Inbreeding and Reproduction in Endangered Ungulates ... · Inbreeding and Reproduction in...
Transcript of Inbreeding and Reproduction in Endangered Ungulates ... · Inbreeding and Reproduction in...

Inbreeding and Reproduction in Endangered Ungulates: Preservation of Genetic
Variation through the Organization of Genetic Resource Banks
ERS Roldan1, M Gomendio
1, JJ Garde
2, G Espeso
3, S Ledda
4, F Berlinguer
4, A del Olmo
1, AJ Soler
2, L Arregui
1, C Crespo
1and
R Gonzalez1
1Grupo de Ecologıa y Biologıa de la Reproduccion, Museo Nacional de Ciencias Naturales (CSIC), Madrid; 2Grupo de Biologıa de la Reproduccion,Institututo de Investigacion en Recursos Cinegeticos, (CSIC-UCLM-JCCM), Albacete; 3Estacion Experimental de Zonas Aridas (CSIC), Almeria,Spain; 4Universita degli Studi di Sassari, Sassari, Italy
Contents
There is a constant increase in the number of species sufferingmarked reductions in population size. This reduction in sizeand the lack of genetic flow may lead to a decrease in geneticvariability and to matings between close relatives (i.e. inbreed-ing) with an ensuing reduction in fitness. It is thus important tounderstand the mechanism underlying the deleterious effects ofinbreeding and to develop reproductive biotechnologies thatwill allow the reduction of inbreeding depression by facilitatinggene exchange between populations. The study of threeendangered species of gazelles, Cuvier’s gazelle (Gazellacuvieri), Mohor gazelle (Gazella dama mhorr) and dorcasgazelle (Gazella dorcas neglecta) has revealed that inbreedingnegatively affects several semen parameters (motility, spermmorphology, acrosome integrity). Semen cryopreservation hasbeen achieved in the three species but success varies dependingon the diluent employed and the level of inbreeding. Artificialinsemination of Mohor gazelles have led to the birth of the firstgazelle born using frozen-thawed semen but improvements areneeded before this technology can be applied on a routine basisfor the genetic management of the populations. Collection ofoocytes after ovarian stimulation, followed by in vitro matur-ation, fertilization and culture has met with some initialsuccess in the Mohor gazelle. These, together with otherreproductive technologies, will offer an invaluable help inpreserving the maximum of genetic diversity of these andrelated endangered ungulate species.
The Biodiversity Crisis and Captive BreedingProgrammes
Extinction rates have accelerated in recent years due tothe exponential growth of the human population andthe intensive use of natural resources that it conveys(May et al. 1995). Within mammals, out of 4856evaluated species, we know that 1093 are threatened orvulnerable, that is 23% of all species for which we havesufficient information (IUCN 2006), although the realfigures are probably much higher. This proportion ofthreatened species is much higher in mammals than thatin other groups such as birds, probably because mam-mals are over-exploited through hunting (Mace andBalmford 2000). Ungulates are one of the mammaliangroups with a higher percentage of threatened species,mainly as a consequence of intensive hunting to obtainfood, fur and horns. In fact, it is the only mammaliangroup in which hunting is a more frequent cause ofthreat than loss of habitat.
In those cases in which it is not possible to halt thecauses that are provoking declines in natural popula-tions, and when these have been reduced to the extent
that they can no longer be considered as viablepopulations, it is advisable to start a captive breedingprogramme for those species. Captive breeding pro-grammes have saved a number of species from extinc-tion, even in those cases in which the magnitude of thedecline in natural populations was such that thebreeding programme was started with just a fewindividuals. This is the case of the black footed ferretwhich started with a founding population of sixindividuals, the Prezwalski horse whose founding pop-ulation consisted of 13 individuals, and the Speke’sgazelle which started with four individuals and later onincorporated three more individuals captured from thewild (reviewed in Hedrick and Kalinowski 2000). Someof these species were later reintroduced to their naturalhabitats, but to date the success of such reintroductionattempts has been very low (Mackinnon 2000).
Traditionally, it has been considered that the mainobjective of captive breeding programmes was to pro-duce a large number of individuals, in order to reintro-duce a sufficiently high number to the natural habitat toensure the long-term viability of reintroduced popula-tions. However, this approach has met with seriousobstacles. On the one hand, when a captive breedingprogramme is started as an emergency response to animminent extinction, generally so little is known aboutthe reproductive biology of the species that it is difficultto know how to start reproducing the animals success-fully. On the other hand, the costs of maintaining a largenumber of individuals in captivity are high, and theavailable space is limited, so there are not enoughresources to establish all the captive breeding pro-grammes that would be needed. Finally, the ultimateobjective of this approach has encountered very limitedsuccess, given that only five reintroduction programmesinvolving mammals have been successful (representing11% of all attempts) leading to the establishment ofviable populations in the wild (Balmford 2000).
These problems have led to a re-evaluation of theobjectives of captive breeding programmes. Nowadayssuch programmes are regarded as a complementarystrategy to the protection of natural populations, ratherthan as an alternative strategy. Within this framework,one of the main objectives of captive breeding pro-grammes is to use a limited number of individuals toimprove our understanding of their reproductive bio-logy; this allows the development of assisted reproduc-tive techniques which facilitate gene flow between
Reprod Dom Anim 41 (Suppl. 2), 82–92 (2006)
� 2006 The Authors. Journal compilation � 2006 Blackwell Verlag

captive populations, as well as between these andnatural populations. The use of reproductive biotech-nologies allows the preservation of genetic diversity, andmay help prevent the effects of inbreeding.
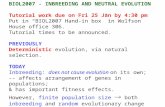
Three captive breeding programmes for endangeredgazelles are under way at the Estacion Experimental deZonas Aridas (CSIC). Between 1971 and 1975, breedingprogrammes were established for three species ofgazelles from the North of Africa, namely Cuvier’sgazelle (Gazella cuvieri), Mohor gazelle (Gazella damamhorr) and a subspecies of dorcas gazelle (Gazelladorcas neglecta) (Fig. 1). Cuvier’s gazelle inhabits themountains in the Atlas region, where small anddispersed groups remain. It is categorized by the IUCNsRed List as ‘Endangered’ (IUCN 2006). Gazella dorcasneglecta is smaller in size and inhabits deserts; thespecies is categorized as ‘Vulnerable’, although thestatus of the subspecies neglecta is not specified, but itis believed to be endangered because of intensivehunting (IUCN 2006). Lastly, the dama gazelle (ofwhich the Mohor gazelle is a subspecies) is larger inbody size, and it inhabits semideserts. Dama gazelle isnow categorized as ‘Critically Endangered’ (IUCN2006) and no Mohor gazelles have been observed inthe wild since 1968 (Barbosa and Espeso 2005). Thesuccess of these breeding programmes, and the handlingprotocols developed, make it possible to carry outstudies which are generally unfeasible in endangeredspecies. For this reason, these species are ideal modelsfor reproductive studies that can then be applied toother endangered ungulates.
Inbreeding Depression and Heterozygosis
It is common for a captive breeding programme to bestarted only when it is perceived that natural popula-tions face an imminent risk of extinction. When a speciesreaches this extreme, and reproduction in captivity startswith just a few individuals, inbreeding is unavoidable.The success of the captive breeding programme underthese conditions depends largely on the chances ofavoiding the deleterious effects of inbreeding on repro-duction and survival (i.e., ‘inbreeding depression’). Theterm ‘inbreeding’ refers to the mating between relatedindividuals and its negative effects are because of thedecrease in heterozygosis that takes place when inbredindividuals are generated (Charlesworth and Charles-worth 1987).
