosis of the submu-cosa. Initially, the blood vessels may bemoderately engorged but later the vascu-lar endothelium proliferates and mayreduce the lumen of the vessel. At first,bony change consists of osteoblastosisbeneath the periosteum. The osteoblastsincrease in number as the bony lamellaeare replaced by fibrous bands. Thereappears to be no enhanced activity ofosteoclasts. Nervous lesions consisting ofganglionitis of the sphenopalatine andGasserian ganglia have been described byBrion et al. (14). Bjorklund describedlesions in bones other than those of thehead. Changes in the lachrymal and sali-vary glands comprised inflammation andfibrosis. Degenerative lesions of theparenchymatous organs have been asso-ciated with AR (9). McNabb reportedthat there were no significant hematologi-cal changes associated with AR (78).
DIAGNOSIS
(a) Rhinoscopic examination. Thismethod has been used in Maryland and isconsidered to be about 75% accurate (29).
(b) Radiographic examination. Radio-graphs have been used but their accuracyis limited (13, 55).
(c) Trichomonads. Lyubimoya suggeststhat the presence of trichomonads in swinenasal passages may be diagnostic (72).
(d) Allergic tests. An allergen fromchick embryos inoculated with nasalmaterial filtrate derived from affected pigshas been described (117).
TREATMENT
Sulphapyridine was used by Aitken totreat pigs with AR (2). After five days oftreatment clinical recovery from the diseaseoccurred. B. bronchiseptica has beeneliminated from the nasal passage of arti-
246

INFECTIOUS PORCINE A.R.
ficially-infected pigs using sulphametha-zine at the rate of 100 grams per ton offeed (111). It has been found that strepto-mycin sulphate, administered by intra-muscular injection on four successive days,reduced the incidence but did not elimi-nate the disease in experimentally infectedpiglets (60). By giving streptomycin as atopical application to young pigs, it wasdemonstrated that ten out of twelve un-treated pigs developed AR whereas onlyone out of ten pigs in the treated groupdid so (43). The addition of chlortetra-cycline at the rate of 50 grams per ton offeed has been reported to reduce the timetaken by pigs affected by AR to reachmarket weight (40). Bugeac et al. foundthat an inoculum consisting of a mixtureof nasal secretion and triturated respiratorymucous membrane became noninfectiveafter incubation with penicillin, strepto-mycin and chlortetracycline (16). Trialswith benzathine penicillin, oxytetracycline,and chlortetracycline have demonstratedthat these drugs cause a sharp reductionin the incidence of the disease in treatedanimals (4). Erythromycin given intra-muscularly each week for five weeks hasnot been an effective medication (73).
EPIDEMIOLOGY
In some areas, the disease may reachepidemic proportions, then subside (76).Both Lukashev et al. (71) and Bendixen(6) considered that the disease incidencewas highest in litters born during thewinter and spring months. Lamnikhov etal. published figures which suggested thatthe gilt's litters were more susceptible thanthose of mature sows (67). Gwatkin (48)stated that whether the sows themselveshave turbinate atrophy is not significantas he observed that sows with AR couldraise normal litters. Outbreaks of AR havebeen blamed on the introduction of in-fected animals (113). In a limited num-ber of experiments, it was difficult totransmit the disease by contact with arti-ficially infected pigs and premises whichhoused infected animals rapidly lost theirinfectivity (41, 94). Pashov et al. (84)were able to produce turbinate atrophy inrats and rabbits following intranasal instil-lation of nasal washings from pigs withAR. Nasal washings from rats were foundto be capable of causing AR in pigs. These
workers noticed that when rabbits weregiven nasal washings from pigs with AR,pasteurellosis was a common sequel. Ithas been reported that three sheep de-veloped rhinitis after sharing a pen withtwo pigs infected with AR. One year later,the sheep were still suffering from rhinitis.A transmission experiment using nasalwashings from sheep with rhinitis instilledinto the nasal passages of pigs elevenweeks of age resulted in acute AR (118).Once present on a farm, AR may beasymptomatic for two years (102). Ex-perience with several outbreaks of AR inherds previously free of the disease hasshown that the incidence rose steadily overa period of four to six months to a peakaverage of 40%, and subsequently fell to30%. Within one large herd examinedclosely for the disease, 70% of a total of90 litters were affected. An average of38% of the pigs in each litter were affected(86).
