Identified early stage mycosis fungoides from psoriasis and atopic dermatitis...
Transcript of Identified early stage mycosis fungoides from psoriasis and atopic dermatitis...

Opt Quant ElectronDOI 10.1007/s11082-014-0017-x
Identified early stage mycosis fungoides from psoriasisand atopic dermatitis using non-invasive color contrastenhancement by LEDs lighting
Yu-Ping Hsiao · Hsiang-Chen Wang · Shih-Hua Chen ·Chung-Hung Tsai · Jen-Hung Yang
Received: 26 June 2014 / Accepted: 3 September 2014© Springer Science+Business Media New York 2014
Abstract Mycosis fungoides (MF) is the most common type of cutaneous T cell lymphoma(CTCL) and may progress internally over time. MF is clinically categorized as poorly definedareas of erythema, flat patches, thin plaques, and tumors. The diagnosis of early stage of MF(patch and early plaque mycosis fungoides) has been a major diagnostic challenge in der-matology because of the lack of highly characteristic immunophenotypical markers and the
Y.-P. HsiaoDermatology Department, Chung Shan Medical University Hospital,No.110, Sec. 1, Jianguo N. Rd., South Dist., Taichung City 40201, Taiwan
Y.-P. Hsiao · C.-H. TsaiSchool of Medicine, Institute of Medicine, Chung Shan Medical University,No.110, Sec. 1, Jianguo N. Rd., South Dist., Taichung City 40201, Taiwan
H.-C. Wang · S.-H. ChenGraduate Institute of Opto-Mechatronics, National Chung Cheng University,168, University Rd., Min-Hsiung, Chia-Yi 62102, Taiwane-mail: [email protected]
C.-H. TsaiDepartment of Pathology, Chung Shan Medical University Hospital,Taichung City 40201, Taiwan
J.-H. YangSchool of Medicine, Tzu Chi University, No.701, Zhongyang Rd., Sec. 3,Hualien 97004, Taiwan
J.-H. Yang (B)Department of Dermatology, Buddhist Tzu Chi General Hospital, No. 707, Sec. 3,Chung Yang Rd., Hualien 97004, Taiwane-mail: [email protected]
H.-C. Wang · S.-H. ChenAdvanced Institute of Manufacturing with High-tech Innovations (AIM-HI),National Chung Cheng University, 168, University Rd., Min-Hsiung,Chia-Yi 62102, Taiwan
123

Y.-P. Hsiao et al.
heterogeneity of clinical presentations of MF. In this study, the spectrum of each picture ele-ment of the patient’s skin image was obtained by multi-spectral imaging technology (MSI).Spectra of normal or pathological skin were collected from 30 patients (10 early stage MF,10 psoriasis vulgaris, and 10 atopic dermatitis). An algorithm combined with multi-spectralimaging and color reproduction technique is applied to find the best enhancement of the dif-ference between normal and abnormal skin regions. Accordingly, an illuminant with specificintensity ratio of red, green, and blue LEDs is proposed, which has optimal color enhance-ment for MF detection. Compared with the fluorescent lighting commonly in the use now, thecolor difference between normal and inflamed skin can be improved from 11.8414 to 17.4002with a 47 % increase, 8.7671 to 12.8544 with a 26.3 % increase, and 11.0735 to 17.2634 witha 34.3 % increase for MF, psoriasis, and atopic dermatitis patients, respectively, thus makingmedical diagnosis more efficient, so helping patients receive early treatment.
Keywords Mycosis fungoides · Biomedical optical imaging · Color contrast enhancement ·Multispectral imaging · Image reconstruction techniques · Illumination design
1 Introduction
Mycosis fungoides (MF) is a heterogeneous group of malignant lymphomas that revealedthe propensity for neoplasmic T lymphocytes to infiltrate the skin. The clinical diagnosis isalways a challenge for physicians because the diversity of manifestations that MF could be thered, scaly patches or thickened plaques that often mimic chronic eczema, atopic dermatitis,or psoriasis (Willemze et al. 2005; Frye et al. 2012; Lee and Hwang 2012). A study amongcutaneous lymphoma patients in Holland and Austria showed that 1,476 of 1,905 cases wereMF patients, constituting 77.5 % of the total number of patients. The study was conducted bythe World Health Organization and the European Organization for Research and Treatmentof Cancer in 2005 (Willemze et al. 2005). MF commonly occurred in patients aged from 40to 69 and were often diagnosed in males, with an occurrence rate of two males for every onefemale. People with dark skin also exhibited higher susceptibility to the disease than thosewith white skin (with an incidence ratio of 1.6) (Willemze et al. 2005).