The traditional way to study the effects of inbreedingupon individual fitness has been to calculate thecoefficient of inbreeding f using information fromgenealogies (Wright 1922). The coefficient of inbreedingis defined as the probability that two alleles in a locusare identical by descent, and it represents the expectedvalue of homozygosis for all the genome. Inbreedingdepression has been recognized for some time in captiveand domestic animals (Charlesworth and Charlesworth1987; Thornhill 1993), the most frequently detectedeffect being a decrease in fecundity or an increase injuvenile mortality (Ralls et al. 1979; Ralls and Ballou1986; Mitton 1993).
It is less clear how inbreeding affects male reproduc-tive performance. The widespread occurrence of female
Fig. 1. Gazelle species in the captive breeding programmes (from topto bottom): Cuvier’s gazelle (Gazella cuvieri), Mohor gazelle (Gazelladama mhorr) and dorcas gazelle (Gazella dorcas neglecta)
Inbreeding and Genetic Resource Banks for Endangered Ungulates 83
� 2006 The Authors. Journal compilation � 2006 Blackwell Verlag

promiscuity and, therefore, of sperm competition(Gomendio et al. 1998) constitutes a problem in studiestrying to understand the effect of inbreeding on malereproductive success in natural populations. Therefore,studies analysing the possible effects of inbreeding focuson male reproductive traits, including the analysis ofsemen parameters. Most work carried out in the pasthas concentrated on carnivores with the cheetah and thelion being the centre of a series of studies postulating thepossible relation between the loss of genetic variationand the poor semen quality observed in the males ofthese species (Wildt et al. 1983, 1987a,b; O’Brien et al.1983, 1985, 1987; Menotti-Raymond and O’Brien 1993).This suggestion is based on a series of assumptions thathave been questioned on various grounds (Caughley1994; Caro and Laurenson 1994; Merola 1994; May1995), with the most relevant ones being that levels ofgenetic variability were determined by using levels ofheterozygosity at a few allozyme loci (which may not bea good indicator of heterozygosity at the genomic level)and the lack of control populations that would allow theestablishment of a causal link between genetic variabilityand poor semen quality. In addition, analyses have beenperformed at the population level and did not take intoaccount factors such as history of the populations,environment or behaviour (Roldan et al. 1998).
To understand how inbreeding may affect malereproduction, we have examined the effects of inbreed-ing upon semen parameters in the three populations ofgazelles for which detailed records have been kept andindividual coefficients of inbreeding are known. Wehave shown that males with high inbreeding coefficientssuffer a reduction in the proportion of motile sperma-tozoa, the proportion of morphologically normal sper-matozoa and in the proportion of spermatozoa withintact acrosomes, traits that are important for fertiliza-tion success (Cassinello et al. 1998; Roldan et al. 1998;Gomendio et al. 2000). Average values for inbreedingcoefficient differ significantly between the three species(populations). However, although there were differencesbetween average values of various sperm parameters forthe three species (populations), no clear trend associ-ated with average inbreeding coefficients was detectedbetween populations (Cassinello et al. 1998). On theother hand, when analyses were carried out withinpopulations, a relation between individual levels ofinbreeding and sperm parameters was found in Cuvier’sgazelle, the species with the highest average level ofinbreeding (Fig. 2) (Roldan et al. 1998; Gomendioet al. 2000). Possible reasons for this finding couldrelate to deleterious effects being seen only after athreshold of inbreeding is reached, or that in popula-tions with intermediate or low average levels ofinbreeding there were a few or even no individualscovering the full range of inbreeding coefficients(Gomendio et al. 2000).
We have also shown that individuals with higherinbreeding coefficients are more vulnerable to parasites(Cassinello et al. 2001), which may increase the mortal-ity risks. In this case too, a relation between theindividual coefficient of inbreeding and parasite countwas found only within the species with higher averagevalues of inbreeding (Cassinello et al. 1998) suggesting
again the possibility that effects are seen only after athreshold value of inbreeding is reached.
Inbreeding is not limited to captive populations, asinbreeding depression has been found to occur also innatural populations. Given the difficulties associatedwith constructing pedigrees over several generations innatural populations, an alternative approach has been toexploit the fact that inbreeding reduces heterozygosity.Thus, inbreeding depression can be detected by correla-
Fig. 2. Relationship bewteen inbreeding of Cuvier’s gazelle males and(a)% motility (n ¼ 14, r2 ¼ 0.31, p ¼ 0.04), (b)% normal spermato-zoa (n ¼ 14, r2 ¼ 0.48, p ¼ 0.01), (c)% spermatozoa with intactacrosomes (n ¼ 14, r2 ¼ 0.30, p ¼ 0.04). For analyses, inbreedingcoefficients were log-transformed and percentage values were trans-formed into the arcsine of their square root. Modified from Gomendioet al. (2000)
84 ERS Roldan, M Gomendio, JJ Garde, G Espeso, S Ledda, F Berlinguer, A del Olmo, AJ Soler, L Arregui, C Crespoand R Gonzalez
� 2006 The Authors. Journal compilation � 2006 Blackwell Verlag

ting multilocus marker heterozygosity of individuals witha trait presumed to be associated with fitness. Studiescarried out in natural populations in different specieshave found associations between heterozygosity andseveral components of fitness such as birth weight andneonatal survival (Coltman et al. 1998; Coulson et al.1998; Rossiter et al. 2001), juvenile survival (Coulsonet al. 1999), female lifetime breeding success (Slate et al.2000), male lifetime copulation success (Hoglund et al.2002), and vulnerability to parasites and pathogens(Coltman et al. 1999; Acevedo-Whitehouse et al. 2003;Reid et al. 2003). In a recent study usingmicrosatellites toestimate heterozygosity, a significant negative relation-ship was found between heterozygosity and the propor-tion of normal spermatozoa in wild rabbit populations(Gage et al. 2006). The effects of inbreeding upon fitnesscomponents may lead natural populations to extinction,as in the case of a large metapopulation of butterflies(Saccheri et al. 1998). A recent study carried out by ourresearch group in populations of Iberian deer fragmentedby the use of fences, has shown that individuals with lowlevels of heterozygosis have higher parasite loads (Go-mendio M, Malo A, Garde J, Vicente J, Rey I, GortazarC, Roldan ERS, unpublished data). Thus, there is a largevolume of evidence showing that heterozygosis measuredwith molecular markers and fitness components areassociated.
However, the view that individual heterozygosityacross a few microsatellite loci reflects inbreedingdepression has been recently challenged (Slate andPemberton 2002; Coltman and Slate 2003; Pemberton2004) and its predictive value of known inbreedingcoefficients questioned (Slate et al. 2004). Individual-based simulations show that heterozygosity markers andinbreeding are likely to be correlated under a narrow setof conditions which require frequent and severe inbreed-ing events, such as under small population sizes, strongpopulation subdivision and high levels of polygyny(Balloux et al. 2004). The studies in which heterozygos-ity markers and fitness have been found to be correlatedmay fulfil these restrictive conditions as they includeisland populations (e.g., Mandarte, Rum, St. Kilda),breeding colonies with high longevity and philopatry(harbour seals, grey seals, albatrosses), or species withstrong polygyny (red deer, sea lions, fur seals) (reviewedin Balloux et al. 2004).