CONTROL
Attempts to control AR have involvedthe use of naturally-born, artificially-rearedpiglets, and caesarean-derived, artificially-reared piglets. Attempts to eliminate thedisease by allowing sows to farrow inisolation have been unsuccessful (3). Onemethod tried by Johnson and co-workerswas to allow the piglets to suckle theirdams for five days, then remove the pigsand raise them in isolation on a syntheticdiet. This method was unsuccessful. Re-moving piglets from the sow immediatelyafter birth but prior to suckling, thenraising them in isolation on synthetic sow-milk was also unsuccessful due to a highmortality rate. The last method theseworkers tried was to remove the pigs atbirth and return them at frequent intervalsto suckle during the first 24 hours of life,then rear the piglets on artificial diets.This method was successful (58). Young(119) and others (3) delivered pigs byhysterectomy or caesarean section andraised them in isolation units on syntheticsow-milk replacer. At four weeks of agethe pigs were gradually exposed to theexternal environment and placed on farmswhich were being repopulated.
REFERENCES
1. AFANASOV, I. A. Epidemiology of por-cine atrophic rhinitis. Sborn. Trud.
247

CANADIAN VETERINARY JOURNAL
Leningrad nauchno.-issled. vet. Inst. 9:101-113. Abstr. Vet. Bull. 32: 699.1962.
2. AITKEN, W. A. Observation from prac-tice. J. Am. vet. med. Ass. 122: 8. 1953.
3. ALEXANDER, T. J., and C. K. ROE.Attempts at establishing swine herdsfree from atrophic rhinitis and viruspneumonia. II. Repopulation withspecific pathogen free pigs. Can. vet. J.3: 299. 1962.
4. ANDRIYAN, E. A. Efficacy of Bicillin,Terramycin and Biomycin in porcineatrophic rhinitis. Veterinarya. Moscow(2) 41-42 (1961). Abstr. Vet. Bull.31: 545. 1961.
5. BAKos, KARL, A. L. OBEL, 0. SWAHN,and H. WALZL. Inclusion body rhinitisin piglets with experimental enzooticpneumonia. Zentbl. Vet. Med. 7: 262.1960.
6. BENDIXEN, H. C. Occurrence andaetiology of chronic atrophic rhinitisin pigs. Dt. tierartzl. Wschr. 64: 330,1957. Abstr. Vet. Bull. 28: 205.1957.
7. BENDIXEN, H. C. Disease conditions inyoung pigs, particularly dystrophic con-ditions of the nasal cavity. Medlemsbl.dansk Dyrlaegeforen. 40: 93, 1957.Abstr. Vet. Bull. 28: 144. 1957.
8. BENDIXEN, H. C. The influence ofnutrition on the mother animal andindirectly on the foetus during preg-nancy. Medlemsbl. dansk Dyrlaegeforen.45: 85, 1962. Abstr. Vet. Bull. 32: 382.1962.
9. BJORKLUND, N. E. Atrophic rhinitis ofpigs. A morphologic study includingsome etiological aspects. Thesis. StateVeterinary Medical Institute. Stockholm.1958.
10. BOLOGEPOV, V. I. Significance of intra-nuclear inclusions in infectious atrophicrhinitis of pigs. Trudy vsesoyuz Inst.eksp. Vet. 27: 101-107. Abstr. Vet.Bull. 32: 465. 1962.
11. BORGMAN, R. Infectious atrophic rhini-tis unrelated to swine erysipelas. Vet.Med. 48: 97. 1953.
12. BOTTCHER, H. Z. Schwienz 48: 251.Cited by Bjorklund (1958).
13. BRAEND, M., and J. L. FLATLA.Rhinitis infectiosa atrophicans hos gris.Nord. VetMed. 6: 81.1954.
14. BRION, A., M. FONTAINE, and C. LABIE.Presence of nervous lesions in porcineatrophic rhinitis. C. R. Acad. Sci. Paris244: 2870. 1957.