Cancerous lymphocytes are classified as primary or secondary. Primary cancerous lym-phocytes are attracted to the skin through the lymphocytes in blood by the cytohormone,whereas secondary lymphoma cells are brought to the skin by blood circulation from thelymph node (Whittaker et al. 2003). Pathologic changes in mycosis fungoides, the mostcommon type of CTCL, refer to the process in which mutant T lymphocytes break out ofthe corium from the capillaries, continuing upwards to attack the epidermal layer (Girardiet al. 2004). In most MF cases, patients first experience problems such as dry, reddish, anditchy skin. Some patients present with local skin depigmentation, experience itchiness, orhave speckles such as rashes (World Health Organization 2009). It could be misdiagnosedas ordinary psoriasis, atopic dermatitis, or eczema if the doctor lacks relevant experience indifferential diagnosis.
Current methods used to detect MF include incision biopsy, blood tests, and lymphogra-phy (Hong Kong Cancer Fund 2007; Willemze and Dreyling 2010). Lymphography is similarto computerized tomography (CT) in which a radio contrast agent is injected into the lym-phatic vessel; incision biopsy involves cutting open the skin for histopathological diagnosis;and blood tests are conducted using a needle head to pierce the skin. The aforementionedprocedures are invasive, painful and psychologically stressful. These techniques must alsobe performed repeatedly for subsequent monitoring, and the results can be confirmed only
123

Non-invasive color contrast enhancement by LEDs lighting
after a considerable period of time. Among the disadvantages of conventional detectionmethods are that they are invasive, painful, and time-consuming. In addition, conventionaldetection methods can only be performed by doctors with extensive experience and skills.Consequently, real-time diagnosis becomes difficult.
The application of spectroscopy and illumination in biomedicine has recently been inves-tigated (Yamaguchi et al. 2005; Yamaguchi 2001; Lee et al. 2009; Park et al. 2007; Hashimotoet al. 2011). The real-time property of multi-spectral image reproduction technology is shownto exhibit the greatest potential. For one image, we can find the individual spectrum of eachpixel point by multi-spectral imaging (MSI). Calculation of the different spectra can enhancethe color contrast between the normal and the pathological images, thus increasing diagnosticefficiency (Wang and Chen 2012; Wang et al. 2010, 2013).
In this study, we collected pictures from thirty patients (ten patients with MF, ten patientswith psoriasis vulgaris, and ten patients with atopic dermatitis, respectively). The inclusioncriteria were early stage MF with patches or plaques phases, psoriasis vulgaris, and atopicdermatitis. There are four stages of mycosis fungoides depending on the degree of skinmanifestation, nodal involvement, and internal organ metastasis. We recruited patients withearly stage mycosis fungoides with patches or plaques phases. The limitations of this studywere that tumor-stage mycosis fungoides were excluded, and the penetration depth of LEDwas <1 mm (Cowen et al. 2007). After the spectra showing different pathological changes andthe normal region were obtained, spectra of a commercial RGB LED with peak wavelengths at460, 530, and 617 nm for blue, green, and red respectively, are applied to illuminate the normalskin and the pathological skin and calculate the color difference by changing the intensityratio of each peak. Principal component analysis (PCA) was conducted for the normal spectraand the pathological spectra of the three MF patients and then the eigenvector of MF (sixbasic functions) was obtained. The spectra of all patients with similar presentations were thenanalyzed using the MF eigenvector, and the principal component score of all pathologicaland normal spectra was determined. Skin diseases with similar symptoms were distinguishedaccording to use some special LEDs lighting. Thus, skin lymphoma, psoriasis, and atopicdermatitis were identified.
2 Materials and methods
2.1 Images of cutaneous lymphoma, psoriasis, and atopic dermatitis
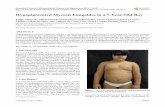
All images of the patients and the pathological sections in this study were provided by theDermatology Department of Chung Shan Medical University Hospital. Initially, pictures often MF patients were taken. However, the initial symptoms of MF were similar to those ofpsoriasis and atopic dermatitis; therefore, images of ten psoriasis and ten atopic dermati-tis patients were also taken to represent the control group. This study was reviewed andapproved by the Chung Shan Medical University Hospital (IRB No CS12126) institutionalreview board. These images, which are clearer than those taken with a camera, also reveal ery-themas in the pathological regions. Figure 1a shows the pathological result of a 35-year-oldman with MF. Figure 1d reveals the pathological changes in patient CA. The yellow arrowsin this figure indicate epidermotropism of atypical lymphocytes. The increased and bizzarelymphocytes rushed into the epidermis; thus, patients CA has been diagnosed with cutaneouslymphoma. Figure 1b shows the pathological region of a 42-year-old male psoriasis patientPA. The symptom of initial psoriasis is similar to that of MF: patches of slight erythemain the pathological region, as shown in Fig. 1b. Figure 1e shows a section image with a
123

Y.-P. Hsiao et al.