An alternative explanation is that heterozygosity doesnot reflect genome wide effects associated with inbreed-ing, but rather that there is linkage disequilibriumbetween the neutral markers used and genes experien-cing balancing selection (Balloux et al. 2004; Hanssonet al. 2004). According to this hypothesis small popu-lations or populations which have suffered bottlenecks,can experience linkage disequilibrium, that is, the non-random association of alleles at different loci in gametes,between a marker and linked fitness loci (Hansson andWesterberg 2002; Balloux et al. 2004). This phenom-enon can arise due to physical linkage or due todemographic processes, being relatively common whenthe effective population size is small.
It is crucial to resolve this debate and to understand ifheterozygosity and the coefficient of inbreeding providethe same kind of information, if both are associated to
fitness-related traits, and which are the underlyingmechanisms that explain the reduction in fitness.
Genetic Resource Banks and AssistedReproductive Techniques
Advantages of using ARTs for conservation
To avoid inbreeding it is necessary to promote gene flowamong different populations and to maintain largepopulations. However, to achieve these objectivesinvolves serious difficulties. The exchange of animalsbetween populations may cause stress, may involvehealth risks, the translocated animals frequently cannotintegrate successfully into the new social groups and,when large-bodied animals are involved, the transpor-tation costs are high. In addition, it is often impossibleto maintain large populations in captive breedingprogrammes given the limited resources available.
Reproductive biotechnologies offer new solutions tofacilitate the genetic management of endangered species,such as the development of genetic resource banks(Loskutoff et al. 1995; Holt et al. 1996b; Wildt et al.1997; Pope and Loskutoff 1999; Wildt and Wemmer1999; Pukazhenthi and Wildt 2004; Pukazhenthi et al.2006). Genetic resource banks allow the preservation ofsemen, oocytes, embryos and other tissues. The mainadvantage of these banks is that they may maintain thegenetic diversity of a given species almost indefinitely.Thus, the semen that is preserved in these banks may beused for many years after the death of an animal. Theexistence of a genetic resource bank reduces consider-ably the number of live individuals which are needed tomaintain a viable population, therefore reducing theamount of space needed to breed a species, minimizingin this way the costs and increasing the number ofspecies which may benefit from breeding programmes.
Sperm cryopreservation
Sperm preservation has been identified as a powerfultool for the conservation of genetic diversity and avaluable aid in the genetic management of captive andfree populations. Cryopreserved semen, together withartificial insemination, allows the storage and use ofsemen from genetically valuable animals, to extendgeneration times, to circumvent husbandry or medicalfactors which may prevent certain animals from breed-ing, and to transfer semen between subpopulations thatmay become geographically or biologically isolated(Wildt 1992; Pope and Loskutoff 1999; Watson andHolt 2001).
It is important to understand the factors that mayinfluence sperm survival during cryopreservation inorder to establish reliable protocols for the establish-ment of genetic resource banks of endangered species.This could require considerable work because differenc-es may exist between species in sperm parameters or intheir susceptibility to cryoinjury. Factors affectingcryopreservation, such as buffer composition, egg-yolkor glycerol concentrations, and refrigeration curves,among others, usually need to be examined in somedetail before a systematic collection and cryopreserva-tion of spermatozoa can be initiated.
Inbreeding and Genetic Resource Banks for Endangered Ungulates 85
� 2006 The Authors. Journal compilation � 2006 Blackwell Verlag

Good protocols of semen handling and cryopreser-vation have been developed for some wild ungulates(reviewed in Holt 2001), with high percentages of spermsurvival and fertility after artificial insemination (e.g.fallow deer: Jabbour et al. 1993; Eld’s deer: Monfortet al. 1993; Iberian deer: Garde et al. 2006) or in vitrofertilization (mouflon: Ptak et al. 2002). Semen preser-vation in true antelopes has received less attention butvarious efforts exist regarding semen cryopreservation inSpeke’s gazelle (Boever et al. 1980), dorcas gazelle(Howard et al. 1981; Garde et al. 2003), blackbuck(Holt et al. 1988), Mohor gazelle (Holt et al. 1996a;Garde et al. 2003), Cuvier’s gazelle (Garde et al. 2003)and gerenuk (Penfold et al. 2005). Fertility after artifi-cial insemination has been seldom tested and, whenperformed, results have been low. Live offspring hasbeen obtained with frozen semen in blackbuck (Holtet al. 1988) and a stillborn calf was delivered afterartificial insemination using cryopreserved semen ingerenuks (Penfold et al. 2005). No live offspring hasbeen reported using cryopreserved gazelle semen. Therehave been attempts of artificial insemination withcryopreserved semen from Speke’s gazelle but no birthswere obtained (Boever et al. 1980). In the Mohorgazelle, pregnancies have been claimed using artificialinsemination with frozen semen, but no live offspringhas been obtained (Holt et al. 1996a; Abaigar and Holt2001).
Semen from other antelopes, such as the addax(Densmore et al. 1987) and the scimitar-horned oryx(Garland et al. 1992; Morrow et al. 2000) has beencollected and frozen successfully, with live offspringobtained after artificial insemination.
As part of an ongoing project for the establishment ofgenetic resource banks for three species of endangeredgazelles (Cuvier’s gazelle, Mohor gazelle and dorcasgazelle), we are exploring factors (including inbreeding)that affect the success of semen cryopreservation. Oneclear factor affecting the results is species differencesbecause when one diluent (Tes-Tris-glucose, with 5%egg yolk and 6% glycerol; TEST) was used forcryopreservation of spermatozoa of the three species,better preservation of sperm motility and acrosomeintegrity were obtained in dorcas gazelles, followed byMohor and Cuvier’s gazelles (Fig. 3). When severaldiluents were examined within a species, differences werealso detected, with best overall recovery rates afterfreezing and thawing found with Triladyl, TEST andTris-trehalose in Cuvier’s gazelle, TEST in Mohorgazelle, and Triladyl and TEST in dorcas gazelle (Gardeet al. 2003). Nevertheless, only a moderate recovery ofsperm motility and acrosome integrity after cryopreser-vation is achieved in Cuvier’s gazelle, in part as a resultof the poor initial quality of semen.
Differences between species in the ability to withstandfreezing and thawing may be related to inbreeding. Wefound that the levels of freezing resistance (i.e. semenparameters after thawing in relation to those in freshsemen) were inversely related to the average values ofinbreeding of these populations (Fig. 4) (Garde et al.2003). Dorcas gazelle males, with the lower averageinbreeding coefficient, showed the best cryopreservationability. On the other hand, Cuvier’s gazelle, having the
highest average level of inbreeding, exhibited the poorestcryosurvival. Cryopreservation of semen from dorcasgazelles yields consistently good results and thus collec-tion and storage for a genome resource bank is nowpossible. For the other two species, improvement is stillnecessary.
Collection of spermatozoa from epididymis of deadanimals also offers an important option for rescuing andpreserving genetic diversity of wildlife. Several studiesreveal that this is indeed possible for ungulates that areculled or hunted, or that died in zoos or captive breedingprogrammes (e.g. Loskutoff et al. 1996; Herrick et al.2004; Bissett and Bernard 2005) with some preliminaryefforts also applied to Mountain gazelle (Gazella gazella)and dorcas gazelle (Saragusty et al. 2006). Thisapproach is being used successfully to preserve geneticdiversity of Iberian ungulates such as Iberian red deer(Soler et al. 2003,2005; Martinez-Pastor et al. 2005), roedeer, chamois (Martinez-Pastor et al. 2005) and Spanishibex (Santiago-Moreno et al. 2006).