15. BRION, A., M. FONTAINE, and C. LABIE.Studies on porcine atrophic rhinitis.The lesions of the nervous system. Can.J. comp. Med. 22: 88. 1958.
16. BUGEAC, T., C. BERBINSCKI, M. CRIS-TESCU, and A. SOLNITZKY. Porcineatrophic rhinitis in Roumania II Anu.Inst. Pat. Igien, anim. 7: 256-264(1955). Abstr. Vet. Bull. 28: 396.1958.
17. CAMERON-STEPHEN, I. D. Inclusion-body rhinitis in swine. Clinical aspects.Aust. vet. J. 37: 87. 1961.
18. CARTER, G. R., and K. A. McKAY. APPLO associated with infectious atrophicrhinitis of swine. Can. J. comp. Med.17: 413. 1953.
19. CARTER, G. R. Observations on PPLOrecovered from swine with atrophicrhinitis and Glassers disease. Can. J.comp. Med. 18: 246. 1954.
20. CARTER, G. R., and J. D. SCHRODER.Pleuropneumonia-like organisms asso-ciated with pneumonia in swine. Can.J. comp. Med. 19: 219. 1955.
21. CARTER, G. R., and J. D. SCHRODER.Virus pneumonia in pigs in Canadawith special reference to the role ofpleuropneumonia-like organism. CornellVet. 46: 344. 1956.
22. CONNELL, R. A disease called "bull-nose" occurring in swine in the PrairieProvinces. Can. J. comp. Med. 9: 224.1945.
23. CORNER, A. H., D. MITCHELL, R. J.JULIAN, and E. B. MEADS. A general-ized disease in piglets associated withthe presence of cytomegalic inclusions.J. comp. Path. Ther. 74: 192. 1964.
24. CRoss, R. F., and R. M. CLAFLIN.Bordetella bronchiseptica-induced por-cine atrophic rhinitis. J. Am. vet. med.Ass. 141: 1467. 1962.
25. DONE, J. T. An "inclusion body"rhinitis of pigs (preliminary report).Vet. Rec. 67: 525. 1955.
26. DORSET, M., C. N. McBRYDE, andG. NYLES. Remarks on "Hog Flu".J. Am. vet. med. Ass. 15: 162. 1922.
27. DOYLE, L. P., C. R. DONHAM, andL. M. HUTCHINGS. Report on a typeof rhinitis of swine. J. Am. vet. med.Ass. 105: 132. 1944.
28. DUTHIE, R. C. Rhinitis of swine. I.Chronic atrophic rhinitis and congenitaldeformity of the skull. Can. J. comp.Med. 11: 250. 1947.
29. EARL, F. L., and R. D. SHUMAN.Atrophic rhinitis. II. The rhinoscopicexamination of swine as a means ofdiagnosing atrophic rhinitis. J. Am. vet.med. Ass. 122: 5. 1953.
30. EARL, F. L., G. E. WHITMORE, R. A.DAMON, H. 0. HETZER, and J. S.TRIBBLE. The effect of atrophic rhinitison the rate of gain of swine. J. Am.vet. med. Ass. 140: 443. 1962.
248

INFECTIOUS PORCINE A.R.
31. EBER, A., and A. MEYN. Acta path.microbiol. scand. 18: 86. 1934. Citedby Bjorklund 1958.
32. ENGLERT, H. K. Done's inclusion bodyrhinitis, enzootic pneumonia and rhinitisatrophicans in swine (inclusionbodiesin nasal mucosa of piglets with enzooticpneumonia). Dt. tierartzl. Wschr. 67:401. 1960.
33. FITZGERALD, P. R., D. M. HAMMOND,and J. SHUPE. Studies on the role ofTrichomonads in the production ofatrophic rhinitis in pigs. Cornell Vet.44: 302. 1954.