Fig. 1 a–c The images of the pathological skin of MF patients CA, psoriasis patients PA, and atopic dermatitispatients AA; d–f the histopathological results of patient CA, PA, and AA, respectively.
pink asterisk representing the parakeratotic corneal layer, a blue dotted arrow indicating theabsence of the granular layer, a hollow green star denoting suprapapillary thinning of the epi-dermis, a solid green star indicating psoriasiform hyperplasia of the epidermis, and a greenarrow denoting tortuous capillaries in the papillary dermis. Given the aforementioned histopathological mechanisms, the condition is confirmed to be psoriasis. Figure 1c shows imagesof pathological regions of atopic dermatitis belonging to a 17-year-old female. Figure 1fshows the section image of (c). The blue asterisk represents a normal basket weave stratumcorneum layer; the blue arrow indicates a granular layer; the purple star denotes spongioticepidermis; and the orange arrow represents lymphocytes with exocytosis. The thicker hornylayer, the present granular layer, and other histopathological mechanisms confirm that thepatients have atopic dermatitis. In the pathological region of a cutaneous lymphoma patient,the characteristics that determine the pathological differences among the three kinds of skindiseases are the monoclonal and atypical lymphocytes gathered into the surface of the epi-dermis, which show epidermotropism. Lymphocytes increase, and abnormal lymphocytescollect in the epidermal layer. The stratum corneum of the pathological region in a psoria-sis patient thickens. The papillary dermis shows dilated capilaries and normal lymphocytesaccumulating in the epidermal layer. The stratum corneum of the pathological region of anatopic dermatitis patient thickens, and the epidermis shows edema and spongiosis. In thesethree pathological changes, keratinocytes show cell edema. The differences among the threepathological changes are obvious. However, patches of slight erythema are a common clinicalcharacteristic, as shown in Fig. 1a–c.
2.2 Multi-spectral imaging technique
Figure 2 shows the estimation processes of the color image reproduction with multi-spectralimage data. The multi-spectral imaging (MSI) can be divided into three parts: the principalcomponents analysis for spectra data reduction, color correction for images, and calculation
123

Non-invasive color contrast enhancement by LEDs lighting
Fig. 2 Schematic diagram of the proposed method used in estimating the spectral reflectance of each pixelof an image using a digital camera
of transformation matrix to find the relationship between the digital camera and spectropho-tometer. The spectral reflectance functions of the 24 patches on the color checker are mea-sured using the spectroradiometer. The color checker is an array of 24 printed color squares,including spectral simulations of light and dark skin, foliage, and others (McCamy et al.1976). Fluorescent lamps (Kino Flo, Mega 4Bank) are used as the illuminant in the setup.The illuminant consists of a ballast and a lamp that has four tubes. The ballast keeps the lampflicker-free at any speed or angle of the camera shutter by controlling the high output powerof the tubes. The correlated color temperature (CCT) of the illuminant is approximately6,500 K. An x–y axis precise-translation-stage table is used in moving the color checker todetermine the uniform measurement of the spectral reflectance function of each color patch.The power of the illuminant is equally distributed on each color patch of the color checker.In the measurement system, the illuminant is warmed up for approximately 30 min beforethe spectrum is measured, and the layout of the setup is based on the illumination/measuring(45◦/0◦) geometry (Berns 2000). The illumination is projected at an incident angle of 45◦.The spectroradiometer is placed at the 0◦ angle, facing the center of the object. After mea-suring the spectrum, the image of the color checker is taken using a digital camera in theconditions (1/5 s exposure time, an opening of the diaphragm with f/4, and ISO value with200), with the reference white color set as the illuminating condition of D65, which is com-monly used as the standard illuminant (International Commission on Illumination) (Lam andXin 2002). Color correction is required to achieve a more accurate estimation of the spectra.The CIE (the Commission Internationale de l’Éclairage) XYZ tristimulus values of the colorchecker are calculated from the spectroradiometer and set as the standard values. The CIEXYZ is one of the first mathematically defined color spaces, created by the InternationalCommission on Illumination (Smith and Guild 1931). The RGB values of each pixel in theimage from the camera are captured using a computer program and transferred into CIE XYZvalues (Wang and Chen 2012).