Evaluation of the fertilizing capacity in cryopreservedspermatozoa is of importance. In domestic species, thisis usually achieved by the ultimate test of fertilizingability, i.e. artificial insemination trials. However, inendangered species, for which the number of individualsavailable for reproductive studies is usually limited, thisapproach is seldom possible. Therefore, it is importantto use laboratory tests of sperm function that evaluatedifferent aspects of sperm behaviour in connection withfertilization-related events. The ability of zona pelluci-da-free hamster oocytes to bind and fuse with a widevariety of mammalian spermatozoa has been proposedas a method to assess the sperm function (Yanagimachi1984). Its use has not been much explored in wildlifespecies (e.g. Soler and Garde 2003) and, in generalterms, is now a less favoured assay because it onlyevaluates one step (sperm–oolema fusion) in the series ofevents during sperm–egg interaction. Furthermore, inorder to bind to the oolema, spermatozoa have toundergo the acrosome reaction either spontaneously
Fig. 3. Comparison of cryopreservation results of spermatozoa fromCuvier’s, Mohor and dorcas gazelles; (a) Sperm motility index[% motility + (quality of motility · 20)/2], (b)% spermatozoa withintact acrosomesspermatozoa. Assessments were made at four stagesduring cryopreservation: T1, fresh semen; T2, after refrigeration,before freezing; T3, after thawing; T4, 2 h after thawing. Based on datain Garde et al. (2003)
86 ERS Roldan, M Gomendio, JJ Garde, G Espeso, S Ledda, F Berlinguer, A del Olmo, AJ Soler, L Arregui, C Crespoand R Gonzalez
� 2006 The Authors. Journal compilation � 2006 Blackwell Verlag

(and this itself is a questionable parameter of spermfunction) or in response to some natural stimulus ormolecular probe. The use of homologous zona-intactoocytes constitutes another possibility for the evaluationof sperm function and this has been used considerably inrecent years in domestic species. However, the availab-ility of oocytes from wildlife species is very limited. Totest the sperm function from wildlife it is possible to useoocytes from related species since heterologous fertil-ization between phylogenetically close species doesoccur. Among ungulates, the fertilizing ability of freshor cryopreserved spermatozoa has been explored byusing oocytes from domestic species, as in the cases ofscimitar-horned oryx (Roth et al. 1998, 1999) or mouf-lon (Berlinguer et al. 2003) using cow or sheep oocytes,respectively. Scimitar-horned oryx spermatozoa canfertilize between 65 and 95% of in vitro matured cowoocytes (Roth et al. 1998). No differences were foundbetween the homologous (bull sperm · cow oocyte) andthe heterologous (scimitar-horned oryx sperm · cowoocyte) in vitro fertilization (Kouba et al. 2001). Inter-estingly, spermatozoa from the related fringe-eared oryxcould not penetrate the zona pellucida of cow oocytesnor fuse with zona-free cow oocytes (Kouba et al. 2001).These species differences may limit the use of cowoocytes for the evaluation of fertilizing capacity ofspermatozoa from some endangered species and alter-native methods should be sought.
Oestrous synchronization and artificial insemination
Artificial insemination presents many advantages forendangered species. It allows for exchange of geneticmaterial among different populations and for theprevention of disease transmission. The possibility ofinseminating females with frozen semen also increasesthe efficiency of captive breeding, as it becomes possibleto choose the matings which will minimize inbreedingwithout having to stress the animals by moving thembetween social groups, and avoiding behaviouralincompatibilities which may otherwise prevent matingswhich may be convenient from the point of view ofgenetic management.
The use of artificial insemination techniques requiresknowledge of the female reproductive cycle and someindication of the timing of ovulation in relation to theonset (or end) of oestrus. Methods for synchronizationof ovarian cycles will be based on the characterzation of
hormonal profiles of the spontaneous or induced cyclesand on hormone levels and female responses to a varietyof synchronization protocols. Methods for non-invasiveanalysis of hormonal profiles in urine and faeces nowexist for various ungulates (Monfort 2003; Pickard 2003;Pukazhenthi and Wildt 2004) and this could facilitateendocrine analysis of cycling activity (or pregnancydiagnosis), but a more traditional characterization ofblood plasma levels of hormones can also be used insome ungulates where captive breeding programmesallow for a routine handling of animals. Analysis ofmetabolized steroids in urine or faeces actually requires,first, an identification of the routes through which theyare excreted and in which proportion.
In wild bovids, oestrous cycles range between 17 and22 days (Citino 2003). Characterization of cycles inMohor gazelles by examining faecal steroids has shownthat the mean duration of the oestrous cycle is18.5 days, with a range of 16–22 days (Pickard et al.2001); no inter-individual differences were found forfaecal progestagen metabolites but variations seem toexist for faecal oestrogens. Synchronization of cycles ingazelles was attempted by using only controlled internaldrug release (CIDR) devices in two studies (Holt et al.1996a; Pickard et al. 2001) but success of synchroniza-tion was low, and no live births were obtained afterartificial insemination with fresh or frozen semen (Holtet al. 1996a; Abaigar and Holt 2001). Perhaps betterresults can be achieved by using in addition to, orinstead of, the CIDRs, one or more injections ofprostaglandin F2alpha and/or low doses of eCG. In astudy on scimitar-horned oryx (Morrow et al. 2000), acomparison of the two treatments (either two injectionsof PGF2alpha or CIDR plus PGF2alpha) revealed thatin the CIDR-treated group, half the females exhibited adelay in luteal development with a possible failure inovulation or luteinization. No pregnancies wereobtained with this treatment whereas several pregnancieswere obtained in the group treated with two injections ofPGF2alpha. Varying degrees of success with the use ofartificial insemination in other ungulates (bongo, eland,addax, gerenuk) have been reported (Schiewe et al. 1991;Loskutoff 2003; Penfold et al. 2005).
To test the fertility of frozen semen and to exploremethods for oestrous synchronization, we have carriedout intrauterine artificial inseminations via laparoscopyunder anaesthesia in the Mohor gazelle. As a result ofthis work, a male calf was born in 2005 after a normal
Fig. 4. Relation between inbreeding and freezing resistance of spermatozoa of Cuvier’s, Mohor and dorcas gazelles cryopreserved in a Tes-Trisbuffer with glucose, 5% egg yok and 6% glycerol; (a) Average coefficient of inbreeding of populations of the three species, (b)%motilespermatozoa, (c)% spermatozoa with intact acrosomes. ‘Freezing resistance’ values are calculated as: (values after thawing/values in freshsemen) · 100. Based on data in Garde et al. (2003)
Inbreeding and Genetic Resource Banks for Endangered Ungulates 87
� 2006 The Authors. Journal compilation � 2006 Blackwell Verlag

gestation of 202 days. The calf is currently healthy(Fig. 5) and represents the first birth of a gazelle afterartificial insemination with frozen semen (Garde JJ,Gomendio M, Espeso G, Roldan ERS, unpublisheddata). The success rate of this trial was low and, thus,although we have been able to show that artificialinsemination with frozen semen is possible, for thetechnique to be useful it is necessary to improve anumber of factors, which are currently limiting itssuccess. Once this is achieved it will be possible to usefrozen semen samples from the genetic resource bank toenhance the genetic management of these populations.
ARTs for oocyte and embryo collection
Much emphasis has been placed on the cryopreservationand use of spermatozoa in conservation efforts relatedto the preservation of genetic resources, partly becauseof the relatively easier access to male gametes thanfemale gametes. However, to preserve as much geneticdiversity as possible, it is also important to develop themethods to obtain, preserve and use oocytes andembryos, a challenge that involves additional technicaldifficulties.