34. FLATLA, J. L., and M. BRAEND. In-fectious atrophic rhinitis in pigs. Studieson the aetiology. 15th Int. Vet. Cong.Proc. Part I. P. 180. 1953.
35. FRANQUE, W. Deutch. Zeitschr. Tier-heilk. 1: 75. 1829. (Cited by Switzerin reference No. 109.)
36. GENDREAU, L. A. Field observationson infectious swine rhinitis. Can. J.comp. Med. 12: 291. 1948.
37. GILMAN, J. W. P. Inherited facialconformation and susceptibility to in-fectious atrophic rhinitis of swine. Can.J. comp. Med. 13: 266. 1949.
:38. GOLDSTEIN, H. E. Progress of atrophicrhinitis studies. Vet. Med. 48: 223.1953.
39. GOTINK, H. M., and J. H. VAN ULSEN.The inclusionbody rhinitis (IBR) inpiglets. Tijdschr. Diergeneesk. 85: 23.1960.
40. GOUGE, H. E., R. BOULTON, and M. C.ALSON. The effect of feeding chlor-tetracycline to pigs with atrophic rhinitis.Antibiotics Annual 1955-56, p. 768.N.Y. Med. Encyclopaedia Inc. 1956.
41. GWATKIN, R., P. J. G. PLUMMER, J. L.BYRNE, and R. V. L. WALKER. Rhinitisof swine. III. Transmission to baby pigs.Can. J. comp. Med. 13: 15. 1949.
42. GWATKIN, R., L. DZENIS, and J. L.BYRNE. Rhinitis of swine. V. Furtherstudies on the aetiology of infectiousatrophic rhinitis. Can. J. comp. Med.15: 32. 1951.
43. GWATKIN, R., and L. DZENIS. Rhinitisof swine. VI. Topical application ofStreptomycin in artificially infected pigs.Can. J. comp. Med. 16: 230. 1952.
44. GWATKIN, R., L. DZENIS, and J. L.BYRNE. Rhinitis of swine. VII. Pro-duction of lesions in pigs and rabbitswith a pure culture of Pasteurella multo-cida. Can. J. comp. Med. 17: 215.1953.
45. GWATKIN, R., and L. DZENIS. Rhinitisof swine. VIII. Experiment with Pas-teurella multocida. Can. J. comp. Med.17: 454. 1953.
46. GWATKIN, R., L. DZENIS, A. S. GRIEG,and C. GRINEWITSCH. Rhinitis of swine.IX. Further studies on aetiologicalagents. Can. J. comp. Med. 18: 341.1954.
47. GWATKIN, R., A. H. CORNER, andC. L'ECUYER. Rhinitis of swine. XI.Search for inclusionbodies during thedevelopment of atrophic rhinitis inartificially infected pigs. Can. J. comp.Med. 23: 84. 1959.
48. GWATKIN, R. Rhinitis of swine. XII.Some practical aspects of the rhinitiscomplex. Can. J. comp. Med. 23: 238.1959.
49. HAMMOND, D. M., P. R. FITZGERALD,and A. E. JOHNSON. Incidence oftrichomonads in the digestive tract andnose of pigs in the western UnitedStates. J. Parasit. 43: 695. 1957.
50. HANSEN, M. A., and J. L. FLATLA.Trichomonader i neshulen hos gris ogderes. Relasjon til rhinitis atroficans.Nord. VetMed. 7: 660. 1955.
51. HARDING, J. D. J. Inclusion bodyrhinitis of swine in Maryland. Am. J.vet. Res. 19: 907. 1958.
52. HEDDLESTON, K. L., R. D. SHUMAN,and F. L. EARL. Atrophic rhinitis. IV.Nasal examination for Pasteurella mul-tocida in two herds affected withatrophic rhinitis. J. Am. vet. med. Ass.125: 225. 1954.
53. HOFLUND, S. Orientering over sjuk-donsen myssjuka (Rhinitis chronicaatrophicans). Hos svin uv Klinisksynpunkt. Svensk. Vet. Tidskr. (1937a)42: 189. Abstr. Vet. Bull. 8: 526.1938.