The color relationship between the spectrophotometer and the camera is found by usingthe third-order polynomial regression for the red, green and blue components separately, andthe regression matrix[C]is determined as follows:
[C] = [A]pinv[F] (1)
123

Y.-P. Hsiao et al.
where
[F] = [1, R, G, B, RG, G B, B R, R2, G2, B2, RG B, R3, G3, B3, RG2, RB2, G R2,
G B2, B R2, BG2]T(2)
and “R”, “G”, and “B” are the respective RGB values of the color checkers captured by thecamera. The corrected RGB values are obtained from Eq. 3. Here [K] presents the RGBvalues captured from any image that expanded into a format such as the original matrix [F].The calculation processes of the CMCCAT2000-corrected RGB values of the digital cameraare obtained from reference (Li et al. 2002). The corrected RGB values of the color checkersare then transferred into CIE XYZ values and arrayed as a matrix, [β].
[Corrected RGB] = [C] [K] (3)
Finally, a transform matrix [M] between the spectrophotometer and the camera is obtainedas follows:
[M] = [α]pinv[β] (4)
The spectral reflectance functions can be determined using the linear combination ofseveral principal components (PCs) after the PC analysis method. The coefficients of the linearcombination are referred to as the PC coefficients in this study. The PC coefficients and thenew RGB values of the digital camera exhibit a linear relationship. The new RGB values andthe corresponding PC coefficients are analyzed to obtain the transformation matrix betweenthe digital camera and the spectroradiometer. After obtaining the transformation matrix, thespectral reflectance functions of any given object surface can be reconstructed using a multi-spectral image acquisition system. The PC coefficients of the spectral reflectance functionof the object can be calculated using the new RGB values and the obtained transformationmatrix. Spectral reflectance can be reconstructed by calculating the linear combination of thePCs with the obtained PC coefficients. The process involved in obtaining the RGB values ofeach pixel after capturing the image is corrected to achieve a more accurate estimation of thespectra. Color correction is implemented to match the color performance of the camera withthat of the spectroradiometer. From the spectroradiometer, the CIE XYZ tristimulus valuesand corresponding RGB values from the spectra of 24 color checkers are calculated and setas standard values. From the camera, images under the same lighting conditions are captured,and RGB values of each pixel can be retrieved using the computer program. Finally, the colorrelationship between these two devices can be determined using the third-order polynomialregression for red, green, and blue components separately. The output format of commercialdigital cameras is sRGB (RAW image files), wherein the reference white color is illuminatedby D65 (Anderson et al. 1996), different from the artificial lights used in measuring thespectra of the 24 color checkers in the present study.
3 Results and discussions
3.1 Reflection spectra reproduction
Figure 1 show three clinical photographs of patients for MF, psoriasis, and atopic dermatitispatients. All images have been captured under the same conditions (light source: fluorescentlamp; illuminance: 200 Lux). From these images, the locations of erythema and their colorchanges can be clearly identified. By using the color reproduction system mentioned above,
123

Non-invasive color contrast enhancement by LEDs lighting
Fig. 3 The simulated reflection spectra of the abnormal skin of the ten MF patients (a), ten psoriasis patients(b), and ten atopic dermatitis patients (c). Thirty points of each skin lesion location is calculated in eachpatient’s body through doctor’s discrimination. d The average spectrum calculation results of normal skin,MF, psoriasis, and atopic dermatitis
the reflection spectrum of each lesion at any site in these images can be estimated rapidly.Figure 3 show simulated reflection spectra of the abnormal skin of the ten MF patients (a),ten psoriasis patients (b), and ten atopic dermatitis patients (c). Thirty points of each skinlesion location is calculated in each patient’s body through doctor’s discrimination. Figure 3dpresents the calculation results of the average spectrum of the normal skin and the skin withthree kinds of pathological changes. The average spectrum of the latter has a lower reflectionrate than that of the former. The reason is that pathological changes alter the structures of thestratum corneum, epidermal layer, and dermis, affecting the reflection of light on the skinsurface. The very dark color of the pathological skin is also attributed to this alteration. Thedifferences among the three types of skin disease are visually slight; therefore, the averagespectra of MF, psoriasis, and atopic dermatitis are almost the same. Their main differenceis in the adsorption of hemoglobin near the 530 nm wave band. An increase in hemochromecauses a reduction in the reflection rate in this wave band. The difference near the short wave(about 450 nm) mainly comes from the increase in the number of abnormal lymphocytesand the reduction in reflection rate. The cell nucleus of the cancer cell becomes enlarged,increasing the adsorption of the short wave and reducing the reflection rate (Jen et al. 2014;van der Poel et al. 1990; Gorgidze et al. 1998).