One approach to maximize the female reproductivepotential is, as is performed in the cattle industry, tostimulate the production of excess number of embryosthat are then transferred to surrogate recipient females.This, however, has a number of difficulties, including theneed for an adequate number of recipients. To overcomethis limitation, efforts were devoted in the past tointerspecies embryo transfer in such a way that thefemales of domestic species or non-endangered wildlifecould serve as the recipients. Some success has beenachieved, as in the cases of a bongo calf being born to adomestic cow, or a bongo calf born to an eland cow(Pope and Loskutoff 1999).
Although in vitro fertilization (of in vivo superovulat-ed oocytes) and embryo transfer have met with somesuccess, there is more potential in in vitro oocytematuration, followed by in vitro fertilization, andembryo culture and transfer (Pukazhenthi and Wildt
2004). Success after in vitro maturation has beenachieved in some non-domestic ungulates such as waterbuffalo, gaur, klipspringer, bongo, addax, red deer andmouflon (Pope and Loskutoff 1999; Holt 2001; Ptaket al. 2002; Loskutoff 2003), but progress has been slowbecause of a number of technical difficulties related,among others, to adequate conditions for oocytematuration or embryo culture in vitro. In vitro producedembryos may also present problems related to cyto-genetic abnormalities, alterations in foetal developmentand abortion, or perinatal losses and large offspring(Loskutoff 2003). Furthermore, inbreeding may negat-ively affect the quality of oocytes and embryos pro-duced, as it happens with spermatozoa. There is yet notmuch evidence on this issue but data from cattle suggestthat inbreeding does lower ovarian responses and theyield of transferable embryos (Alvarez et al. 2005).
Little has been performed so far on the collection andpreservation of antelope oocytes or in the in vitroproduction of antelope embryos. We have begun aprogramme to obtain, mature, fertilize and culturein vitro oocytes from the endangered gazelles to allowfor the preservation of female gametes and embryos in agenetic resource bank. Initial results have shown thefeasibility of FSH-induced ovarian stimulation ofMohor gazelles and the semi-laparoscopic recovery ofoocytes, followed by in vitro maturation and fertilizationand embryo development in vitro. Although the embryodevelopment was limited, reaching only the 6–8 cellstage (Berlinguer F, Gonzalez R, Succu S, del Olmo A,Garde JJ, Espeso G, Gomendio M, Ledda S, RoldanERS, unpublished data), this constitutes a promisingresult towards the establishment of genetic resourcebanks in these endangered gazelles.
In vitro maturation and fertilization represent animportant technology to rescue oocytes from femalesthat die prematurely or undergo ovariohysterectomy.We have started a programme to rescue oocytes fromanimals that die in the captive breeding programme.Initial studies have allowed us to obtain oocytes 7 hafter the death of a dorcas gazelle who died of naturalcauses and cultured them in vitro for 24 h in TCM-199with 10% heat-treated oestrous sheep serum, 10 lg/mlovine FSH/LH, 1 lg/ml estradiol and 0.1 mg/ml gluta-mine at 38.5�C under 5% CO2 in air achieving 50%maturation of oocytes (Fig. 6) (Ledda S, Berlinguer F,Succu S, Gonzalez R, del Olmo A, Espeso G, GomendioM, Roldan ERS, unpublished data). Although these arepreliminary results, they demonstrate the feasibility ofrescuing some genetic material from valuable endan-gered animals.
ARTs for the future
Cloning by nuclear transfer is perhaps one obviousdevelopment in the future of ARTs for endangeredspecies. This technology now receives considerableattention in domestic animals both for its potential ofreproducing outstanding individuals and applications inpharmaceutical industries. It has been shown thatnuclear transfer can be used in non-domestic ungulatesas demonstrated by the birth of a gaur (Lanza et al.2000) and a banteng calf using cow oocytes and
Fig. 5. First gazelle calf born after artificial insemination withcryopreserved spermatozoa
88 ERS Roldan, M Gomendio, JJ Garde, G Espeso, S Ledda, F Berlinguer, A del Olmo, AJ Soler, L Arregui, C Crespoand R Gonzalez
� 2006 The Authors. Journal compilation � 2006 Blackwell Verlag

surrogate mothers, and mouflons using sheep oocytesand recipient mothers (Loi et al. 2001).
The advantages and the disadvantages of usingnuclear transfer for species conservation are beingdebated. Both technological aspects and conceptualissues have been identified and analysed in some detail(Critser et al. 2003; Pukazhenthi and Wildt 2004).Among the technical aspects, factors such as nuclearpreparation and reprogramming, oocyte maturation andactivation, nuclear–mitochondrial interactions andembryo-foetus development are the important areas toimprove upon. Of particular importance for endangeredspecies is the limited availability of recipient oocytes andsurrogate females that will carry the embryo to term.Perhaps a more heated debate is generated by theconceptual issues that relate to the use of nucleartransfer in endangered wildlife. One frequent criticism inthis context is the perception that nuclear transfertechniques may reduce genetic diversity whereas, in fact,it could be argued that this technology could allow thereproduction of individuals that would never have suchchance as, for example, in the case of animals that diebefore puberty. Furthermore, it may be possible tocollect and preserve tissue samples from the animals inthe wild (without actually removing the animal from itshabitat) and use these cells for the generation of animalsfor captive breeding programmes that will then breednaturally or using more conventional technologies.There is also the perception that resources used inARTs could be better spent in habitat preservation or,for some social groups, a dislike for invasive technol-ogies. These arguments may oppose, or outbalance,under certain circumstances, the value of reproductivebiotechnologies for the genetic management and con-servation of endangered species and should be takeninto consideration.
The currently accepted consesus thus far regardingcloning seems to be that nuclear transfer is a challengingtechnology, and that we must ask what can be learnt asthe technology develops and how it could fit within therepertoire of various assisted reproductive technologies(Critser et al. 2003; Pukazhenthi and Wildt 2004). Asthere is still much to be learnt about the technical and
the biological factors that affect the success of cloningby nuclear transfer, efforts in this direction will help toobtain a better understanding of reproductive processes.Based on the potential benefits of using nuclear transfer,it will be advisable to collect and preserve somatictissues and cells as part of a general strategy of genomeresource banking of endangered species.
Conclusions
The organization of genetic resource banks for thepreservation of genetic diversity is now a priority formany endangered species. Much can be gained by thesystematic and organized collection and preservation ofgametes, embryos and somatic tissues, with a widerepresentation of genotypes that can be used to manageand preserve this genetic diversity for the future bymeans of assisted reproductive techniques. As part ofthis endeavour, it is essential to take the opportunity tolearn about the basic reproductive biology of the speciesto be preserved. The idea of samples collected (and used)in a genetic resource bank should not be restricted toanimals in captive breeding programmes (in zoos orfauna reserves), but should also include animals living inthe wild. Furthermore, every effort should be made torescue and preserve biomaterials from animals dyingaccidentally, or through culls, or management pro-grammes, especially if they have not experienced areproductive opportunity. Use of ARTs, and exchangeof genetic materials should, therefore, not be restrictedto animals in captive breeding programmes but need toinclude free-living animals. Only in this way would themaximum of genetic diversity be managed and con-served.
Acknowledgements
Funding from the Spanish Ministry of Education and Science(REN2000-1470; REN 2003-01587), Acciones Integradas(HI20030336), the National Institute for Agricultural and FoodResearch and the Ministry of the Environment is acknowledged, aswell as support from Ford Espana (Novauto). We thank EulaliaMoreno, Jesus Benzal and Andres Barbosa from the EEZA-CSIC, andstaff at the Parque de Rescate de Fauna Sahariana (EEZA-CSIC) inAlmeria. We are grateful to Juana Ortiz, Aurelio Malo, Sara Succuand Federica Ariu for their help during the work summarized here.