54. HOFLUND, S. Nyssjukans etiologi.Svensk. Vet. Tidskr. 42: 364. 1937.
55. HoJovEc, J. X-ray diagnosis of porcineatrophic rhinitis. Veterinarstri II: 45-51. 1961. Abstr. Vet. Bull. 31: 344.1961.
56. IVANOV, V. P., and C. M. BLAZHEVICH.Histological changes in the parenchy-matous organs of pigs with atrophicrhinitis. Veterinarya. Moscow. 10: 42.Abstr. Vet. Bull. 31: 215. 1960.
57. JENSEN, C. 0. (1916). (Cited byBjorklund in reference No. 9.)
58. JOHNSON, T. K., J. R. BONE, and T. OLD-FIELD. Atrophic rhinitis in swine. I.Methods of control in a purebred herd.N. Am. Vet. 36: 191. 1955.
59. JONEs, T. L. Rhinitis in swine. Agric.inst. Rev. 2: 274. 1947.
60. JoNEs, T. L. Streptomycin and otheragents used in infectious atrophic rhinitisof swine. J. Am. vet. med. Ass. 121:192. 1952.
61. KASZUBKIEWICZ, C., and J. LIPANOWICZ.
249

CANADIAN VETERINARY JOURNAL
Porcine atrophic rhinitis in Poland.Weterynaria. Wroclaw 9: 95 (1961).Abstr. Vet. Bull. 32: 110. 1961.
62. KERRUISH, D. W. Atrophic rhinitis inpigs other than landrace. Vet. Ree. 68:541. 1956.
63. KosKE (1906). (Cited by McKay inreference No. 76.)
64. KRAGE, P. Tierartzl Rdsch. 40: 317.(Cited by Bjorklund in reference No.9.)
65. KRAGE, P. Dt. tierartzl Wschr. 45: 129,1937. (Cited by Bjorklund in referenceNo. 9.)
66. KRISTJANSSON, F. K., and R. GWATKIN.The effect of infectious rhinitis onweight for age of swine. Can. J. Agric.Sci. 35: 139. 1955.
67. LAMNIKHOV, K. F., and I. I. FELDMAN.The role of breeding sows in porcineatrophic rhinitis. Veterinarya. Moscow.2: 38-41. 1961. Abstr. Vet. Bull. 31:545. 1961.
68. LARSEN, S. Atrophic rhinitis and en-vironment. Medlemsbl. dansk. Dyrlaege-foren. 41: 304. 1958.
69. LEVINE, N. D., W. C. MARQUARDT, andP. D. BEAMER. Failure of bacteria freetrichomonads to cause atrophic rhinitisin young pigs. J. Am. vet. med. Ass.125: 61. 1954.
70. LIVESTOCK COMMISSIONER's OFFICE,Parliament Bldgs., Toronto. Records andRegulations. 1960-62.
71. LUKASHEV, I. I., and G. G. PETRENKO.Aetiology and pathogenesis of porcineatrophic rhinitis. Veterinarya. Moscow.9: 36. 1960. Abstr. Vet. Bull. 32: 387.1960.
72. LYUBINIOYA, L. K. Porcine atrophicrhinitis. Sborn Trnd. Leningrad. In-auchno-vet Inst. 9: 115-145. 1961.Abstr. Vet. Bull. 32: 699. 1962.
73. MAcDONALD, M. A. The use of Galla-mycin for controlling infectious atrophicrhinitis in bacon pigs. Can. J. comp.Med. 27: 147. 1963.
74. McKAY, K. A., and G. R. CARTER.Some observations on the isolation, culti-vation and variation of Spherophorusnecrophorus associated with infectiousatrophic rhinitis, liver abscesses andnecrotic enteritis. Can. J. comp. Med.17: 299. 1953.
75. McKAY, K. A., and G. R. CARTER. Apreliminary note on the bacteriologyand experimental production of in-fectious atrophic rhinitis of swine. Vet.Med. 48: 351. 1953.
