Ig VENA - kongres.uakis.org.rs
Transcript of Ig VENA - kongres.uakis.org.rs

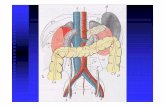
Ig VENAH U M A N N O R M A L I M M U N O G L O B U L I N

2 Ig VENA DESCRIPTION
THERAPEUTIC INDICATIONS(1)
REPLACEMENT THERAPY in adults, children and adolescents (0-18 years) in:
• Primary immunodeficiency syndromes with impaired antibody production (PID)
• Secondary immunodeficiencies (SID) in patients who suffer from severe or recurrent infections, ineffective antimicrobial treatment and either proven specific antibody failure (PSAF) or serum IgG level of <4 g/l
IMMUNOMODULATION in adults, children and adolescents (0-18 years) in:
Neurology
• Guillain Barré syndrome (GBS)
• Chronic inflammatory demyelinating poliradiculoneuropathy (CIDP)
• Multifocal motor neuropathy (MMN)
Other autoimmune disorders
• Primary immune thrombocytopenia (ITP), in patients at high risk of bleeding or prior to surgery to correct the platelet count
• Kawasaki disease (in conjunction with acetylsalicylic acid)
PHARMACOLOGICAL PROPERTIES(1)
• Ig VENA contains mainly immunoglobulin G (IgG ≥98%) with a broad spectrum of antibodies against infectious agents(2)
• The distribution of immunoglobulin G subclasses is closely proportional to that in native human plasma
• Prepared from pooled plasma from not fewer than 1000 donations
• Adequate doses may restore abnormally low immunoglobulin G levels to their normal range
• Immediately and completely bioavailable in the recipient’s circulation after intravenous administration
1. Ig VENA summary of product characteristics (SmPC) - 2019. 2. Analisi dei dati di composizione proteica e contenuto IgA Ig VENA 50 g/l 01/01/2018 al 01/04/2020.

3Ig VENA KEDRION QUALITY PROGRAMME*
• Compliance with Good Manufacturing Practice (GMP), as certified by the Italian Medicines Authority (AIFA)
STEP 1Selected donors
STEP 2Testing of each single
plasma donation
STEP 3Inventory Hold and
Look Back procedures
STEP 4Nucleic Acid Test (NAT)
on plasma poolsSTEP 5
GMP
STEP 6Validated viral
inactivations/removal
STEP 7Official Batch Release
STEP 8Pharmacovigilance
• Regular inspections by Italian and international health authorities
Viral inactivation and removal steps are validated in accordance with national and international guidelines
Viral and prions clearance of IgVENA satisfy current regulatory requirements
Virus inactivation/removal steps(3)
GLOBALQUALITY
3 phases of Cohn’s cold ethanol fractionation process are VALIDATED for inactivation/removal of enveloped and non-enveloped viruses
Global Quality Programme: The measures taken are considered effective for enveloped viruses such as HIV, HBV, HCV and for the non-enveloped virus HAV. It is also assumed that the antibody content makes an important contribution to the viral safety of HAV and B19V(1)
2 manufacturing process phases are VALIDATED for inactivation of enveloped viruses: 1 treatment with solvent/detergent and 1 treatment at low pH
2 phases of the cold ethanol precipitation are also validated for prions removal
*The beginning of data collection is subject to the requirements defined in the Italian ordinance no. 4471997 (U.G. 06.03.1997, no. 54). This ordinance implements Council Directi-ve 93/39/EEC, which harmonises pharmacovigilance procedures and obligations within the European Union.1. Ig VENA summary of product characteristics (SmPC) - 2019. 3. Ig VENA, Solution for infusion – 3.2.A.2 – Adventitious Agents Safety Evaluation.

4 Ig VENA CHARACTERISTICS
Protein chemical composition of Ig VENA(1,4)
Distribution of IgG subclasses
Physiological IgG distribution
Average value Ig VENA(1)
IgG1 60% 62,1%
IgG2 32% 34,8%
IgG3 4% 2,5%
IgG4 4% 0,6%
Limits marked ** are specified by Ph.Eur, any other values are based on internal specifications.
*Data analysis for proteic composition and IgA content - Ig VENA 50 g/l.
Measured Value RequirementsAverage value
Ig VENA(4)
IgA ≤50 µg/ml 7 µg/ml (tested on 173 batches)*
IgM ≤10 μg/ml <1 μg/ml
Monomers/Dimers ≥90%** 100%
Molecular profile fragments
≤5%** 1%
Polymers ≤3%** 1%
Fc Fragment integrity ≥60%** 93%
Compatibility parameters for Ig VENA(4)
Measured Value RequirementsAverage value
Ig VENA(4)
Anticomplementary activity (ACA%)
≤50%** 34%
Prekallikrein activator(PKA)
≤35 IU/ml** 2 IU/ml
1. Ig VENA Summary of Product Characteristics (SmPC) - 2019. 4. Product quality review: liquid i.v. standard immunoglobulin (01/03/2018- 28/02/2019).

5
Other product specifications(4)
Measured Value RequirementsAverage value
Ig VENA(4)
Osmolality ≥240 mOsmol/kg** 353 mOsmol/kg
pH‡ 4.0 - 5.5* 4.0 - 4.5
Maltose§ 9 – 11% 9,6%
TNBP ≤1,2 ppm 0.4 ppm
Sodium Cholate ≤70 ppm 7 ppm
Aluminium ≤200 ppb 17ppb
§Ig VENA is stabilized with maltose instead of sucrose, which avoids the risk of renal failure(3)
Ig VENA contains approximately 3 mmol/liter (or 69 mg/liter) sodium. To be taken into consideration by patients on a controlled sodium diet(1)
Limits marked ** are specified by Ph.Eur, any other values are based on internal specifications.
1. Ig VENA Summary of Product Characteristics (SmPC) - 2019. 3. Ig VENA, Solution for infusion - 2.5 - Clinical Overview. 4. Product quality review: liquid i.v. standard immunoglobulin (01/03/2018 - 28/02/2019). 5. Kroez M et al. Hypotension with intravenous immunoglobulin therapy: importance of pH and dimer formation. Biologicals 2003; 31(4):277-286.
Antimicrobial specifications(4)
Measured Value RequirementsAverage value
Ig VENA(4)
Anti-HAV ≥7.0 IU/ml 14 IU/ml
Anti-tetanus antibody ≥10.0 IU/ml 17 IU/ml
Anti-HBsAg ≥0.05 IU/ml 2.48 IU/ml
*Limit identified by Kedrion. ‡Preparation of IVIg at a low pH substantially reduces immunoglobulin G dimerization; this effect significantly decreases the potential to induce hypotension(5).