3.2 Color contrast enhancement by LEDs lighting
Skin cancers are a significant health problem worldwide, and researchs are constantly lookingfor methods of early diagnosis. The traditional biopsy method is invasive, as well as requiring
123

Y.-P. Hsiao et al.
a time-consuming and high-cost pathological analysis. Skin cancer, for example, is studied bymany researchers for the possibility of using illuminants for diagnosis. These studies are basedon confirmed cases of skin cancer; studies of illumination for early detection are few. Previousstudies show that LEDs have good performance not only for exciting autofluorescence, butalso for enhancing the color contrast of microvasculature in narrowband imaging (Park et al.2007; Nishino et al. 2012; Lee et al. 2009; Li 2005; Kaarna et al. 2010).
The main idea of this study is to find the optimal color lighting which can enhance the colorcontrast between the abnormal and normal skin for optimal visual perception and detectionof MF. However, as indicated in previous studies, white light might not be the best choice,so multicolored LEDs should be tested in the same way. A suitable mix of colored LEDs canbe expected to be found. A computer program has been written to resolve the complex com-putation process. Spectra of a commercial RGBY LED with peak wavelengths at 617, 530,460, and 586 nm for red, green, blue, and yellow, respectively, are applied to illuminate theskin and calculate the color difference by changing the intensity ratio of each peak. By usingthis method, the color enhancement ability of every single point in the CIE (the Commis-sion Internationale de l’Éclairage) 1931 chromaticity diagram is calculated (Smith and Guild1931). The distribution of the maximum color difference also is found. After the computationof color difference for all illuminants, the maximum, the minimum and the median valuesare determined, and denoted as �E∗
ab max, �E∗ab min, and �E∗
abmid , respectively. Finally, acolor difference distribution diagram is plotted by the program, which represents the averagecolor differences by using the formula of RGB digital counts (Rd, Gd, Bd) as follows :
Rd = 255 − (�E∗ab max − �E∗
ab)[255/(�E∗ab max − �E∗
ab min)] (5)
Gd = 255 − ∣∣�E∗ab − �E∗
abmid
∣∣ [255/(�E∗ab max − �E∗
abmid)] (6)
Bd = 255 − (�E∗ab − �E∗
ab min)[255/(�E∗ab max − �E∗
ab min)] (7)
where “Rd”, “Gd”, and “Bd” are the degrees of difference between a color difference �E∗ab
and �E∗ab max, �E∗
ab min, and �E∗abmid , respectively.
The program is applied to the selected regions in Fig. 1a–c. By changing the intensity ratioof the red, green, blue, and yellow peaks about 2.5 million different light sources spectra arecalculated for RGBY LEDs by varying the intensity ratio by intervals of 0.025, the colordifferences between the normal and inflamed regions are calculated. Figure 4a shows colordifference distribution diagram which represents the average color difference between normaland inflamed skins of ten MF patients under the illumination of commercial RGBY LEDs,in which “x” and “y” are the chromaticity coordinates of the illuminant in the CIE 1931standard colorimetric system (colour figure online). The color bar at the right-side of thediagram refers to the color of the corresponding average color difference calculated usingEqs. 5–7, “R”, “G”, “B”, and “Y” denote the chromaticity coordinates of red, green, blue, andyellow LEDs, respectively. The chromaticity coordinates of any mixing ratio of these LEDswill be bounded in the area of these four points. A large color difference will appear red, asmall one, blue. It can be seen that the illuminant with a specific intensity ratio of red, green,blue, and yellow LEDs with (x, y)=(0.4205, 0.5198) has the best color contrast enhancementability, producing a color difference of 17.4002, as shown in the inserted plate at the top rightin the diagram. Figure 4b, c shows a color difference distribution diagram which representsthe average color difference between normal and inflamed skins of ten psoriasis and tenatopic dermatitis patients, where the distribution of the maximum values appears at a similarposition to that in Fig. 4a, and the color difference is 11.0735 and 17.2634, respectively.From color difference distribution diagrams, Fig. 4a–c can be found to the trend with the areacovered by the maximum color difference is too large, the reason is because the resolution
123

Non-invasive color contrast enhancement by LEDs lighting
Fig. 4 Color difference distribution diagrams of normal and inflamed regions in the skin of (a) ten MFpatients, (b) ten psoriasis patients, and (c) ten atopic dermatitis patients under the illumination of differenttypes of LEDs. d Chromaticity distribution diagram of the illumination light sources of the maximum colordifference for MF, psoriasis, and atopic dermatitis as green, blue, and red regions, respectively
of the scale bar is not obvious. Therefore, we take advantage of the multi-spectral imagingtechnology to produce excellent to poor color difference distribution diagram. A 2.5 millioncolor difference values for each patient, and each value corresponds to a set of chromaticitycoordinates. Using this correspondence, 30 patients with maximum color difference valuetaken out before 1,000 group, and presented in the graph of the chromaticity coordinates. Onthe graph will have each patient area by 1,000 chromaticity coordinates overlapping part ofthe area reserved for the same lesion, to skin lymphoma marked in green; Similarly availableatopic dermatitis marked red compared to psoriasis to blue in Fig. 4d. Therefore, we canknow that the similar visual symptoms of skin lesions, color difference can enhance visualrecognition through different color lighting sources, further identify different diseases.