ReferencesAbaigar T, Holt WV, 2001: Towards the development of agenetic resource bank for the Mohor gazelle: putting theoryinto practice. In: Watson PF, Holt WV (eds), Cryobankingthe Genetic Resource. Wildlife Conservation for the Future?Taylor and Francis, London, pp. 123–139.
Acevedo-Whitehouse K, Gulland F, Greig D, Amos W, 2003:Disease susceptibility in California sea lions. Nature 422,
35.Alvarez RH, Barbosa da Silva MVG, Pereira de Carvalho JB,Binelli M, 2005: Effects of inbreeding on ovarian responsesand embryo production from superovulated Mantiqueirabreed cows. Theriogenology 64, 1669–1676.
Balmford A, 2000: Priorities for captive breeding – whichmammals should board the ark? In: Entwistle A, DunstoneN (eds), Priorities for the Conservation of MammalianDiversity. Cambridge University Press, Cambridge, pp. 291–307.
Fig. 6. Oocytes from dorcas gazelle, after 24 h of in vitro maturation
Inbreeding and Genetic Resource Banks for Endangered Ungulates 89
� 2006 The Authors. Journal compilation � 2006 Blackwell Verlag

Balloux F, Amos W, Coulson T, 2004: Does heterozygosityestimate inbreeding in real populations? Mol Ecol 13, 3021–3031.
Barbosa A, Espeso G, 2005: International Studbook of Gazelladama mhorr. Estacion Experimental de Zonas Aridas. CSIC,Almeria.
Berlinguer F, Ledda S, Rosati I, Bogliolo L, Leoni G, NaitanaS, 2003: Superoxide dismutase affects the viability of thawedEuropean mouflon (Ovis g. musimon) semen and theheterologous fertilization using both IVF and intracytoplas-matic sperm injection. Reprod Fertil Dev 15, 19–25.
Bissett C, Bernard RT, 2005: The effect of prolonged coldstorage of eland (Taurotragus oryx) cauda epididymides onthe spermatozoa: possible implications for the conservationof biodiversity. Theriogenology 63, 1592–1604.
Boever J, Knox D, Merilan C, Read B, 1980: Estrus inductionand artificial insemination with successful pregnancy inSpeke’s gazelle. Proc 9th Int Congr Anim Reprod ArtifInsem 2, 565–569.
Caro TM, Laurenson MK, 1994: Ecological and geneticfactors in conservation: a cautionary tale. Science 263, 485–486.
Cassinello J, Abaigar T, Gomendio M, Roldan ERS, 1998:Characteristics of the semen of three endangered species ofgazelles. J Reprod Fertil 113, 35–45.
Cassinello J, Gomendio M, Roldan ERS, 2001: The relation-ship between coefficient of inbreeding and parasite burden inendangered ungulates. Conserv Biol 15, 1171–1174.
Caughley G, 1994: Directions in conservation biology. J AnimEcol 63, 215–244.
Charlesworth D, Charlesworth B, 1987: Inbreeding depressionand its evolutionary consequences. Annu Rev Ecol Syst 18,237–268.
Citino SB, 2003: Bovidae (except sheep and goats) andAntilocapridae. In: Fowler ME, Miller RE (eds), Zoo andWildlife Animal Medicine, 5th edn. WB Saunders, Philadel-phia, pp. 649–674.
Coltman DW, Don Bowen W, Wright JM, 1998: Birth weightand neonatal survival of harbour seal pups are positivelycorrelated with genetic variation measured by microsatel-lites. Proc Roy Soc Lond B 265, 803–809.
Coltman DW, Pilkington JG, Smith JA, Pemberton JM, 1999:Parasite-mediated selection against inbred soay sheep in afree-living, island population. Evolution 53, 1259–1267.
Coltman DW, Slate J, 2003: Microsatellite measures ofinbreeding: a meta-analysis. Evolution 57, 971–983.
Coulson T, Albon SD, Slate J, Pemberton J, 1999: Microsat-ellite loci reveal sex-dependent responses to inbreeding andoutbreeding in red deer calves. Evolution 53, 1951–1960.
Coulson TN, Pemberton JM, Albon SD, Beaumont M,Marshall TC, Slate J, Guinness FE, Clutton-Brock TH,1998: Microsatellites reveal heterosis in red deer. Proc RoySoc Lond B 265, 489–495.
Critser JK, Riley LK, Prather RS, 2003: Application ofnuclear transfer technology to wildlife species. In: Holt WV,Pickard A, Rodger JC, Wildt DE (eds), ReproductiveScience and Integrated Conservation. Cambridge UniversityPress, Cambridge, pp. 195–208.
Densmore MA, Owen MJ, Magyar SJ, Amos MS, RobinsonRM, Harms PG, Kraemer DC, 1987: Artificial inseminationwith frozen, thawed semen and pregnancy diagnosis inaddax (Addax nasomaculatus). Zoo Biol 6, 21–29.
Gage MJG, Surridge AK, Tomkins JL, Green E, Wiskin L,Bell DJ, Hewitt GM, 2006: Reduced heterozygosity depres-ses sperm quality in wild rabbits, Oryctolagus cuniculus.Curr Biol 16, 612–617.
Garde JJ, Martınez-Pastor F, Gomendio M, Malo AF, SolerAJ, Fernandez-Santos MR, Esteso MC, Anel L, RoldanERS, 2006: The application of reproductive technologies to
natural populations of red deer. Reprod Dom Anim41(Suppl), 93–102.
Garde JJ, Soler AJ, Cassinello J, Crespo C, Malo AF, EspesoG, Gomendio M, Roldan ERS, 2003: Sperm cryopreserva-tion in three species of endangered gazelles (Gazella cuvieri,Gazella dama mhorr and Gazella dorcas neglecta). BiolReprod 69, 602–611.
Garland P, Frazer L, Sanderson N, Mehren K, Kroetsch T,1992: Artificial insemiantion of scimitar-horned oryx atOmaha Park with frozen semen from Metro Toronto Zoo.Symp Zool Soc Lond 64, 37–43.
Gomendio M, Cassinello J, Roldan ERS, 2000: A comparativestudy of ejaculate traits in three endangered ungulates withdifferent levels of inbreeding: fluctuating asymmetry as anindicator of reproductive and genetic stress. Proc R SocLond B 267, 875–882.
Gomendio M, Harcourt AH, Roldan ERS, 1998: Spermcompetition in mammals. In: Birkhead TR, Møller AP (eds),Sperm Competition and Sexual Selection. Academic Press,London, pp. 667–751.
Hansson B, Westerberg L, 2002: On the correlation betweenheterozygosity and fitness in natural populations. Mol Ecol11, 2467–2474.
Hansson B, Westerdahl H, Hasselquist D, Akesson M, BenschS, 2004: Does linkage disequilibrium generate heterozygos-ity-fitness correlations in great reed warblers? Evolution 58,870–879.
Hedrick PW, Kalinowski ST, 2000: Inbreeding depression inconservation biology. Annu Rev Ecol Syst 31, 139–162.
Herrick JR, Bartels P, Krisher RL, 2004: Postthaw evaluationof in vitro function of epididymal spermatozoa from fourspecies of free-ranging African bovids. Biol Reprod 71, 948–958.
Hoglund J, Piertney SB, Alatalo RV, Lindell J, Lundberg A,Rintamaki PT, 2002: Inbreeding depression and male fitnessin black grouse. Proc R Soc Lond B 269, 711–715.