6
Ig VENA AND REPLACEMENT THERAPYFROM GENERAL TO SPECIFIC
Ig VENA is indicated for PID(1)
Data elaborated from Figure 2 and 3 of reference (6)
The overall number of serious infections for the 20 patients during the pre-treatment was 17, whereas, the total number of serious infections for the same 20 patients observed during the treatment period was 2(7)
All patients received a dose of between 0.4-0.8 g/kg per month for each infusion(7)
Data elaborated from page 4 of reference (7)
1. Ig VENA Summary of Product Characteristics (SmPC) - 2019. 6. Orange Jordan S et al. Impact of trough IgG on pneumonia incidence in primary immunodeficiency: A meta-analysis of clinical studies. Clinical immunology 2010; 137(1): 21-30. 7. Ricci S, Lippi F, Canessa C et al. Efficacy and safety of human intravenous immunoglobulin 5% (Ig VENA) in pediatric patients affected by primary immunodeficiency. International Journal of Immunopathology and Pharmacology, 2020; 34, 205873842094300. https://doi.org/10.1177/2058738420943006.
IMPACT OF TROUGH IgG ON PNEUMONIA INCIDENCE IN PRIMARY IMMUNODEFICIENCY: A META-ANALYSIS OF CLINICAL STUDIES(6)
500 600 700 800 900 1000Trough IgG (mg/dL)
Pre-treatment Post-treatment
Pne
umon
ia in
cid
ence
per
pat
ient
-yea
rN
. of p
atie
nts
0.15
0.10
0.05
0.00
30
20
10
0
5-fold
-27%
p=0.021
Infections
No infections
Pneumonia incidence in primary immunodeficient patients progressively declined after treatment with intravenous immunoglobulins(6)
The median administered dose of IVIG was 492 mg/kg (IQR, 328–529 mg/kg) per 28 days(6)
RETROSPECTIVE OBSERVATIONAL STUDY TO ASSESS THE EFFICACY AND SAFETY OF Ig VENA IN PEDIATRIC PATIENTS AFFECTED BY PRIMARY IMMUNODEFICIENCY (PID)(7)
17 2

7
Ig VENA is indicated for SID(1)
Ig VENA protects patients with multiple myeloma in plateau phase against life-threatening infections, and could thus be useful for long-term prevention(8)
The recommended dose is 0.2-0.4 g/kg every three to four weeks. IgG trough levels should be measured and assessed in conjunction with the incidence of infection. Dose should be adjusted as necessary to achieve optimal protection against infections, an increase may be necessary in patients with persisting infection; a dose decrease can be considered when the patient remains infection free(1)
1. Ig VENA Summary of Product Characteristics (SmPC) - 2019. 8. Musto P, Brugiatelli M, Carotenuto M. Prophylaxis against infections with intravenous immunoglobulins in multiple myeloma. British Journal of Haematology 1995; 89(4):945-946.
No-treatment group(n=250 patients-months)
Ig VENA(n=261 patients-months)
Num
ber
of s
erio
us in
fect
ions
oc
curr
ed
50
40
30
20
10
0
IVIg in Secondary Immunodeficiency Syndrome (SID)(8)
2-years double cross-over study in 25 patients with MM (multiple myeloma). The patients were randomly allocated to receive a 2 h single infusion of 0.3 g/kg Ig VENA every 4 weeks for 6 months or no therapy; then they were switched to observation or Ig VENA for another 12 months; finally, they were switched again: they received Ig VENA or no therapy for a further 6 months. Data collected from text or reference(8)
30
10
p<0.002

8 Ig VENA & IMMUNOMODULATION
• IVIg is now considered as treatment of first choice by many authorities given its efficacy, ease of administration, and the low incidence of side effects of this drug(9)
• IVIg remain the elective therapy for GBS because it’s more likely to be completed than Plasma Exchange(10)
Treatment of CIDP with IVIg for 6 months is less frequently discontinued because of inefficacy, adverse events, or intolerance than was treatment with intravenous methylprednisolone(11)
Seven of eight patients who did not respond to intravenous methylpred-nisolone were shifted to Ig VENA and improved on this therapy(11)
Ten (48%) of 21 patients treated with intravenous methylprednisolone completed the 6-month study period com-pared with 21 (88%) of 24 treated with IVIg. The cumulative probability of treatment discontinuation was significantly higher with intravenous methylprednisolone than with IVIg at 15 days, 2 months, and 6 months(11)
Baseline(randomization)
0.5 1 2 3 4 5 6Time (months)
Sur
viva
l pro
bab
ility
1.0
0.8
0.6
0.4
0.2
0
Treatment with IVIg is well tolerated by the patients(11)
Intravenous methylprednisolone
p=0.008588%
48%
Data elaborated from figure 2 of reference (11)
1. Ig VENA Summary of Product Characteristics (SmPC) - 2019. 9. Kieseier, Bernd & Hartung, Hans-Peter. Therapeutic Strategies in the Guillain-Barré Syndrome. Seminars in neurology 2003; 23; 159-68. 10.1055/s-2003-41132. 10. Hughes, Richard & Swan, Anthony & Doorn, Pieter. Intravenous immunoglobulin for Guillain-Barre syndrome. Cochrane database of syste-matic reviews (Online) 2014; 7. CD002063. 10.1002/14651858.CD002063.pub5. 11. Nobile-Orazio, Eduardo et al. Intravenous immunoglobulin versus intravenous methylprednisolone for chronic inflammatory demyelinating polyradiculoneuropathy: a randomised controlled trial. The Lancet Neurology 2012; 11:493-502.
Ig VENA
Ig VENA is indicated for CIDP(1)
Ig VENA is indicated for GBS(1)

9
• Ig VENA is also efficacious and well tolerated in MMN with a proportion of responders comparable to that reported in previous controlled studies(12)
• Thirty days after the first infusion 45% of patients improved on both scales(12)
• At 6 months 83% improved on both scales(12)
*MRC; Medical Research Council Scale§ONLS; Overall Neuropathy Limitations Scale
MRC* ONLS§ MRC* ONLS§
75% 50% 100% 79%
N. o
f pat
ient
s
30
20
10
0
30 daysafter 1° infusion
6 months
Worsening
No change
Improvement (%)
1. Ig VENA Summary of Product Characteristics (SmPC) - 2019. 12. Nobile-Orazio, Eduardo et al. High-dose Ig VENA is well tolerated and efficacious in patients with multifocal motor neuropathy.Neurological Sciences 2017; 38:899-902.
Ig VENA is indicated for MMN(1)

10 Ig VENA & IMMUNOMODULATION
Ig VENA is indicated for ITP(1)
• 93.3% of patients achieved a platelet count of ≥50x109/L(13)
• Platelet counts started to increase on day 2 of treatment(13)
Mean and median values of platelet count in 15 patients with idiopathic thrombocytopenic purpura treated with Ig VENA 400 mg/kg for 5 days. Prospective, open-label phase III study(13)
1. Ig VENA Summary of Product Characteristics (SmPC) - 2019. 13. Grossi A, Balestri F, Tognoni D et al. Intravenous immunoglobulin therapy in the treatment of the acute phase of chronic idiopathic thrombocytopenic purpura in adults. Haema 2006; 9 (4): 567-57.
T0 T2 T4 T6 End Day 0 Day 2 Day 4 Day 10
Pla
tele
t co
unt
x 10
9 /L
200
150
100
50
0
Mean Median Target platelet count value