76. McKAY, K. A. Unpublished data.1953.
77. McKAY, K. A. Personal communica-tion. 1964.
78. McNABB, A. L. Rept. Ont. Vet. Coll.1948.
79. MESSMORE, H. L. Erysipelas in swine.N. Am. Vet. 33: 308. 1952.
80. MESSMORE, H. L. Erysipelas in swine.N. Am. Vet. 33: 385. 1952.
81. MOYNIHAN, I. W. Rhinitis of swine.II. An effort to transmit chronic atrophicrhinitis of swine. Can. J. comp. Med.II: 260. 1947.
82. NORRISH, J. G. Observations in a herdof SPF swine. Paper presented Can.Soc. An. Prod. Mtg., Ridgetown. Feb.1964.
83. OBEL, A. L. (1961). (Cited by Corneret al. in reference No. 23.)
84. PASHov, T. B., M. Y. KURBALA, andE. I. SEREDA. Adaptation of the casualagent of infectious atrophic rhinitis.Veterinarya. Moscow 2: 31. 1963. Abstr.Vet. Bull. 33: 1963.
85. PEARCE, H. G., and K. A. McKAY.Can. Vet. J. 1966. In preparation.
86. PEARCE, H. G., and C. K. ROE. Can.Vet. J. 1966. In preparation.
87. PHILLIPS, C. E. Alcaligenes (Brucella)bronchiseptica as a factor in porcinepneumonia. Can. J. comp. Med. 7: 58.1943.
88. PHILLIPS, C. E. Infectious rhinitis ofswine (bull nose). Can. J. comp. Med.10: 33. 1946.
89. PHILLIPS, C. E., H. F. LONGFIELD, andJ. E. MILTIMORE. Porcine infectiousatrophic rhinitis experiments. Can. J.comp. Med. 12: 268. 1948.
90. PINK, A. N., and G. A. YAROSEVICH.An outbreak of porcine rhinitis causedby trichomonads. Veterinarya Moscow.34: 27-29. 1957. Abstr. Vet. Bull. 28:281. 1957.
91. RADTKE, G. Arch. wiss. Prakt. Tier-heilk. 72: 371 (1938). (Cited byBjorklund in reference No. 9.)
92. ROE, C. K., and T. J. L. ALEXANDER.Attempts at establishing swine herdsfree from atrophic rhinitis and viruspneumonia. A review of initial workat the Ontario Veterinary College. Can.vet. J. 2: 139. 1961.
93. Ross, R. F., W. P. SWITZER, and C. J.MARE. The incidence of certain micro-organisms in Iowa swine. Vet. Med.58: 562. 1963.
94. Ross, R. F., J. R. DUNCAN, and W. P.SWIIZER. Turbinate atrophy in swineproduced by pure cultures of Borde-tella bronchiseptica. Vet. Med. 58: 566.1963.
95. SCHOFIELD, F. W., and T. L. JONES.The pathology and bacteriology of in-fectious atrophic rhinitis in swine. J.Am. vet. med. Ass. 116: 120. 1950.
250

INFECTIOUS PORCINE A.R.
96. SCHOFIELD, F. W. Rep. Ont. Vet Coll.p. 57. 1952.
97. SCHOFIELD, F. W., and A. ROBERTSON.Further studies on the pathology andbacteriology of infectious atrophic rhini-tis of swine. Proc. Am. vet. med. Ass.p. 155. 1953.
98. SHUMAN, R. D., F. L. EARL, W. T.SHALKOP, and C. G. BURBIN. Atrophicrhinitis. I. A herd survey. J. Am. vet.med. Ass. 122: 1. 1953.
99. SHUMAN, R. D., and F. L. EARL.Atrophic rhinitis. III. The evaluationof the rhinoscopic examination for itsdiagnosis. J. Am. vet. med. Ass. 122:7. 1953.
100. SHUMAN, R. D., and F. L. EARL.Atrophic rhinitis. V. An effort to obtainatrophic rhinitis free pigs by selectionand isolation. J. Am. vet. med. Ass. 127:427. 1955.