3.3 Color images reproduction under the new illumination
In order to know the real visual perception of MF, psoriasis, and atopic dermatitis afterthe color contrast enhancement from the complex computation process above. Figure 5show the optimal lighting spectra of RGBY LEDs for (a) MF, (b) psoriasis, and (c)atopic dermatitis color contrast enhancement. The chromaticity coordinates of the threelight are (x,y)=(0.4271,0.5251), (x,y)=(0.3486,0.3872), and (x,y)=(0.3450,0.3805) of MF,psoriasis, and atopic dermatitis, respectively. The relative intensity ratio of RGBY LEDsare 0.5815:0.9361:0.0308:0.9999, 0.5791:0.9358:0.7523:0.9688, and 0.5922:0.9068:0.7494:
123

Y.-P. Hsiao et al.
Fig. 5 Optimal color lighting spectra for (a) MF, (b) psoriasis, and (c) atopic dermatitis with color contrastenhancement
0.9701 for MF, psoriasis, and atopic dermatitis, respectively. In color science, the CIE XYZcolor space defines all the colors in terms of three imaginary primaries based on the humanvisual system. X, Y and Z denote tristimulus values of a color stimulus (S(λ)) and areexpressed as
X = k
780nm∫
380nm
S(λ)x(λ)dλ, Y = k
780nm∫
380nm
S(λ)y(λ)dλ, Z = k
780nm∫
380nm
S(λ)z(λ)dλ (8)
where, x(λ), y(λ), and z(λ) are the color matching functions, S(λ) represents the spectralradiance at a certain wavelength λ which is the product of the spectral power distribution ofthe illuminant I(λ) and the spectral reflectance function of the object R(λ), and k is a constant(Wyszecki and Stiles 1982). The unit of the spectral power distribution is measured in powerunits, watts. The normalization constant k in equation is defined differently for relative andabsolute colorimetry. In absolute colorimetry, k is set equal to 683 lumen/W, and making thesystem of colorimetry compatible with the system of photometry. For relative colorimetry, kis defined by equation
k = 100780∫
380S(λ)y(λ)dλ
(9)
The normalization for relative colorimetry in Eq. (9) results in tristimulus values thatare scaled from zero to approximately 100 for various materials. In this study, we userelative colorimetry during estimation processes. The spectral radiometric quantity foreach pixel of one color image is simulated, then the new light source spectrum in S(λ)
(as Fig. 5a–c) is replaced to reproduce new color images with different light sources. Colorimages of the same position of skin based on different color light sources in the same con-ditions are demonstrated. In Fig. 6a, c, e show the color images from camera under theillumination of a fluorescent lamp for MF, psoriasis, and atopic dermatitis patients respec-tively. In Fig. 6b, d, f show color images reproduction of MF, psoriasis, and atopic dermatitispatients are under replacing the optimal LEDs light source of Fig. 4a–c, respectively. InFig. 6a, b, the blue arrows present the inflamed skins of a MF patient for highlighting theareas of interest. Under the illumination of the fluorescent lamp and optimal LEDs lamp,the average color difference between the normal and inflamed skins of the ten MF patientsis 11.8414 and 17.4002, respectively. The color contrast enhancement is about 47 % for MFpatients. For psoriasis, and atopic dermatitis patients, the average color difference between thenormal and inflamed skins is about 8.7671 and 12.8544 under the fluorescent lamp, 11.0735and 17.2634 under the optimal LEDs lamp, respectively. The color contrast enhancement is
123

Non-invasive color contrast enhancement by LEDs lighting
Fig. 6 Color images from camera are under fluorescent lamps of (a) MF, (c) psoriasis, and (e) atopic dermatitispatients. The color images reproduction of (b) MF, (d) psoriasis, and (f) atopic dermatitis patients are underreplacing the optimal LEDs light source of Fig. 3a–c, respectively
about 26.3 and 34.3 % for psoriasis, and atopic dermatitis patients, respectively. It is obviousfrom visual inspection that the color enhancement ability of the optimal LEDs in Fig. 6b, d,f are better than that of the fluorescent lamp in Fig. 6a, c, e. The significant differences in thevisual inspection, the normal and inflamed skins in color changes from skin color to slightlyred under the illumination of the fluorescent lamp and from yellow-green to slightly blackunder the illumination of the optimal LEDs lamp. For the color lighting, the optiaml colorilluminates of psoriasis and atopic dermatitis are correlated color temperature 4,000–5,000 Kof white lights. The illumination color for visual recognition of MF is yellow-green colorlighting. In the future, if we can develop a smart lighting environment through changes inlighting colors, doctors will be able to work in a more efficient visual identification space.