11
Ig VENA is indicated for Kawasaki disease(1)
• Physicians should treat patients with IVIg as soon as possible when a diagnosis of Kawasaki disease is strongly suspected(14)
• The American Heart Association (AHA) recommends treating with IVIg C 10 days of illness, and ideally within 7 days(16)
• A dose of IVIg of 2 g/kg in a single infusion is well established(15)
One hundred and fifty patients were studied: a group treated with IVIg on days 11 to 20 and pair matched control subjects of the same gender and age treated with IVIg on days 4 to 8 with the same dose at same institutions. Data collected from text of reference (14)
1. Ig VENA Summary of Product Characteristics (SmPC) - 2019. 14. Muta H, Ishii M, Yashiro M al. Late Intravenous Immunoglobulin Treatment in Patients With Kawasaki Disease. Pediatrics 2012; 129(2): 291-297. 15. Dhanrajani, Anita & Chan, Mercedes & Pau, Stephanie & Ellsworth, Janet & Petty, Ross & Guzman, Jaime. Aspirin Dose in Kawasaki Disease: The Ongoing Battle. Arthritis Care & Research 2017. 70. 10.1002/acr.23504. 16. Lo, MS, & Newburger, JW. Role of intravenous immunoglobulin in the treatment of Kawasaki disease. International Journal of Rheumatic Diseases 2017; 21(1): 64-69. https://doi.org/10.1111/1756-185x.13220.
Late IVIg (days 11-20) Early IVIg (days 4-8)
27%
1%
Pop
ulat
ion
of C
ALs
dur
ing
conv
ales
cent
pha
se
30%
25%
20%
15%
10%
5%
0
Proportion of coronary artery lesion (CALs)

12 PHARMACOVIGILANCE DATA
Pharmacovigilance Risk Assessment Committee (PRAC) of European Medicines Agency (EMA) confirmed the safety profile of Kedrion S.p.A human immunoglobulin(17)
Data elaborated from page 3 of reference (17)
Only the 1.8% (Kg) of worldwide delivered Ig VENA were associated with ADRs(17)
Post-marketing data refer to the following period: 01 January 2001 to 31 December 2019(17)
The reported ADRs are NOT included in the Important Medical Events (IME) list released by the European Medical Agency (EMA)(17)
NO ADRs - 98.2%
ADRs - 1.8%
17. Riassunto dati di Farmacovigilanza per IVIG KEDRION.
Percentage of IVIg in Kg delivered worldwide

13INFUSION RATE IN REPLACEMENT THERAPY
In PID patients who tolerate the infusion rate of 0.92 ml/kg/h, the rate of administration may be gradually increased to 2 ml/kg/h, 4 ml/kg/h, up to a maximum of 6 ml/kg/h, every 20-30 minutes and only if the patient tolerates the infusion well(1)
1. Ig VENA Summary of Product Characteristics (SmPC) - 2019. 18. Spadaro, Giuseppe et al. Corrigendum to “Rapid infusions of human normal immunoglobulin 50 g/l are safe and well tolerated in immunodeficiencies and immune thrombocytopenia [Int. Immunopharmacol. 44 (2017) 38-42]. International immunopharmacology 2017; 57:201.
Ig VENA rapid infusion and tolerability
1. Help to minimise the time spent in outpatient hospital visits(18)
2. Maintain patient’s quality of life(18)
PID PATIENTS, INTRAVENOUS USE(1)
Infusion rate
0.92 ml/kg/h
2 ml/kg/h
4 ml/kg/h
6 ml/kg/hMAX
START
ONLY IF
WELL TOLERATED
Every20-30
min
Every20-30
min
Every20-30
min

14 INFUSION RATE IN IMMUNOMODULATION THERAPY
Ig VENA rapid infusion and tolerabilityHelp to minimise the time spent in outpatient hospital visits(18)
Maintain patient’s quality of life(18)
Ig VENA administered at increasing infusion speed was well tolerated both in patients with ID and in those with ITP and the infusion speed could be ramped up to a maximum of 6 ml/kg/hr and, in a limited number of patients, to 8 ml/kg/hr.(1)
PATIENTS WITH ID AND ITP, INTRAVENOUS USE(1)
Infusion rate
6 ml/kg/h
8 ml/kg/hin a limited number of patients
MAX
ONLY IFWELL TOLERATED
GRADUALLY INCREASE EVERY
20-30 MIN
1. Ig VENA Summary of Product Characteristics (SmPC) - 2019. 18. Spadaro, Giuseppe et al. Corrigendum to “Rapid infusions of human normal immunoglobulin 50 g/l are safe and well tolerated in immunodeficiencies and immune thrombocytopenia [Int. Immunopharmacol. 44 (2017) 38-42]. International immunopharmacology 2017; 57:201.

15Ig VENA PACK-SIZES/STORAGETEMPERATURE(1)
• 20 ml (1 g)
• 50 ml (2.5 g)
• 100 ml (5 g)
• 200 ml (10 g)
Ig VENA is available in four vial sizes:(1)
1. Ig VENA Summary of Product Characteristics (SmPC) - 2019.
Storage & Shelf Life(1)
• Store in a refrigerator (2°C - 8°C)
• Before use and within the shelf-life, the vials of 50, 100 and 200 ml can be stored at room temperature, not exceeding 25 °C, for a maximum of 6 consecutive months
• After this period, the product must be discarded. In any case, the product can no longer be put back in the refrigerator if kept at room temperature
• Do not freeze
• Shelf life 3 years