Holt WV, 2001: Germplasm preservation in elephants and wildungulates. In: Watson PF, Holt WV (eds), Cryobanking theGenetic Resource. Wildlife Conservation for the Future?Taylor and Francis, London, pp. 317–348.
Holt WV, Abaigar T, Jabbour HN, 1996a: Oestrus synchron-ization, semen preservation and artificial insemination in theMohor gazelle (Gazella dama mhorr) for the establishmentof a genome resource bank programme. Reprod Fertil Dev8, 1215–1222.
Holt WV, Bennett PM, Volobouev V, 1996b: Genetic resourcebanks in wildlife conservation. J Zool Lond 238, 531–544.
Holt WV, Moore HDM, North RD, Hartman TD, HodgesJK, 1988: Hormonal and behavioural detection of oestrus inblackbuck (Antilopre cervicapra) and successful artificialinsemination with fresh and frozen semen. J Reprod Fertil82, 717–725.
Howard J, Pursel VG, Wildt D, Chakraborty P, Bush M, 1981:Comparative of various extenders for freeze-preservation ofsemen from selective captive wild ungulates. J Am Vet MedAssoc 179, 1157–1161.
IUCN 2006: IUCN red list of threatened animals. Available inhttp://www.redlist.org/.
Jabbour HN, Argo CM, Brinklow BR, Loudon ASI, HootonJ, 1993: Conception rates following intrauterine insemin-ation of European (Dama dama dama) fallow deer does withfresh or frozen-thawed Mesopotamian (Dama dama meso-potamica) fallow deer spermatozoa. J Zool 230, 379–384.
Kouba AJ, Atkinson MW, Gandolf AR, Roth TL, 2001:Species-specific sperm-egg interaction affects the utility of aheterologous bovine in vitro fertilization system for evalu-ating antelope sperm. Biol Reprod 65, 1246–1251.
Lanza RP, Cibelli JB, Diaz F, Moraes CT, Farin PW, FarinCE, Hammer CJ, West MD, Damiani P, 2000: Cloning of
90 ERS Roldan, M Gomendio, JJ Garde, G Espeso, S Ledda, F Berlinguer, A del Olmo, AJ Soler, L Arregui, C Crespoand R Gonzalez
� 2006 The Authors. Journal compilation � 2006 Blackwell Verlag

an endangered species (Bos gaurus) using interspeciesnuclear transfer. Cloning 2, 79–90.
Loi P, Ptak G, Barboni B, Fulka J Jr, Cappai P, Clinton M,2001: Genetic rescue of an endangered mammal by cross-species nuclear transfer using post-mortem somatic cells.Nat Biotechnol 19, 962–964.
Loskutoff NM, 2003: Role of embryo technologies in geneticmanagement and conservation of wildlife. In: Holt WV,Pickard AR, Rodger JC, Wildt DE (eds), ReproductiveScience and Integrated Conservation. Cambridge UniversityPress, Cambridge, pp. 147–165.
Loskutoff NM, Bartels P, Meintjes M, Godke RA, SchieweMC, 1995: Assited reproductive technology in nondomesticungulates: a model approach to preserving and managinggenetic diversity. Theriogenology 43, 3–12.
Loskutoff NM, Simmons HA, Goulding M, Thompson G,Jongh TD, Simmons LG, 1996: Species and individualvariations in cryoprotectant toxicities and freezing resistanceof epididymal sperm from African antelope. Anim ReprodSci 42, 527–535.
Mace GM, Balmford A, 2000: Patterns and processes incontemporary mammalian extinction. In: Entwistle A,Dunstone N (eds), Priorities for the Conservation ofMammalian Diversity. Cambridge University Press, Cam-bridge, pp. 27–52.
Mackinnon K, 2000: Never say die: fighting species extinction.In: Entwistle A, Dunstone N (eds), Priorities for theConservation of Mammalian Diversity. Cambridge Univer-sity Press, Cambridge, pp. 335–353.
Martinez-Pastor F, Guerra C, Kaabi M, Garcia-Macias V, dePaz P, Alvarez M, Herraez P, Anel L, 2005: Season effect ongenitalia and epididymal sperm from Iberian red deer, roedeer and Cantabrian chamois. Theriogenology 63, 1857–1875.
May RM, 1995: The cheetah controversy. Nature 374, 309–310.
May RM, Lawton JH, Stork NE, 1995: Assessing extinctionrates. In: Lawton JH, May RM (eds), Extinction Rates.Oxford University Press, Oxford, pp. 1–24.
Menotti-Raymond M, O’Brien SJ, 1993: Dating the geneticbottleneck of the African cheetah. Proc Natl Acad Sci USA90, 3172–3176.
MerolaMA, 1994:A reassessment of homozygosity and the casefor inbreeding depression in the cheetah, Acinonyx jubatus:implications for conservation. Conserv Biol 8, 961–971.
Mitton JB, 1993: Theory and data pertinent to the relationshipbetween heterozygosity and fitness. In: Thornhill NW (ed.),The Natural History of Inbreeding and Outbreeding.University of Chicago Press, Chicago, pp. 17–41.
Monfort SL, 2003: Non-invasive endocrine measures ofreproduction and stress in wild populations. In: Holt WV,Pickard A, Rodger JC, Wildt DE (eds), ReproductiveScience and Integrated Conservation. Cambridge UniversityPress, Cambridge, pp. 145–165.
Monfort SL, Asher GW, Wildt DE, Wood TC, Schiewe MC,Williamson LR, Bush M, Rall WF, 1993: Successfulintrauterine insemination of Eld’s deer (Cervus eldi thamin)with frozen-thawed spermatozoa. J Reprod Fertil 99, 459–465.
Morrow CJ, Wolfe BA, Roth TL, Wildt DE, Bush M, BlumerES, Atkinson MW, Monfort SL, 2000: Comparing ovula-tion synchronization protocols for artificial insemination inthe scimitar-horned oryx (Oryx dammah). Anim Reprod Sci59, 71–86.
O’Brien SJ, Wildt DE, Goldman D, Merril CR, Bush M, 1983:The cheetah is depauperate in genetic variation. Science 221,459–462.
O’Brien SJ, Roelke ME, Newman A, Winkler CA, Meltzer D,Colly L, Everman J, Bush M, Wildt DE, 1985: Genetic basis
for species vulnerability in the cheetah. Science 227, 1428–1434.
O’Brien SJ, Wildt DE, Bush M, Caro TM, FitzGibbon C,Aggundey I, Leakey RE, 1987: East African cheetahs:evidence for two population bottlenecks? Proc Natl AcadSci USA 84, 508–511.
Pemberton JM, 2004: Measuring inbreeding depression in thewild: the old ways are the best. Trends Ecol Evol 19, 613–615.
Penfold LM, L Monfort S, Wolfe BA, Citino SB, Wildt DE,2005: Reproductive physiology and artificial inseminationstudies in wild and captive gerenuk (Litocranius walleriwalleri). Reprod Fertil Dev 17, 707–714.
Pickard AR, 2003: Reproductive and welfare monitoring forthe management of ex situ populations. In: Holt WV,Pickard A, Rodger JC, Wildt DE (eds), ReproductiveScience and Integrated Conservation. Cambridge UniversityPress, Cambridge, pp. 132–146.
Pickard AR, Abaigar T, Green DI, Holt WV, Cano M, 2001:Hormonal characterization of the reproductive cycle andpregnancy in the female Mohor gazelle (Gazella damamhorr). Reproduction 122, 571–580.