4 Conclusion
In this study, we analyzed the trend of the average spectrum of the three diseases, skin lesionsof 30 patients by the MSI technique. The differences among the three types of skin disease
123

Y.-P. Hsiao et al.
are visually slight; therefore, the average spectra of MF, psoriasis, and atopic dermatitis arealmost the same. The difference near the short wave (about 450 nm) mainly comes from theincrease in the number of abnormal lymphocytes and the reduction in reflection rate. The cellnucleus of the cancer cell becomes enlarged, increasing the adsorption of the short wave andreducing the reflection rate. We use the computer program to the proportion of the RGBYLEDs individual strength to enhance the color contrast in the images of the three diseases,lesions and normal areas. The color contrast enhancement is about 47, 26.3 and 34.3 % forMF, psoriasis, and atopic dermatitis patients, respectively. The chromaticity coordinates ofoptimal color lighting are (0.4205,0.5198), (0.3476, 0.3785), and (0.3486,0.3872) for MF,psoriasis, and atopic dermatitis patients. This study provides a novel idea, the use of differentlighting colors to increase the doctors on the visual identification MF lesions.
Acknowledgments This research was supported by National Science Council, The Republic of China, underthe Grants of NSC 100-2221-E-194-043, 101-2221-E-194-049, 102-2221-E-194-045, 102-2622-E-194-004-CC3 and Chung Shan Medical University Hospital (CSH-2011-C-024 and CSH-2013-C -018).
References
Anderson, M., Motta, R., Chandrasekar, S., Stokes, M.: Proposal for a standard default color space for theinternet: sRGB. In: IS&T/SID 4th Color Imaging Conference Proceedings, vol. 238 (1996)
Berns, R.S.: Principles of Color Technology. Wiley, Taipei (2000)Cowen, E.W., Liu, C.W., Steinberg, S.M., Kang, S., Vonderheid, E.C., Kwak, H.S., Booher, S., Petricoin,
E.F., Liotta, L.A., Whiteley, G., Hwang, S.T.: Differentiation of tumour-stage mycosis fungoides, psoriasisvulgaris and normal controls in a pilot study using serum proteomic analysis. Br. J. Dermatol. 157, 946–953(2007)
Frye, R., Myers, M., Axelrod, K.C., Ness, E.A., Piekarz, R.L., Bates, S.E., Booher, S.: Romidepsin: a newdrug for the treatment of cutaneous T-cell lymphoma. Clin. J. Oncol. Nurs. 2, 195–204 (2012)
Girardi, M., Heald, P.W., Wilson, L.D.: The pathogenesis of mycosis fungoides. N. Engl. J. Med. 350, 1978–1988 (2004)
Gorgidze, L.A., Oshemkova, S.A., Vorobjev, I.A.: Blue light inhibits mitosis in tissue culture cells. Biosci.Rep. 18, 215–224 (1998)
Hashimoto, N., Murakami, Y., Bautista, P.A., Yamaguchi, M., Obi, T., Ohyama, N., Uto, K., Kosugi, Y.:Multispectral image enhancement for effective visualization. Opt. Express 19, 9315–9329 (2011)
Hong Kong Cancer Fund. Understanding: lymphoma (2007). http://www.cancer-fund.orgJen, C.P., Huang, C.T., Chen, Y.S., Wang, H.C.: Diagnosis of human bladder cancer cells at different stages
using multispectral imaging microscopy. Ieee J. Sel. Top. Quantum Electron. 20, 6800808 (2014)Kaarna, A., Nishino, K., Miyazawa, K., Nakauchi, S.: Michromatic scope for enhancement of color difference.