Ig VENASUMMARY OF PRODUCT CHARACTERISTICS
1. NAME OF THE MEDICINAL PRODUCT
Ig VENA 50 g/l Solution for infusion.
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
Human normal immunoglobulin (IVIg). One ml of solution contains:Human normal immunoglobulin 50 mg(purity of at least 95% IgG).Each vial of 20 ml contains: 1 g of human normal immunoglobulin. Each vial of 50 ml contains: 2.5 g of human normal immunoglobulin. Each vial of 100 ml contains: 5 g of human normal immunoglobulin. Each vial of 200 ml contains:10 g of human normal immunoglobulin.Distribution of the IgG subclasses (approx. values):IgG1 62,1 %IgG2 34,8 %IgG3 2,5 %IgG4 0,6 %
The maximum IgA content is 50 micrograms/ml. Produced from the plasma of human donors.
Excipients with known effect:The product contains 100 mg of maltose per ml. For the full list of excipients, see section 6.1.
3. PHARMACEUTICAL FORM
Solution for infusion.The solution should be clear or slightly opalescent, colourless or pale yellow.
4. CLINICAL PARTICULARS
4.1 Therapeutic indications
Replacement therapy in adults, and children and adolescents (0-18 years) in:• Primary immunodeficiency syndromes (PID) with impaired
antibody production• Secondary immunodeficiencies (SID) in patients who suffer from
severe or recurrent infections, ineffective antimicrobial treatment and either proven specific antibody failure (PSAF)* or serum IgG level of <4 g/l
* PSAF = failure to mount at least a 2-fold rise in IgG antibody titre to pneumococcal polysaccharide and polypeptide antigen vaccines
Immunomodulation in adults, and children and adolescents (0-18 years) in:• Primary immune thrombocytopenia (ITP), in patients at high risk
of bleeding or prior to surgery to correct the platelet count• Guillain Barré syndrome• Kawasaki disease (in conjunction with acetylsalicylic acid; see
4.2)• Chronic inflammatory demyelinating poliradiculoneuropathy
(CIDP)• Multifocal motor neuropathy (MMN)
4.2 Posology and method of administration
Replacement therapy should be initiated and monitored under the supervision of a physician experienced in the treatment of immunodeficiency.
PosologyThe dose and dose regimen is dependent on the indication.The dose may need to be individualised for each patient dependent on the clinical response. Dose based on bodyweight may require adjustment in underweight or overweight patients.The following dose regimens are given as a guideline.
Replacement therapy in primary immunodeficiency syndromesThe dose regimen should achieve a trough level of IgG (measured before the next infusion) of at least 6 g/L or within the normal reference range for the population age. Three to six months are required after the initiation of therapy for equilibration (steady-state IgG levels) to occur. The recommended starting dose is 0.4-0.8 g/kg given once followed by at least 0.2g/kg given every three to four weeks.The dose required to achieve a trough level of IgG 6 g/L is of the order of 0.2-0.8 g/kg/month. The dosage interval when steady state has been reached varies from 3-4 weeks.IgG trough levels should be measured and assessed in conjunction with the incidence of infection. To reduce the rate of bacterial infections, it may be necessary to increase the dosage and aim for higher trough levels.
Secondary immunodeficiencies (as defined in 4.1.)The recommended dose is 0.2-0.4 g/kg every three to four weeks.
IgG trough levels should be measured and assessed in conjunction with the incidence of infection. Dose should be adjusted as necessary to achieve optimal protection against infections, an increase may be necessary in patients with persisting infection; a dose decrease can be considered when the patient remains infection free.
Primary immune thrombocytopeniaThere are two alternative treatment schedules:• 0.8-1 g/kg given on day one; this dose may be repeated once
within 3 days• 0.4 g/kg given daily for two to five days. The treatment can be
repeated if relapse occurs.
Guillain Barré syndrome0.4 g/kg/day over 5 days (possible repeat of dosing in case of relapse).
Kawasaki disease2.0 g/kg should be administered as a single dose. Patients should receive concomitant treatment with acetylsalicylic acid.
Chronic inflammatory demyelinating polyneuropathy (CIDP)Starting dose: 2 g/kg in 2-5 consecutive days. Maintenance doses:1 g/kg over 1-2 consecutive days every 3 weeks.The treatment effect should be evaluated after each cycle; if no treatment effect is seen after 6 months, the treatment should be discontinued.If the treatment is effective long term treatment should be subject to the physicians discretion based upon the patient response and maintenance response. The dosing and intervals may have to be adapted according to the individual course of the disease.
Multifocal Motor Neuropathy (MMN)Starting dose: 2 g/kg given over 2-5 consecutive days.Maintenance dose: 1 g/kg every 2 to 4 weeks or 2 g/kg every 4 to 8 weeks.The treatment effect should be evaluated after each cycle; if no treatment effect is seen after 6 months, the treatment should be discontinued.If the treatment is effective long term treatment should be subject to the physicians discretion based upon the patient response and maintenance response. The dosing and intervals may have to be adapted according to the individual course of the disease.
The dosage recommendations are summarised in the following table:
Indication Dose Frequency of injections
Replacement therapyPrimary immunodeficiencysyndromes
Starting dose: 0.4 - 0.8 g/kg
every 3 - 4 weeksMaintenance dose:0.2- 0.8 g/kg

Secondary Immunodeficiencies (as defined in 4.1.)
0.2 - 0.4 g/kg every 3 - 4 weeks
Immunomodulation:
Primary immune thrombocytopenia
0.8 -1 g/kg on day 1, possibly repeated once within 3 days
Or0.4 g/kg/d
for 2 - 5 days
Guillan Barré syndrome 0.4 g/kg/d for 5 daysKawasaki disease 2 g/kg in one dose in
association with acetylsalicylic acid
Chronic inflammatory demyelinating polyradiculoneuropathy (CIDP)
Starting dose: 2 g/kg
Maintenance dose: 1 g/kg
in divided doses over 2-5 days
every 3 weeks over 1-2 days
Multifocal Motor Neuropathy (MMN)
Starting dose: 2 g/kg
over 2-5 consecutive days
Maintenance dose: 1 g/kgor2 g/kg
every 2-4 weeksorevery 4-8 weeks over 2-5 days
Paediatric populationThe posology in children and adolescents (0-18 years) is not different to that of adults as the posology for each indication is given by body weight and adjusted to the clinical outcome of the above mentioned conditions.
Hepatic impairmentNo evidence is available to require a dose adjustment.
Renal impairmentNo dose adjustment unless clinically warranted, see section 4.4.
ElderlyNo dose adjustment unless clinically warranted, see section 4.4.
CIDPDue to the rarity of the disease and consequently the overall low number of patients, only limited experience is available of use of intravenous immunoglobulins in children with CIDP; therefore, only data from literature are available. However, published data are all consistent in showing that the IVIg treatment is equally effective in adults and children, as it is the case for the IVIg established indications.
Method of administration
For intravenous use.Human normal immunoglobulin should be infused intravenously at an initial rate of 0.46 – 0.92 ml/kg/hr (10 - 20 drops per minute) for 20 - 30 minutes. See section 4.4. In case of adverse reaction, either the rate of administration must be reduced or the infusion stopped. If well tolerated, the rate of administration may be gradually increased to a maximum of 1.85 ml/kg/hr (40 drops/minute).
In PID patients who tolerate the infusion rate of 0.92 ml/kg/hr, the rate of administration may be gradually increased to 2 ml/kg/hr, 4 ml/kg/hr, up to a maximum of 6 ml/kg/hr, every 20-30 minutes and only if the patient tolerates the infusion well.
In general, dosage and infusion rates have to be individually tailored according to the patient’s needs. Depending on body weight, dosage and occurrence of adverse reactions, the patient may not reach the maximum infusion speed. In case of adverse reactions, the infusion should be immediately stopped and it should be resumed at the appropriate infusion rate for the patient.
See also paragraph 6.6
Special populationsIn paediatric patients (0-18 years) and elderly (>64 years of age),
the initial rate of administration should be 0.46 – 0.92 ml/kg/hr (10 - 20 drops per minute) for 20 - 30 minutes. If well tolerated and considering patient’s clinical conditions, the rate may be gradually increased to a maximum of 1.85 ml/kg/hr (40 drops/minute).
4.3 Contraindications
Hypersensitivity to the active substance (human immunoglobulins) or to any of the excipients (see sections 4.4 and 6.1).Patients with selective IgA deficiency who developed antibodies to IgA, as administering an IgA- containing product can result in anaphylaxis.
4.4 Special warnings and precautions for use
This medicinal product contains 100 mg of maltose per ml as an excipient. The interference of maltose in blood glucose assays may result in falsely elevated glucose readings and, consequently, in the inappropriate administration of insulin, resulting in life-threatening hypoglycaemia and death. Also, cases of true hypoglycaemia may go untreated if the hypoglycaemic state is masked by falsely elevated glucose readings. For further details, see section 4.5. For acute renal failure see below.
This medicinal product contains approximately 3 mmol/liter (or 69 mg/liter) sodium. To be taken into consideration by patients on a controlled sodium diet.
TraceabilityIn order to improve the traceability of biological medicinal products, the name and the batch number of the administered product should be clearly recorded.
Precautions for usePotential complications can often be avoided by ensuring that patients:• are not sensitive to human normal immunoglobulin by initially
injecting the product slowly (rate of administration 0.46 – 0.92 ml/kg/hr);
• are carefully monitored for any symptoms throughout the infusion period. In particular, patients naive to human normal immunoglobulin, patients switched from an alternative IVIg product or when there has been a long interval since the previous infusion should be monitored at the hospital during the first infusion and for the first hour after the first infusion, in order to detect potential adverse signs. All other patients should be observed for at least 20 minutes after administration.
In all patients, IVIg administration requires:• adequate hydration prior to the initiation of the infusion of IVIg• monitoring of urine output• monitoring of serum creatinine levels• avoidance of concomitant use of loop diuretics (see 4.5).
In case of adverse reaction, either the rate of administration must be reduced or the infusion stopped. The treatment required depends on the nature and severity of the adverse reaction.
Infusion reactionCertain adverse reactions (e.g. headache, flushing, chills, myalgia, wheezing, tachycardia, lower back pain, nausea, and hypotension) may be related to the rate of infusion. The recommended infusion rate given under section 4.2 must be closely followed. Patients must be closely monitored and carefully observed for any symptoms throughout the infusion period.
Adverse reactions may occur more frequently• in patients who receive human normal immunoglobulin
for the first time or, in rare cases, when the human normal immunoglobulin product is switched or when there has been a long interval since the previous infusion
• in patients with an untreated infection or underlying chronic inflammation
HypersensitivityHypersensitivity reactions are rare.Anaphylaxis can develop in patients:

• with undetectable IgA who have anti-IgA antibodies• who had tolerated previous treatment with human normal
immunoglobulin.
In case of shock, standard medical treatment for shock should be implemented.
ThromboembolismThere is clinical evidence of an association between IVIg administration and thromboembolic events such as myocardial infarction, cerebral vascular accident (including stroke), pulmonary embolism and deep vein thromboses which is assumed to be related to a relative increase in blood viscosity through the high influx of immunoglobulin in at-risk patients. Caution should be exercised in prescribing and infusing IVIg in obese patients and in patients with pre-existing risk factors for thrombotic events (such as advanced age, hypertension, diabetes mellitus and a history of vascular disease or thrombotic episodes, patients with acquired or inherited thrombophilic disorders, patients with prolonged periods of immobilisation, severely hypovolemic patients, patients with diseases which increase blood viscosity).
In patients at risk for thromboembolic adverse reactions, IVIg products should be administered at the minimum rate of infusion and dose practicable.
Acute renal failureCases of acute renal failure have been reported in patients receiving IVIg therapy. In most cases, risk factors have been identified, such as pre-existing renal insufficiency, diabetes mellitus, hypovolemia, overweight, concomitant nephrotoxic medicinal products or age over 65.
Renal parameters should be assessed prior to infusion of IVIg, particularly in patients judged to have a potential increased risk for developing acute renal failure, and again at appropriate intervals. In patients at risk for acute renal failure, IVIg products should be administered at the minimum rate of infusion and dose practicable. In case of renal impairment, IVIg discontinuation should be considered.
While reports of renal dysfunction and acute renal failure have been associated with the use of many of the licensed IVIg products containing various excipients such as sucrose, glucose and maltose, those containing sucrose as a stabiliser accounted for a disproportionate share of the total number. In patients at risk, the use of IVIg products that do not contain these excipients may be considered. Ig VENA contains maltose. (See excipients above).
Aseptic meningitis syndrome (AMS)Aseptic meningitis syndrome has been reported to occur in association with IVIg treatment.The syndrome usually begins within several hours to 2 days following IVIg treatment. Cerebrospinal fluid studies are frequently positive with pleocytosis up to several thousand cells per mm3, predominantly from the granulocytic series, and elevated protein levels up to several hundred mg/dl.AMS may occur more frequently in association with high-dose (2 g/kg) IVIg treatment.
Patients exhibiting such signs and symptoms should receive a thorough neurological examination, including CSF studies, to rule out other causes of meningitis.
Discontinuation of IVIg treatment has resulted in remission of AMS within several days without sequelae.
Haemolytic anaemiaIVIg products can contain blood group antibodies which may act as haemolysins and induce in vivo coating of red blood cells with immunoglobulin, causing a positive direct antiglobulin reaction (Coombs’test) and, rarely, haemolysis. Haemolytic anaemia can develop subsequent to IVIg therapy due to enhanced red blood cells (RBC) sequestration. IVIg recipients should be monitored for clinical signs and symptoms of haemolysis. (See section 4.8.).
Neutropenia/LeukopeniaA transient decrease in neutrophil count and/or episodes of neutropenia, sometimes severe, have been reported after treatment
with IVIgs. This typically occurs within hours or days after IVIg administration and resolves spontaneously within 7 to 14 days.
Transfusion related acute lung injury (TRALI)In patients receiving IVIg, there have been some reports of acute non-cardiogenic pulmonary oedema [Transfusion Related Acute Lung Injury (TRALI)]. TRALI is characterised by severe hypoxia, dyspnoea, tachypnoea, cyanosis, fever and hypotension. Symptoms of TRALI typically develop during or within 6 hours of a transfusion, often within 1-2 hours. Therefore, IVIg recipients must be monitored for and IVIg infusion must be immediately stopped in case of pulmonary adverse reactions. TRALI is a potentially life-threatening condition requiring immediate intensive- care-unit management.
Interference with serological testingAfter the administration of immunoglobulin the transitory rise of the various passively transferred antibodies in the patient’s blood may result in misleading positive results in serological testing.
Passive transmission of antibodies to erythrocyte antigens, e.g. A, B, D may interfere with some serological tests for red cell antibodies for example the direct antiglobulin test (DAT, direct Coombs’ test).
Transmissible agentsStandard measures to prevent infections resulting from the use of medicinal products prepared from human blood or plasma include selection of donors, screening of individual donations and plasma pools for specific markers of infection and the inclusion of effective manufacturing steps for the inactivation/removal of viruses.Despite this, when medicinal products prepared from human blood or plasma are administered, the possibility of transmitting infective agents cannot be totally excluded. This also applies to unknown or emerging viruses and other pathogens.The measures taken are considered effective for enveloped viruses such as HIV, HBV, HCV and for the non-enveloped virus HAV.
The measures taken may be of limited value against non-enveloped viruses such as parvovirus B19. There is reassuring clinical experience regarding the lack of hepatitis A or parvovirus B19 transmission with immunoglobulins and it is also assumed that the antibody content makes an important contribution to the viral safety.
It is strongly recommended that every time that Ig VENA is administered to a patient, the name and batch number of the product are recorded in order to maintain a link between the patient and the batch of the product.
Paediatric populationCases of glycosuria have been reported in paediatric patients after administration of Ig VENA. These events are usually mild and transient with no clinical signs.Ig VENA contains 100 mg of maltose per ml as an excipient. In the renal tubules, maltose is hydrolysed to glucose which is reabsorbed and generally very little is excreted in the urine. The glucose reabsorption is age dependent. The transitory increase of maltose in plasma may exceed the renal capacity of sugar re-absorption, and result in positive testing for glucose in the urine.
4.5 Interaction with other medicinal products and other forms of interaction
Live attenuated virus vaccinesImmunoglobulin administration may impair for a period of at least 6 weeks and up to 3 months the efficacy of live attenuated virus vaccines such as measles, rubella, mumps and varicella. After administration of this medicinal product, an interval of 3 months should elapse before vaccination with live attenuated virus vaccines. In the case of measles, this impairment may persist for up to 1 year. Therefore patients receiving measles vaccine should have their antibody status checked.
Loop diureticsAvoidance of concomitant use of loop diuretics.
Blood Glucose TestingSome types of blood glucose testing systems (for example, those