101. SHUMAN, R. D., and F. L. EARL.Atrophic rhinitis. VII. A study on theeconomic effect in a swine herd. J. Am.vet. med. Ass. 129-220. 1956.
102. SMILEY, R. S. Infectious atrophicrhinitis in Ohio. Vet. Med. 48: 10.1953.
103. SPINDLER, L. A., D. A. SHORB, andC. H. HILL. The role of trichomonadsin atrophic rhinitis of swine. J. Am. vet.med. Ass. 122: 151. 1963.
104. SWITZER, W. P. Atrophic rhinitis andtrichomonads. Vet. Med. 46: 478. 1951.
105. SwITZER, W. P. Studies on infectiousatrophic rhinitis of swine. I. Isolationof a filterable agent from the nasalcavity of swine with atrophic rhinitis.J. Am. vet. med. Ass. 123: 45. 1953.
106. SWITZER, W. P. Studies on infectiousatrophic rhinitis of swine. II. Intraperi-toneal and intranasal inoculation ofyoung pigs with a filterable agent iso-lated from the nasal mucosa of swine.Vet. Med. 48: 392. 1953.
107. SWITZER, W. P. A suspected PPLOin Iowa swine. Iowa Vet. 25: 9. 1954.
108. SwITzER, W. P. Observations on infec-tious atrophic rhinitis. Proc. U.S. Live-stock San. Assn. p. 363. 1954.
109. SwIIZER, W. P. Infectious atrophic
rhinitis. V. Concept that several agentsmay cause turbinate atrophy. Am. Jour.vet. Res. 17: 478. 1956.
110. SWITZER, W. P., E. D. ROBERTS, and C.L'ECUYER. Site of localization andeffects of swine nasal virus in experi-mental pigs. Am. J. vet. Res. 22: 67.1961.
111. SWITZER, W. P. Elimination of Borde-tella bronchiseptica from the nasalcavity of swine by Sulphonamidetherapy. Vet. Med. 58: 571. 1963.
112. THUNBERG, E. Bidrag till myssjukanseitiologi. Svensk. Vet. Tidskr. 42: 360.1937.
113. THUNBERG, E., and B. CARLSTROM. Theepizoology of chronic rhinitis in swine.Skand. Vet. Tidskr. 48: 404. 1940.
114. TSAREV, S. G., V. V. NIKOLAEVA, andV. V. VIOTSEKHOVSKII. Aetiology andclinical manifestation of porcine infec-tious atrophic rhinitis. Trudy. Dal'ne-vost. nouchno-issled. vet. Inst. 4: 25.1962. Abstr. Vet. Bull. 33: 459. 1962.
115. VAN DER SCHAAF, A. Atrophic rhinitisin pigs. Tijdschr. Diergeneesk. 85: 14-37-48. 1960. Abstr. Vet. Bull. 31: 156.1961.
116. WURZINGER, H. Atrophic rhinitis inpigs in Czechoslovakia. Sborn. ces Akad.zemedelsk. vet. Med. 6: 173. 1961.Abstr. Vet. Bull. 31: 480. 1961.
117. ZOTov, A. P., and P. N. BLINOV. Por-cine atrophic rhinitis: Isolation of afilterable agent and preparation of adiagnostic allergen. Veterinarya. Mos-cow. 3: 32. 1961. Abstr. Vet. Bull.31: 546. 1961.
118. SOLOVEV, S. I., and V. V. KVANOSHCHEK.Epidemiology of porcine infectiousatrophic rhinitis. Veterinarya. Moscow2: 31. 1963. Abstr. Vet. Bull. 33: 518.1963.
119. YOUNG, B. A., N. R. UNDERDAHL, L. S.SUMPTION, E. R. PEO, JR., L. S.OLSEN, C. W. KELLEY, D. B. HUDMAN,J. D. CALDWELL, and C. H. ADAMS.Swine repopulation I. Performance with-in a "disease-free" experiment stationherd. J. Am. vet. med. Ass. 134: 491.1959.
251