Pope CE, Loskutoff NM, 1999: Embryo transfer and sementechnology from cattle applied to nondomestic artiodactyls.In: Fowler ME, Miller RE (eds), Zoo and Wildlife Medicine.WB Saunders, Philadelphia, pp. 597–604.
Ptak G, Clinton M, Barboni B, Muzzeddu M, Cappai P,Tischner M, Loi P, 2002: Preservation of the wild Europeanmouflon: the first example of genetic management using acomplete program of reproductive biotechnologies. BiolReprod 66, 796–801.
Pukazhenthi B, Comizzoli P, Travis AJ, Wildt DE, 2006:Applications of emerging technologies to the study ofconservation of threatened and endangered species. ReprodFertil Dev 18, 77–90.
Pukazhenthi B, Wildt DE, 2004: Which reproductive technol-ogies are most relevant to studying, managing and conser-ving wildlife? Reprod Fertil Dev 16, 33–46.
Ralls K, Ballou JD, 1986: Captive breeding programs forpopulations with a small number of founders. Trends EcolEvol 1, 19–22.
Ralls K, Brugger K, Ballou JD, 1979: Inbreeding and juvenilemortality in small populations of ungulates. Science 206,
1101–1103.Reid JM, Arcese P, Keller LF, 2003: Inbreeding depressesimmune response in song sparrows (Melospiza melodia):direct and inter-generational effects. Proc R Soc Lond B270, 2151–2157.
Roldan ERS, Cassinello J, Abaigar T, Gomendio M, 1998:Inbreeding, fluctuating asymmetry, and ejaculate quality inan endangered ungulate. Proc R Soc Lond B 265, 243–248.
Rossiter SJ, Jones G, Ransome RD, Barrat EM, 2001:Outbreeding increases offspring survival in wild greaterhorseshoe bats (Rhinolophus ferrumequinum). Proc R SocLond B 268, 1055–1061.
Roth TL, Bush LM, Wildt DE, Weiss RB, 1999: Scimitar-horned oryx (Oryx dammah) spermatozoa are functionallycompetent in a heterologous bovine in vitro fertilizationsystem after cryopreservation on dry ice, in a dry shipper, orover liquid nitrogen vapor. Biol Reprod 60, 493–498.
Roth TL, Weiss RB, Buff JL, Bush LM, Wildt DE, Bush M,1998: Heterologous in vitro fertilization and spermcapacitation in an endangered african antelope, the scimi-tar-horned oryx (Oryx dammah). Biol Reprod 58, 475–482.
Saccheri I, Kuussaari M, Kankare M, Vikman P, Fortelius W,Hanski I, 1998: Inbreeding and extinction in a butterflymetapopulation. Nature 392, 491–494.
Santiago-Moreno J, Toledano-Diaz A, Pulido-Pastor A,Gomez-Brunet A, Lopez-Sebastian A, 2006: Birth of live
Inbreeding and Genetic Resource Banks for Endangered Ungulates 91
� 2006 The Authors. Journal compilation � 2006 Blackwell Verlag

Spanish ibex (Capra pyrenaica hispanica) derived fromartificial insemination with epididymal spermatozoaretrieved after death. Theriogenology 66, 283–291.
Saragusty J, Gacitua H, King R, Arav A, 2006: Post-mortemsemen cryopreservation and characterization in two differ-ent endangered gazelle species (Gazella gazella and Gazelladorcas) and one subspecies (Gazella gazelle acaiae). Theri-ogenology (in press).
Schiewe MC, Bush M, Phillips LG, Citino SB, Wildt DE,1991: Comparative aspects of estrus synchronization, ovu-lation induction and embryo cryopreservation in the scimi-tar-horned oryx, bongo, eland and geater kudu. J Exp Zool258, 75–88.
Slate J, David P, Dodds KG, Veenvliet BA, Glass BC, BroadTE, McEwan JC, 2004: Understanding the relationshipbetween the inbreeding coefficient and multilocus hetero-zygosity: theoretical expectations and empirical data. Her-edity 93, 255–265.
Slate J, Kruuk LEB, Marshall TC, Pemberton JM, Clutton-Brock TH, 2000: Inbreeding depression influences lifetimebreeding success in a wild population of red deer (Cervuselaphus). Proc R Soc Lond B 67, 1657–1662.
Slate J, Pemberton J, 2002: Comparing molecular measures fordetecting inbreeding depression. J Evol Biol 15, 20–31.
Soler AJ, Esteso MC, Fernandez-Santos MR, Garde JJ, 2005:Characteristics of Iberian red deer (Cervus elaphus hispani-cus) spermatozoa cryopreserved after storage at 5�C in theepididymis for several days. Theriogenology 64, 1503–1517.
Soler AJ, Garcia AJ, Fernandez-Santos MR, Esteso MC,Garde JJ, 2003: Effects of thawing procedure on postthawedin vitro viability and in vivo fertility of red deer epididymalspermatozoa cryopreserved at )196�C. J Androl 24, 746–756.
Soler AJ, Garde JJ, 2003: Relationship between the charac-teristics of epididymal red deer spermatozoa and penetra-bility into zona-free hamster ova. J Androl 24, 393–400.
Thornhill NW, 1993: The Natural History of Inbreeding andOutbreeding. The University of Chicago Press, Chicago.
Watson PF, Holt WV (eds) 2001: Cryobanking the GeneticResource. Wildlife Conservation for the Future? Taylor andFrancis, London.
Wildt DE, 1992: Genetic resource banks for conservingwildlife species: justification, examples and becoming organ-ized on a global basis. Anim Reprod Sci 28, 247–257.
Wildt DE, Bush M, Goodrowe KL, Packer C, Pusey AE,Brown JL, Joslin P, O’Brien SJ, 1987b: Reproductive andgenetic consequences of founding isolated lion populations.Nature 329, 328–331.
Wildt DE, Bush M, Howard JG, O’Brien SJ, Meltzer D, vanDyk A, Ebedes H, Brand DJ, 1983: Unique seminal qualityin the south african cheetah and a comparative evaluation inthe domestic cat. Biol Reprod 29, 1019–1025.
Wildt DE, O’Brien SJ, Howard JG, Caro TM, Roelke ME,Brown JL, Bush M, 1987a: Similarity in ejaculate-endocrinecharacteristics in captive versus free-ranging cheetahs of twosubspecies. Biol Reprod 36, 351–360.
Wildt DE, Rall WF, Critser JK, Monfort SL, Seal US, 1997:Genome resource banks. Living collections for biodiversityconservation. Bioscience 47, 689–698.
Wildt DE, Wemmer C, 1999: Sex and wildlife: the role ofreproductive science in conservation. Biodivers Conserv 8,965–976.
Wright S, 1922: Coefficients of inbreeding and relationship.Am Nat 56, 330–338.
Yanagimachi R, 1984: Zona-free hamster eggs: Their use inassessing fertilizing capacity and examining chromosomes ofhuman spermatozoa. Gamete Res 10, 187–232.
Submitted: 05 May 2006
Author’s address (for correspondence): Eduardo RS Roldan, Grupode Ecologıa y Biologıa de la Reproduccion, Museo Nacional deCiencias Naturales (CSIC), c/Jose Gutierrez Abascal 2, 28006 Madrid,Spain. E-mail: [email protected]
92 ERS Roldan, M Gomendio, JJ Garde, G Espeso, S Ledda, F Berlinguer, A del Olmo, AJ Soler, L Arregui, C Crespoand R Gonzalez
� 2006 The Authors. Journal compilation � 2006 Blackwell Verlag