based on the glucose dehydrogenase pyrroloquinolinequinone (GDH-PQQ) or glucose-dye-oxidoreductase methods) falsely interpret the maltose (100 mg/ml) contained in Ig VENA as glucose. This may result in falsely elevated glucose readings during an infusion and for a period of about 15 hours after the end of the infusion and, consequently, in the inappropriate administration of insulin, resulting in life- threatening or even fatal hypoglycemia. Also, cases of true hypoglycemia may go untreated if the hypoglycemic state is masked by falsely elevated glucose readings. Accordingly, when administering Ig VENA or other parenteral maltose-containing products, the measurement of blood glucose must be done with a glucose-specific method.The product information of the blood glucose testing system, including that of the test strips, should be carefully reviewed to determine if the system is appropriate for use with maltose-containing parenteral products. If any uncertainty exists, contact the manufacturer of the testing system to determine if the system is appropriate for use with maltose-containing parenteral products.
Paediatric populationAlthough specific interaction studies have not been performed in the paediatric population, no differences between adults and children are to be expected.
4.6 Fertility, pregnancy and lactation
PregnancyThe safety of this medicinal product for use in human pregnancy has not been established in controlled clinical trials and therefore should only be given with caution to pregnant women and breast-feeding mothers. IVIg products have been shown to cross the placenta, increasingly during the third trimester.Clinical experience with immunoglobulins suggests that no harmful effects on the course of pregnancy, or on the foetus and the neonate are expected.
Breast-feedingImmunoglobulins are excreted into human milk. No negative effects on the breastfed newborns/infants are anticipated.
FertilityClinical experience with immunoglobulins suggests that no harmful effects on fertility are to be expected.
4.7 Effects on ability to drive and use machines
The ability to drive and operate machines may be impaired by some adverse reactions associated with Ig VENA. Patients who experience adverse reactions during treatment should wait for these to resolve before driving or operating machines.
4.7 Undesirable effects
Summary of the safety profileAdverse reactions caused by human normal immunoglobulins (in decreasing frequency) encompass (see also Section 4.4):• chills, headache, dizziness, fever, vomiting, allergic reactions,
nausea, arthralgia, low blood pressure and moderate low back pain
• reversible haemolytic reactions; especially in those patients with blood groups A, B, and AB and (rarely) haemolytic anaemia requiring transfusion
• (rarely) a sudden fall in blood pressure and, in isolated cases, anaphylactic shock, even when the patient has shown no hypersensitivity to previous administration
• (rarely) transient cutaneous reactions (including cutaneous lupus erythematosus - frequency unknown)
• (very rarely) thromboembolic reactions such as myocardial infarction, stroke, pulmonary embolism, deep vein thromboses
• cases of reversible aseptic meningitis• cases of increased serum creatinine level and/or occurrence of
acute renal failure• cases of Transfusion Related Acute Lung Injury (TRALI).
The safety of Ig VENA was evaluated in four clinical trials in which a total of 1189 infusions was administered. The CIDP study enrolled 24 patients with Chronic Inflammatory Demyelinating Poliradiculoneuropathy (CIDP) receiving Ig VENA, for a total of 840
infusions administered. In the PID study, 16 patients with Primary Immunodeficiency (PID) were enrolled and received a total of 145 infusions. The ITP study enrolled 15 subjects with Immune Thrombocytopenia (ITP) with a total of 80 infusions administered. In the ID/ITP study, 43 patients with either Immunodeficiency (ID) or ITP were enrolled and received a total of 124 infusions.
Tabulated list of adverse reactionsThe tables presented below are according to the MedDRA system organ classification (SOC and Preferred Term Level).
Table 1 shows the adverse reactions from clinical trials and Table 2 shows the post-marketing adverse reactions.
Frequencies have been evaluated according to the following convention: very common (≥1/10); common (≥1/100 to <1/10); uncommon (≥1/1,000 to <1/100); rare (≥1/10,000 to <1/1,000); very rare (<1/10,000), not known (cannot be estimated from the available data).
Frequencies of undesirable effects from clinical trials are based on percentage per infusions (total number of infusions: 1189).
Adverse reactions from post-marketing experience are listed with unknown frequency as post- marketing reporting of adverse reactions is voluntary and from a population of uncertain size and it is not possible to reliably estimate the frequency of these reactions.
Source of the safety database (e.g. from clinical trials, post-authorisation safety studies and/or spontaneous reporting)
Table 1Frequency of Adverse Reactions from Clinical Trials
MedDRA System Organ Class (SOC)
Adverse reaction
Frequency per patient
Frequency per infusion
Nervous system disorders
Headache, somnolence
Common Rare
Gastrointestinal disorders
Nausea Common Rare
Musculoskeletal and connective tissue disorders
Back pain Common Uncommon
Myalgia Common Rare
General disorders andadministration site conditions
Asthenia, fatigue,pyrexia
Common Rare
Table 2Post-marketing Adverse Reactions
MedDRA SOC Adverse reaction
Frequency per patient
Frequency per infusion
Infections and infestations
Meningitis aseptic
Not known Not known
Blood and lymphatic systemdisorders
Haemolysis,haemolytic anaemia
Not known Not known
Immune system disorders
Anaphylactic shock,hypersensitivity
Not known Not known
Psychiatric disorders
Confusional state
Not known Not known
Nervous system disorders
Cerebrovascular accident, headache, dizziness, tremor,paraesthesia
Not known Not known
Cardiac disorders
Myocardial infarction, cyanosis, tachycardia, bradycardia,palpitations
Not known Not known