Color Res. Appl. 35, 101–109 (2010)Lam, Y.M., Xin, J.H.: Evaluation of the quality of different D65 simulators for visual assessment. Color Res.
Appl. 27, 243–251 (2002)Lee, C.H., Hwang, S.T.Y.: Pathophysiology of chemokines and chemokine receptors in dermatological science:
a focus on psoriasis and cutaneous T-cell lymphoma. Dermatol. Sin. 30, 128–135 (2012)Lee, M.H., Seo, D.K., Seo, B.K., Park, J.I.: Optimal illumination for discriminating objects with different
spectra. Opt. Lett. 34, 2664–2666 (2009)Li, X.M.: Image enhancement algorithm based on retinex theory. Appl. Res. Comput. 2, 235–237 (2005)Li, C.J., Luo, M.R., Rigg, B., Hunt, R.W.G.: CMC 2000 chromatic adaptation transform: CMCCAT2000.
Color Res. Appl. 27, 49–58 (2002)McCamy, C.S., Marcus, H., Davidson, J.G.: A color rendition chart. J. Appl. Photogr. Eng. 11, 95–99 (1976)Nishino, K., Kaarna, A., Miyazawa, K., Oda, H., Nakauchi, S.: Optical implementation of spectral filtering
for the enhancement of skin color discrimination. Color Res. Appl. 37, 53–58 (2012)Park, J.I., Lee, M.H., Grossberg, M.D., Nayar, S.K.: Multispectral imaging using multiplexed illumination.
In: Proceedings of ICCV, pp. 1–8 (2007)Smith, T., Guild, J.: The C.I.E. colorimetric standards and their use. Trans. Opt. Soc. 33, 73–134 (1931)van der Poel, H.G., Boon, M.E., van der Meulen, E.A., Wijsman-Grootendorst, A.: The reproducibility of
cytomorphometrical grading of bladder tumours. Virchows Arch. A Pathol. Anat. Histopathol. 416, 521–525 (1990)
123

Non-invasive color contrast enhancement by LEDs lighting
Wang, H.C., Chen, Y.T., Lin, J.T., Chiang, C.P., Cheng, F.H.: Enhanced visualization of oral cavity for earlyinflamed tissue detection. Opt. Express 18, 11800–11809 (2010)
Wang, H.C., Chen, Y.T.: Optimal lighting of RGB LEDs for oral cavity detection. Opt. Express 20, 10186–10199 (2012)
Wang, H.C., Tsai, M.T., Chiang, C.P.: Visual perception enhancement for detection of cancerous oral tissueby multi-spectral imaging. J. Opt. 15, 055301–055312 (2013)
Whittaker, S.J., Marsden, J.R., Spittle, M., Russell, J.R.: Joint British Association of Dermatologists and U.K.Cutaneous Lymphoma Group guidelines for the management of primary cutaneous T-cell lymphomas. Br.J. Dermatol. 149, 1095–1107 (2003)
Willemze, R., Dreyling, M.: Primary cutaneous lymphomas: ESMO Clinical practice guidelines f or diagnosis,treatment and follow-up. Ann. Oncol. 21, 177–180 (2010)
Willemze, R., Jaffe, E.S., Burg, G., Cerroni, L., Berti, E., Swerdlow, S.H., Ralfkiaer, E., Chimenti, S., Diaz-Perez, J.L., Duncan, L.M., Grange, F., Harris, N.L., Kempf, W., Kerl, H., Kurrer, M., Knobler, R., Pimpinelli,N., Sander, C., Santucci, M., Sterry, W., Vermeer, M.H., Wechsler, J., Whittaker, S., Meijer, C.J.: WHO-EORTC classification for cutaneous lymphomas. Blood 105, 3768–3785 (2005)
World Health Organization.: Romidepsin: approved for cutaneous T-cell lymphoma. WHO Drug Inform. 23,306–307 (2009)
Wyszecki, G., Stiles, W.: Color Science - Concepts and Methods, Quantitative Data and Formulate, 2nd edn.Wiley, New York (1982)
Yamaguchi, M.: Medical application of a color reproduction system with a multispectral camera. Digit. ColorImaging Biomed. 2, 33–38 (2001)
Yamaguchi, M., Mitsui, M., Murakami, Y., Fukuda, H., Ohyama, N., Kubota, Y.: Multispectral color imagingfor dermatology: application in inflammatory and immunologic diseases. In: IS&T/SID 13th Color ImagingConference, Scottsdale, Arizona, vol. 39, pp. 52–58 (2005)
123