MedDRA SOC Adverse reaction
Frequency per patient
Frequency per infusion
Vascular disorders
Deep vein thrombosis, embolism, hypotension,hypertension, pallor
Not known Not known
Respiratory, thoracic and mediastinal disorders
Pulmonary embolism, pulmonary oedema, bronchospasm,dyspnoea, cough
Not known Not known
Gastrointestinal disorders
Vomiting, diarrhoea, nausea, abdominalpain
Not known Not known
Skin and subcutaneous tissue disorders
Angioedema, urticaria, erythema, dermatitis, rash, pruritus, eczema,hyperhidrosis
Not known Not known
Musculoskeletal and connective tissue disorders
Arthralgia, back pain, myalgia, neck pain, muscoloskeletalstiffness
Not known Not known
Renal and urinary disorders
Acute kidney injury
Not known Not known
General disorders and administration site conditions
Injection site phlebitis, pyrexia, chills, chest pain, face oedema,malaise
Not known Not known
Investigations Blood pressure decreased, bloodcreatinine increased
Not known Not known
For safety with respect to transmissible agents, see section 4.4.
Paediatric populationFrequency, type and severity of adverse reactions in children are expected to be the same as in adults.Transient glycosuria has been observed after administration of Ig VENA in paediatric patients.This event could be due to the maltose contained in Ig VENA and to the different capacity of renal tubules to reabsorb glucose, that is an age dependent mechanism.
Reporting of suspected adverse reactionsReporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the national reporting system.
4.9 Overdose
Overdose may lead to fluid overload and hyperviscosity, particularly in patients at risk, including elderly patients or patients with cardiac or renal impairment (see section 4.4.).
5. PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: immune sera and immunoglobulins: immunoglobulins, normal human, for intravascular administration; ATC code: J06BA02.Human normal immunoglobulin contains mainly immunoglobulin G (IgG) with a broad spectrum of antibodies against infectious agents.Human normal immunoglobulin contains the IgG antibodies present in the normal population. It is usually prepared from pooled plasma from not fewer than 1000 donations. It has a distribution of immunoglobulin G subclasses closely proportional to that in native human plasma. Adequate doses of this medicinal product may restore abnormally low immunoglobulin G levels to the normal range.The mechanism of action in indications other than replacement therapy is not fully elucidated.
Clinical efficacy and safetyFour clinical trials were performed with Ig VENA: three trials on efficacy and safety in patients with Primary Immunodeficiency (PID), Immune Thrombocytopenia (ITP) and Chronic Inflammatory Demyelinating Poliradiculoneuropathy (CIDP); and one study on safety and tolerance of Ig VENA at increased infusions rates in patients with Immunodeficiency (ID) or ITP.
A phase III, prospective, open-label study in patients with primary Immunodeficiency syndromes (KB028) evaluated the pharmacokinetic profile of Ig VENA as the primary objective. Secondary objectives were therapeutic efficacy in terms of prophylaxis of episodes of infection, and safety in terms of short term-tolerability. Fifteen patients out of 16 enrolled, aged 28-60 years old, were evaluated for efficacy and were treated for 24 weeks with Ig VENA (total of 140 infusions).The pharmacokinetic profile of Ig VENA showed a terminal half-life fairly consistent with the data reported in the literature, being 26.4 days. One patient developed pneumonia after 18 weeks of therapy with Ig VENA but this patient suffered of severe pulmonary infections also in the previous 10 years. No serious infections were reported for the other patients enrolled.The data generated in KB028 study indicate that Ig VENA is safe and effective for treatment of primary immunodeficiency syndromes.
The ITP Study (KB027) was a phase III, open-label, prospective trial for the evaluation of efficacy and tolerability of Ig VENA in adult patients with chronic idiopathic thrombocytopenic purpura. The primary objective was the evaluation of platelet count increase. Secondary objectives were: reduction in haemorrhagic events, duration of platelet response, and the incidence of AEs. Fifteen patients received a total dose of 2 g/kg each, subdivided into 5 daily infusions of 400 mg/kg on consecutive days. A second cycle of 2 g/kg body weight was given to one patient within the first 14 days. The total number of infusions applied was 80.All enrolled patients achieved a platelet count ≥ 50x109/L, except one who received a second cycle of therapy but did not achieve the target platelet count (response rate 93.3%, 90% CI from 68.1 to 99.8). No adverse events were reported.The results obtained from KB027 study provided evidence of tolerability and therapeutic efficacy of Ig VENA in ITP patients.
In the phase III study KB057 for the evaluation of tolerability and safety of Ig VENA at increased infusion rates, 43 adult patients were enrolled: 38 ID and 5 ITP patients who received Ig VENA at the dosages approved according to both indications.
Thirty-seven ID patients were observed for 3 infusions and 1 ID patient for 2 infusions. Four ITP patients received their planned dose over 2 daily infusions, while 1 patient was infused over 3 days. (total of 124 infusions).At infusion 2, twenty-eight patients out of 43 were infused at the maximum speed of 8 ml/kg/hr; 13 patients out of 43 reached only a maximum infusion speed of 6 ml/kg/hr, because their infusion was finished before they could be ramped up to the next increment in infusion speed. During the clinical trial, two patients did not reach 8 ml/kg/h because they developed 3 adverse events during the infusion at lower infusion rates.

Results obtained from this study show that Ig VENA administered at increasing infusion speed was well tolerated both in patients with ID and in those with ITP and that the infusion speed could be ramped up to a maximum of 6 ml/kg/hr and, in a limited number of patients, to 8 ml/kg/hr.Adverse drug reactions were reported in less than 10% of ID patients and were reactions generally related with IVIg administration (e.g. pyrexia, back pain, myalgia, asthenia, somnolence and fatigue).No serious ADR was reported as well as local reactions at the infusion site.
Clinical trial conducted with Ig VENA on patients with Chronic Inflammatory Demyelinating Poliradiculoneuropathy (CIDP):The double-blind controlled Phase III study on the tolerability and efficacy of long-term treatment with high doses intravenous immunoglobulins versus high doses intravenous methylprednisolone (IVMP) in CIDP (KB034) was conducted of a total of 46 CIDP adult patients, randomised to receive either Ig VENA (dosage: 2g/Kg/month in 4 consecutive days for 6 months) or IVMP (dosage: 2g/month in 4 consecutive days for 6 months).
Ten of the 21 patients treated with IVMP (47.6%) completed the 6 months of the study compared to 21/24 on Ig Vena (87.5%) (p=0.0085). The cumulative probability of treatment discontinuation was significantly higher with IVMP than with Ig VENA at 15 days, 2 months and 6 months. Of the 11 patients who discontinued IVMP, eight did so because of progressive worsening after treatment start (5 patients) or failure to improve after two courses of therapy (3 patients), while one had adverse events (gastritis) (9.1%) and two voluntarily withdrew (18.2%). Three patients discontinued Ig VENA for progressive worsening after starting the therapy (two patients), or absence of improvement after two courses of therapy (one patient). All the patients worsening or not improving after IVMP or IVIg were shifted to the alternative therapy while the three patients who discontinued IVMP for adverse event or who voluntary withdrew after IVMP refused further therapy.
Results regarding the study secondary endpoints are summarized in the table reported below (statistically significant differences in bold):
Intention To Treat Population (ITT)
Per Protocol Population (PP)
Secondary endpoints
Ig VENA 10g/200 ml
MPIV p-value Ig VENA 10g/200 ml
MPIV p-value
Relapses rate*
45.8%(n 11/24)
52.4%(n 11/21)
0.7683 38.1%(n 8/21)
0%(n 0/10)
0.0317
MRC sum score [delta (p-value)]
+4.7(0.0078)
+1.8(0.1250)
0.6148 +4.0(0.0469)
+2.0(0.5000)
0.5473
INCAT (p-value)
0.0004 0.1877 0.3444 0.0057 0.2622 0.9065
Vibratory score - Right medial malleolus (p-value)
<0.0001 0.6515 0.0380 0.0009 0.2160 0.4051
Fist strength right [delta (p- value)]
+19.4(0.0005)
+5.4(0.6169)
0.0641 +16.5(0.0044)
+14.7(0.0156)
0.5012
Fist strength left [delta (p-value)]
+16.9(0.0011)
+8.8(0.1170)
0.1358 +12.7(0.0014)
+10.5(0.0156)
0.3330
Time on 10 meters [delta (p- value)]
-3.2(0.0025)
-0.5(0.2051)
0.0800 -3.5(0.0043)
-2.0(0.4453)
0.2899
ONLS scale (p-value)
0.0006 0.0876 0.4030 0.0033 0.0661 0.8884
Rankin scale (p-value)
0.0006 0.0220 0.3542 0.0132 0.2543 0.8360
Rotterdam scale [delta (p-value)]
+1.4(0.0071)
+1.3(0.0342)
0.6465 +1.1(0.0342)
+1.1(0.0859)
0.4056
SF-36 QoL
+14.2(0.0011)
+16.7(0.0008)
0.3634 +11.1(0.0091)
+16.0(0.1094)
0.6518
*ITT: throughout the study (12 months); PP: follow up phase (6 months)
Paediatric populationPublished data related to efficacy and safety studies have not revealed major differences between adults and children suffering from the same disorder.
5.2 Pharmacokinetic properties
Human normal immunoglobulin is immediately and completely bioavailable in the recipient’s circulation after intravenous administration. It is distributed relatively rapidly between plasma and extravascular fluid, after approximately 3-5 days equilibrium is reached between the intra- and extravascular compartments.Human normal immunoglobulin has a half-life of about 26 days. This half-life may vary from patient to patient, in particular in primary immunodeficiency.
IgG and IgG-complexes are broken down in cells of the reticuloendothelial system.
Paediatric populationPublished data related to PK studies have not revealed major differences between adults and children suffering from the same disorder.There are no data on pharmacokinetic properties in paediatric patients with CIDP.
5.3 Preclinical safety data
Immunoglobulins are normal constituents of the human body. Moreover, as administration of immunoglobulins in animal studies may lead to the formation of antibodies, preclinical safety data are limited. However, the limited animal studies did not show special risks for humans, based on acute and sub-acute toxicity studies.
6. PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Maltose.Water for injections.
6.2 Incompatibilities
In the absence of compatibility studies, this medicinal product must not be mixed with other medicinal products, nor with any other IVIg products.
6.3 Shelf-life
3 years.Once the infusion container has been opened the contents should be used immediately.
6.4 Special precautions for storage
Store in a refrigerator (2°C - 8°C). Keep the vial in the outer carton.Before use and within the shelf-life, the vials of 50, 100 and 200 ml can be stored at room temperature, not exceeding 25 °C, for a maximum of 6 consecutive months.After this period, the product must be discarded. In any case, the product can no longer be put back in the refrigerator if kept at room temperature.

The starting date of the storage at room temperature should be reported on the outer box. Do not freeze.
6.5 Nature and contents of container
20 ml solution in a vial (type I glass) with a stopper (halobutyl rubber); pack size of one vial.50 ml, 100 ml and 200 ml solution in a vial (type I glass) with a stopper (halobutyl rubber): pack size of one vial + hanger.
6.6 Special precautions for disposal and other handling
The product should be brought to room or body temperature before use.The solution should be clear or slightly opalescent, colourless or pale yellow. Solutions that are cloudy or have deposits should not be used.Solution should be inspected visually for particulate matter and discoloration prior to administration.
Instructions for the use of the hanger
1 Initial status of the vial with hanger label2 Turn vial upside down3 Activate the hanger by unfolding it from the label4 Hang the vial on the infusion stand
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
7. MARKETING AUTHORISATION HOLDER
Kedrion S.p.A. Loc. Ai Conti55051 Castelvecchio Pascoli Barga (Lucca)Italy.
8. MARKETING AUTHORISATION NUMBERS
Ig VENA 50 g/l solution for infusion, 20 ml 1 vial n° 025266141.Ig VENA 50 g/l solution for infusion, 50 ml 1 vial + hanger n° 025266154.Ig VENA 50 g/l solution for infusion, 100 ml 1 vial + hanger n° 025266166.Ig VENA 50 g/l solution for infusion, 200 ml 1vial + hanger n° 025266178.
9. DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
Date of first authorisation: April 6th 1984. Date of renewal of authorisation: Jun 1st 2010.
10. DATE OF REVISION OF THE TEXT
07 July 2019.


Kedrion S.p.A.Loc. Ai Conti - 55051 Castelvecchio Pascoli, Barga (Lucca) - Italy.
Vers
ion
n. 2
, 30-
10-2
020,
Cod
e 00
0598
0836
Please add local packshots here
TO WHOM IT MAY CONCERNFor the correct use of this product please refer to the technical documents (e.g. patient information leaflets and SPC) approved in your country by the competent regulatory authorities.
INFORMATION FOR PHYSICIANSPlease do not hesitate to request a copy of said technical documentation to our local representative.
PLEASE BE ADVISED THAT THE CONTENTSOF THIS BROCHURE
MAY BE USED ONLY IF COMPLIANT WITHLOCAL LEGISLATION